Abstract
Vector-borne rickettsial infection is a major cause of febrile illnesses throughout the world. Although vertebrates hosting the vectors play a vital role in the natural cycle of rickettsiae, studies have not been conducted on them in Sri Lanka. Therefore, the present study was designed to determine the exposure of dog population in Rajawatta, Thambavita, and areas of the Western Slopes and Unawatuna of Sri Lanka to rickettsial pathogens. A total of 123 dog blood samples were collected from those areas. Samples were tested for antibodies against Rickettsia conorii (RC) of the spotted fever group (SFG), Rickettsia typhi (RT) of the typhus group (TG), and Orientia tsutsugamushi (OT) of the scrub typhus group (ST) of rickettsiae by indirect immunofluorescence antibody test (IFA). Samples with titers ≥1:64 were considered as positive in this study. Collectively, 49% dogs were found to have antibodies against the rickettsial agents. Of the dogs, 42%, 24%, and 2% had antibodies against RC, OT, and RT, respectively. The seropositive rate of 100% was observed in areas of the Western Slopes, whereas the lowest rate of 20% was in Unawatuna. Among the positive samples, antibody titers against RC and OT ranged from 1/64 to 1/8192. In contrast, the few dogs that tested positive for RT showed very low titers of 1/64 and 1/128. Results of this study show the extent of exposure to the pathogen and its dispersion in the natural ecology. We suggest that dogs could be acting as reservoirs in the rickettsial transmission cycle or could be effective tracer animals that can be used to detect areas with potential for future outbreaks.
Key Words: Spotted fever group, Typhus group, Scrub typhus group, Sri Lanka
Introduction
Two genera, Rickettsia and Orientia, within the family Rickettsiaceae are comprised of intracellular bacteria, many of which cause zoonotic diseases in different parts of the world. The genus Rickettsia includes two immunologic groups, the typhus group (TG), which is composed of Rickettsia prowazekii and Rickettsia typhi associated with lice and fleas, and the spotted fever group (SFG), which includes more than 20 valid species associated with ticks, mites, and fleas (Parola et al. 2005). Genus Orientia includes two species that cause scrub typhus, Orientia tsutsugamushi transmitted by chiggers and the recently discovered Orientia chuto (Tamura et al. 1995, Izzard et al. 2010).
Vertebrates play a vital role in the natural cycle of Rickettsia and Orientia because they are natural hosts of many vectors of rickettsiae (Parola et al. 2005). The dog, which is a common domestic vertebrate, has also been suggested as a probable natural reservoir (Feng et al. 1979, Solano-Gallego et al. 2006). Price (1954) demonstrated that ticks are infected when feeding on dogs during the peak period of rickettsemia. However, Rovery et al. (2008) stated that dogs are not efficient reservoirs but rather act as transient reservoirs because of transient rickettsemia after infection. Nevertheless, dogs as vertebrates living in close proximity to humans could be playing an important role in transmitting rickettsial infection to them by transporting the infected vectors in to the human habitats (Chenchittikul et al. 2000, Nicholson et al. 2010). A report exists of concurrent outbreak of rickettsial infection in both a dog and its owner (Paddock et al. 2002). Furthermore, Mannelli et al. (2003) detected associations between occurrences of infection among humans with the proximity of the dogs.
Several publications exist regarding human rickettsial infection in Sri Lanka. The first recorded reference to rickettsial infection in the country dates back to 1937 in a short report by Nicholls (1940). The report documents scrub typhus patients who were confirmed in the laboratory with the Weil–Felix test. Thereafter, studies have reported cases of scrub typhus, spotted fever, and murine typhus from all 9 provinces of Sri Lanka (Sayers 1948, Van Peenen et al. 1976, Vasanthatilaka and Senanayaka 1994, Kularatne et al. 2003, Punchihewa and Karunanayaka 2003, Premaratna et al. 2008, Murugananthan 2010, Liyanapathirana and Thevanesam 2011). However, investigations on vector or reservoir species of these pathogenic rickettsiae have been minimal. Other than the early studies of Wolff (1939) on rat fleas collected from the port city Colombo and of Jayewickreme and Niles (1946, 1947) on Trombiculid mites, no entomological studies have been carried out thus far. Furthermore, no reports exist on possible rickettsial reservoirs within the country. This is the first study that presents the data from a canine serosurvey of exposure to Rickettsia and Orientia in arbitrarily selected regions of Sri Lanka.
Materials And Methods
Collection of blood samples from dogs
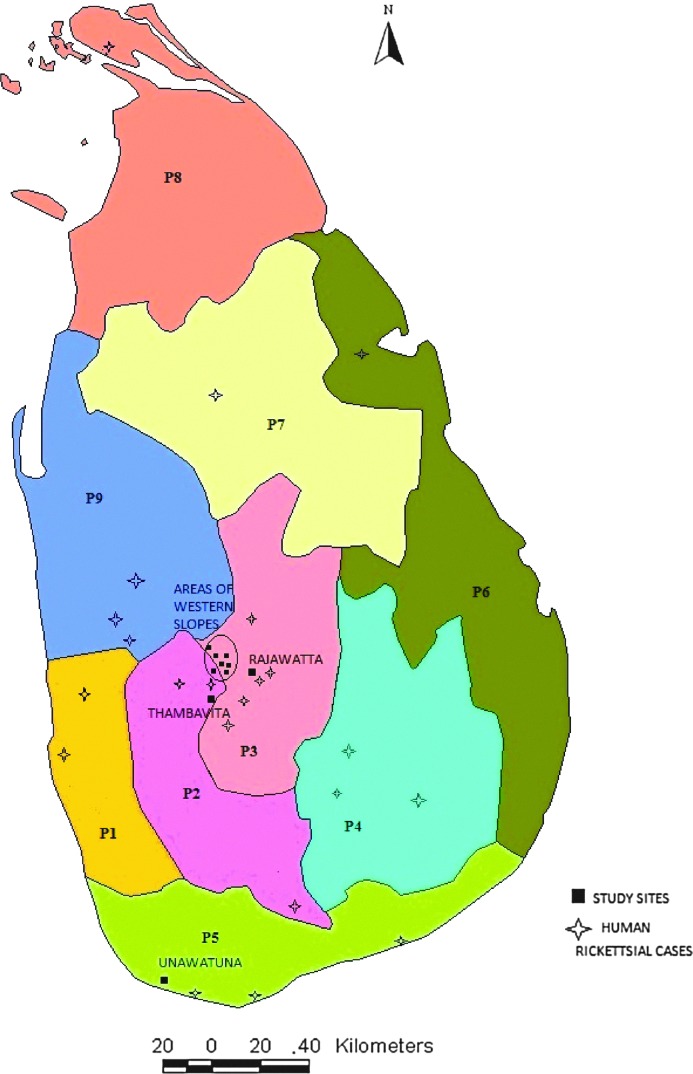
A total of 73 blood samples were collected from the dogs in Rajawatta, Thambavita, and areas of Western Slopes (Pottapitiya, Hatharaliyadda, Kadugannawa, Wattapola, Muruthalawa, Mawanella, and Hingula) in 2010 (Fig. 1). In addition, 50 blood samples were obtained from the mobile sterilization project conducted at Unawatuna in southern Sri Lanka in the same year. All the dogs had owners; however, they were free roaming. Samples were collected from both male and female dogs. Serum was separated by centrifugation at 2200×g for 5 min. Labeled samples were stored at −20°C until use.
FIG. 1.
Locations of reported (published) human rickettsial cases and the sample collection sites of the current study, Sri lanka. P1, Western Province; P2, Sabaragamuwa Province; P3, Central Province; P4, Uva Province; P5, Southern Province; P6, Eastern Province; P7, North Central Province; P8, Northern Province; P9, North Western Province. Color images available online at www.liebertpub.com/vbz
Ethical clearance was obtained from the Ethical Review Committee of Faculty of Veterinary Medicine and Animal Science, University of Peradeniya, Sri Lanka.
Immunoflourescence antibody assay
The serum samples were tested serologically by an immunofluorescence antibody assay (IFA). Frozen antigen pellets of Rickettsia conorii strain Malish (RC) of the spotted fever group, Rickettsia typhi strain Wilmington (RT) of murine typhus group, and Orientia tsutsugamushi strain Karp (OT) of scrub typhus group were obtained from the center for the Rickettsia- and Bartonella-associated diseases (Centers for Disease Control and Prevention, Atlanta, GA). Antigen pellets were diluted in 0.5 mL of antigen-diluting buffer (phosphate-buffered saline [PBS] at pH 7.38 mixed with 0.1% sodium azide and 0.5% gamma globulin–free bovine serum albumin [BSA]). Teflon-coated IFA slides (Cel-Line, Erie Scientific Co.) with wells were first coated with 1% BSA, and then approximately 3 μL of antigen suspension was aliquoted to each well and dried. Test sera were thawed and vortexed briefly. The 1:16 serum dilution was prepared by mixing 5 μL of serum with 75 μL of IFA buffer (PBS at pH 7.38 mixed with 0.1% sodium azide, 1% gamma globulin–free BSA, and 1% heat-inactivated normal goat serum) in a 96-well flat-bottomed plate, after which 1:32 dilution was made by half-diluting the above in 50 μL of IFA buffer. Screening was done at 1:32 dilution. Then diluted (1:32) serum was aliquoted into the wells of the rickettsial antigen-coated slides. Known positive and negative samples at the same dilutions were used as controls. Slides were kept in a damp box and incubated at 37°C for 30 min, and samples were washed three times with PBS (1.7 grams of sodium phosphate, 0.4 gram of potassium phosphate, and 8.5 gram of sodium chloride mixed into 1 L of distilled water, pH adjusted to 7.38) in a slide-staining chamber for 5 min at each washing. After drying the slides, fluorescence-labeled goat anti-dog immunoglobulin G (IgG) (H+L) conjugate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD) diluted to 1:100 with IFA buffer was aliquoted into each well. The conjugant was not aliquoted to the well assigned to be used as a conjugant control.
After incubating these slides at 37°C for 30 min, slides were washed again 3 times with PBS; 5–10 drops of counterstain, Eriochrome Black T, was added for the final wash. Slides were dried, a small amount of antifade medium (90 mL of glycerol, 10 mL of PBS at pH 7.38, and 3.37 grams of DABCO were mixed together) was added to each well, and a cover slip was placed over the slide. Then, slides were observed under 40× magnification with an ultraviolet (UV) light source. Negative wells showed only a faint red counterstain, whereas positive wells showed individual organisms fluorescing as discrete, bright green specks within the cytoplasm and surrounding lysed cells. Positive serum samples were serially diluted to determine their end point, and only those with titers ≥1:64 were considered positive for this study (cutoff of 1:64 was recommended in the standardized protocol of IFA test provided by the Centers for Disease Control and Prevention).
Results
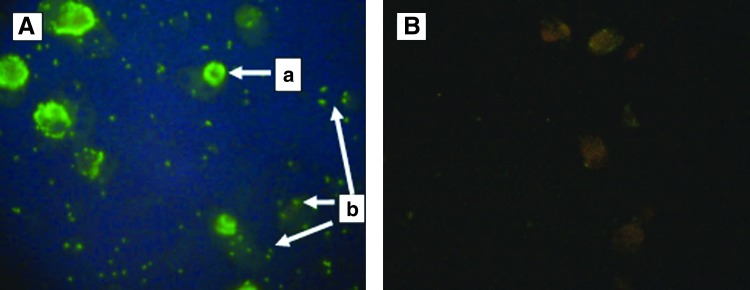
Serum samples with antibodies to rickettsiae showed green fluorescence under the UV microscope (Fig. 2). Of 123 dog serum samples, 60 (49%) sera were detected with anti-rickettsial antibodies. Most of the dogs (42%) had antibodies reacting with the antigen of R. conorii. Twenty-nine (24%) dogs exhibited antibodies against O. tsutsugamushi, whereas just 3 (2%) dogs of the study group had antibodies reacting with R. typhi. The presence of antibodies to rickettsiae varied with location ranging from 20% to 100% (Table 1). All dogs (100%) from areas of the Western Slopes had anti-rickettsial antibodies, whereas the lowest seropositivity was observed in Unawatuna, with 20% of the study group having anti-rickettsial antibodies. Dogs that tested positively for IFA with R. conorii and O. tsutsugamushi were found in all 4 study sites; however, antibodies reacting with R. typhi antigen were detected only in dogs from Rajawatta and in areas of the Western Slopes. In all areas, seroreactivity against the spotted fever group antigen was the most prevalent. Comparably, a substantial number of dogs in Rajawatta and Western Slopes were also positive for scrub typhus.
FIG. 2.
Rickettsiae-infected Vero cells reacted by indirect immunoflourescence with canine antiserum (A) (a, organisms within the cytoplasm of an intact cell; b, organisms from a lysed cell) negative canine serum (control) (B).
Table 1.
Reactions of Dog Serum to IFA in Sri Lanka, 2010
| |
|
|
Serum samples positive for |
|||||
|---|---|---|---|---|---|---|---|---|
| Area | No. of samples | No. of positive samples | RC only | OT only | RT only | RC and OT | RT and OT | RC, OT, and RT |
| Rajawatta | 32 | 15 (47%) | 7 (22%) | 4 (12%) | 1 (3%) | 2 (6%) | 1 (3%) | 0 (0) |
| Thambavita | 18 | 12 (67%) | 10 (55%) | 0 (0) | 0 (0) | 2 (11%) | 0 (0) | 0 (0) |
| Western Slopes | 23 | 23 (100%) | 4 (17%) | 1 (4%) | 0 (0) | 17 (74%) | 0 (0) | 1 (4%) |
| Unawatuna | 50 | 10 (20%) | 9 (18%) | 1 (2%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Total | 123 | 60 (49%) | 30 (24%) | 6 (5%) | 1 (1%) | 21 (17%) | 1 (1%) | 1 (1%) |
IFA, immunofluorescence antibody test; RC, Rickettsia conorii; OT, Orientia tsutsugamushi; RT, Rickettsia typhi.
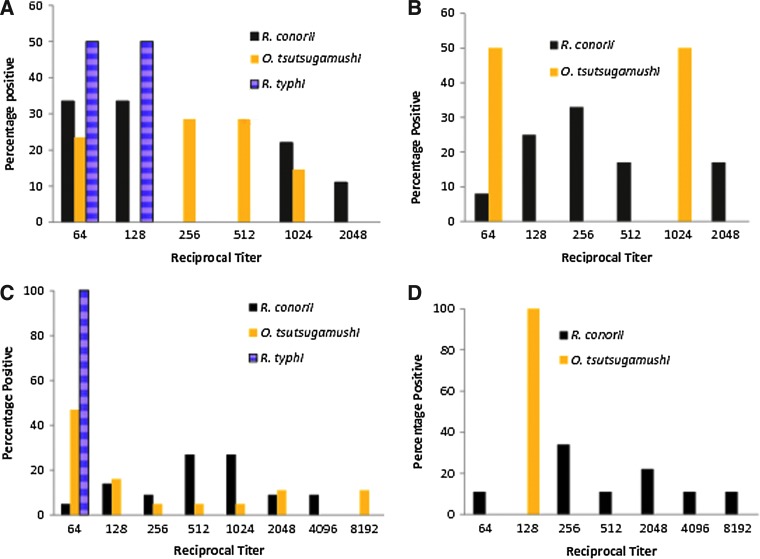
The highest titer levels were reported against R. conorii, in Rajawatta, Thambavita, and Unawatuna, but in areas of Western Slopes the highest titer of 1/8192 was against O. tsutsugamushi. The lowest titers were against R. typhi, and they did not exceed 1/128 (Fig. 3).
FIG. 3.
Titer distribution in dogs positive for Rickettsia in Sri Lanka 2010. (A) Rajawatta, (B) Thambavita, (C) areas of Western Slopes, (D) Unawatuna.
Discussion
In the present study, we determined for the first time the presence of rickettsial antibodies in dogs in Sri Lanka. Antibodies against all 3 antigenic groups of rickettsia were detected. Seropositive rates in dogs varied from place to place. We suggest that this might be due to the variations of vector densities (Kelley et al. 1991) and preference of feeding by different vector species. In our study, the majority of the canines exhibited antibodies against the SFG rickettsia. Because most of the SFG rickettsiae are transmitted by hard ticks, it is possible that the density of responsible vector tick might be high in all study sites. Epidemiology of the infection is linked to particular behavior and ecology of each vector species. Therefore, extensive studies should be conducted to determine the vectors occurring in Sri Lanka.
A few dogs showed high antibody titers, such as 1/2048 and 1/8192, against rickettsiae. The presence of such high titers is probable only with a recent exposure or repeated inoculation with the infectious pathogen (Breitschwerdt et al. 1988). Twenty-three samples gave positive results against 2 or 3 rickettsial antigens in an IFA assay. Twenty-one dogs (17%) had antibodies against both SFG and ST groups. One dog exhibited antibodies against ST and TG (OT, 1/512; RT, 1/64) and 1 had antibodies against all 3 groups (RC, 1/1024; OT, 1/128; and RT, 1/64). The cross-reactions between SFG/TG and the scrub typhus group have not been detected. Antibodies against both groups could be due to exposure to the both groups of rickettsiae. In addition, cross-reactivity could have occurred as a result of similar antigens in SFG and TG (Tissot-Dupont et al. 1995). Thus, dogs exhibiting antibodies against both typhus and SFG might be due to serological cross-reactivity.
Several cases of humans infected with rickettsioses have been documented in proximity to the sites where this canine survey was conducted (Fig. 1). The detection of antibodies to rickettsiae in both human and dog populations indicates the extent of diffusion of the pathogen in the natural ecology of these areas. It also suggests the possibility of using canines as a probable tracer animal to determine the areas with potential risk of rickettsial outbreaks in future. Harrus et al. (2007) revealed higher seroprevalence rates among dogs in endemic zones where human disease have been reported. Also studies by Segura Porta et al. (1998) and Demma et al. (2006) found that canine rickettsioses often precede outbreaks in human populations. Thus, further serological studies on dog population in other provinces should be carried out to identify the possible endemic areas of rickettisioses within the country. Findings of such a study will provide valuable information to plan better strategies for the control and prevention of outbreaks.
Conclusions
In the present study, 49% of dogs exhibited serological reactivity to pathogenic rickettsial species. This indicates the possible use of the dog as a tracer animal to detect endemic zones with the potential risk of human outbreaks in the future. Studies attempting isolation of rickettsial pathogen from dogs should be carried out to determine if dogs are reservoirs in the rickettsial cycle or just an exposed animal with transient disease.
Acknowledgments
This study received financial support by the National Research Council of Sri Lanka (Research Grant 09-05). The authors sincerely acknowledge Dr. G.A. Dasch, CDC, Atlanta for providing antigens for IFA test and technical advice for establishing the serological test.
Author Disclosure Statement
No competing financial interests exist.
References
- Breitschwerdt EB. Walker DH. Levy MG. Burgdorfer W, et al. Clinical, hematologic, and humoral immune response in female dogs inoculated with Rickettsia rickettsii and Rickettsia montana. Am J Vet Res. 1988;49:70–76. [PubMed] [Google Scholar]
- Chenchittikul M. Pangjai D. Warachit P. Prevalence of antibody to Orientia tsutsugamushi in dogs along Thai-Myanmar border. Kasetsart J. 2000;34:91–97. [Google Scholar]
- Demma LJ. Traeger M. Blau D. Gordon R, et al. Serologic evidence for exposure to Rickettsia rickettsii in eastern Arizona and recent emergence of Rocky Mountain spotted fever in this region. Vector Borne Zoonotic Dis. 2006;6:423–429. doi: 10.1089/vbz.2006.6.423. [DOI] [PubMed] [Google Scholar]
- Feng WC. Murray ES. Rosenberg GE. Spielman JM, et al. Natural infection of dogs on Cape Cod with Rickettsia rickettsii. J Clin Microbiol. 1979;10:322–325. doi: 10.1128/jcm.10.3.322-325.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrus S. Lior Y. Ephros M. Grisaru-Soen G. Keysary A, et al. Rickettsia conorii in humans and dogs. A seroepidemiologic survey of two rural villages in Israel. Am J Trop Med Hyg. 2007;77:133–135. [PubMed] [Google Scholar]
- Izzard L. Fuller A. Blacksell SD. Paris DH. Richards AL, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayewickreme SH. Niles WJ. Successful feeding experiments with an adult Trombiculid mites (order Acarina) Nature. 1946;157:878. doi: 10.1038/157878a0. [DOI] [PubMed] [Google Scholar]
- Jayewickreme SH. Niles WJ. Rearing of Trombicula acuscutellaris Walch. Nature. 1947;160:578. doi: 10.1038/160578a0. [DOI] [PubMed] [Google Scholar]
- Kelley PJ. Mason PR. Manning T. Slater S. Role of cattle in the epidemiology of tick-bite fever in Zimbabwe. J Clin Microbiol. 1991;29:256–259. doi: 10.1128/jcm.29.2.256-259.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kularatne SAM. Edirisinghe JS. Gawarammana IB. Urakami H, et al. Emerging rickettsial infections in Sri Lanka: The pattern in the hilly Central Province. Trop Med Int Health. 2003;8:803–811. doi: 10.1046/j.1365-3156.2003.01108.x. [DOI] [PubMed] [Google Scholar]
- Liyanapathirana VC. Thevanesam V. Seroepidemiology of rickettsioses in Sri Lanka: A patient based study. BMC Infect Dis. 2011;11:328. doi: 10.1186/1471-2334-11-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannelli A. Mandola ML. Pedri P. Tripoli M, et al. Associations between dogs that were serologically positive for Rickettsia conorii relative to the residences of two human cases of Mediterranean spotted fever in Piemonte (Italy) Prev Vet Med. 2003;60:13–26. doi: 10.1016/s0167-5877(03)00079-5. [DOI] [PubMed] [Google Scholar]
- Murugananthan K. Enigma of emerging typhus in Jaffna- A public health challenge. Proc Ann Jaffna Sci Assn. 2010:29–32. [Google Scholar]
- Nicholls L. A case of Tsutsugamushi (rural typhus) in Ceylon. Br Med J. 1940;2:490. [Google Scholar]
- Nicholson WL. Allen KE. McQuiston JH. Breitschwerdt EB, et al. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol. 2010;26:205–212. doi: 10.1016/j.pt.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Paddock CD. Brenner O. Vaid C. Boyd DB, et al. Short report: Concurrent Rocky Mountain spotted fever in a dog and its owner. Am J Trop Med Hyg. 2002;66:197–199. doi: 10.4269/ajtmh.2002.66.197. [DOI] [PubMed] [Google Scholar]
- Parola P. Paddock CD. Raoult D. Tick borne rickettsioses around the world. Emerging diseases challenging old concepts. Clin Microbiol Rev. 2005;18:719–756. doi: 10.1128/CMR.18.4.719-756.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premaratna R. Loftis AD. Chandrasena TGAN. Dasch GA, et al. Rickettsial infections and their clinical presentations in the Western Province of Sri Lanka: A hospital-based study. Int J Infect Dis. 2008;12:198–202. doi: 10.1016/j.ijid.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Price WH. The epidemiology of Rocky Mountain spotted fever, studies on the biological survival mechanisms of Rickettsia rickettsii. Am J Hyg. 1954;60:292–319. doi: 10.1093/oxfordjournals.aje.a119723. [DOI] [PubMed] [Google Scholar]
- Punchihewa PMG. Karunanayaka MCG. Tsutsugamushi fever: A substantial health problem in Southern Province. Abstract. 7th Annual Congress of the Sri Lanka College of Paediatricians; 2003. [Google Scholar]
- Rovery C. Brouqui P. Raoult D. Synopsis; Questions on Mediterranean spotted fever a century after its discovery. Emerg Infect Diseases. 2008;14:1360–1367. doi: 10.3201/eid1409.071133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayers MHP. The occurrence and identification of typhus group of fevers in South East Asia Command. J R Army Med Corps. 1948;90:6–22. [PubMed] [Google Scholar]
- Segura-Porta F. Diestre-Ortin G. Ortuno-Romero A. Sanfeliu-Sala I, et al. Prevalence of antibodies to spotted fever group rickettsiae in human beings and dogs from an endemic area of Mediterranean spotted fever in Catalonia, Spain. Eur J Epidemiol. 1998;14:395–398. doi: 10.1023/a:1007479909654. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L. Kidd L. Trotta M. Marco MD. Caldin M, et al. Febrile illness associated with Rickettsia conorii Infection in dogs from Sisily. Emerg Infect Diseases. 2006;12:1985–1988. doi: 10.3201/eid1212.060326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura A. Ohashi N. Urakami H. Miyamura S. Classification of Rickettsia tsutsugamushi in a new genus, Orientia gen. nov., as Orientia tsutsugamushi comb. nov. Int J Syst Bacteriol. 1995;45:589–91. doi: 10.1099/00207713-45-3-589. [DOI] [PubMed] [Google Scholar]
- Tissot-Dupont H. Brouqui P. Faugere B. Raoult D. Prevalence of antibodies to Coxiella burneti, Rickettsia conorii and Rickettsia typhi in seven African countries. Clin Infect Dis. 1995;21:1126–1133. doi: 10.1093/clinids/21.5.1126. [DOI] [PubMed] [Google Scholar]
- Van Peenen PFD. See R. Soysa PE. Irving GS. Seroepidemiological survey of hospital associated populations in Colombo, Sri Lanka. Southeast Asian J Trop Med Public Health. 1976;7:16–20. [PubMed] [Google Scholar]
- Vasanthathilaka VWJK. Senanayaka N. Abstract, Proceedings of the 107th Anniversary Academic Sessions of the Sri Lanka Medical Association. Murine typhus in Sri Lanka. 1994 [Google Scholar]
- Wolff EK. A strain of tropical typhus recovered from rat fleas. Ceylon J Sci. 1939;V:39–45. [Google Scholar]