Abstract
Tuberculosis (TB) remains a serious threat to global public health, largely due to the successful manipulation of the host immunity by its etiological agent Mycobacterium tuberculosis. The PE_PGRS protein family of M. tuberculosis might be a contributing factor. To investigate the roles of PE_PGRS17, the gene of PE_PGRS 17 was expressed in nonpathogenic fast growing Mycobacterium smegmatis. We found that the recombinant strain survives better than the control in macrophage cultures, accompanied by more host cell death and a marked higher secretion of tumor necrosis factor-alpha by a recombinant strain compared with control. Blocking the action of Erk kinase by an inhibitor can abolish the above effects. In brief, our data showed that PE_PGRS 17 might facilitate pathogen survival and disserve the host cell via remodeling the macrophages immune niche largely consisting of inflammatory cytokines. This furnishes a novel insight into the immune role of this mycobacterium unique gene family.
Introduction
Tuberculosis (TB) remains a formidable infectious disease with global significance (Raviglione and others 1995). About one third of the world's population is latently infected with Mycobacterium tuberculosis, the etiological agent of TB. About 3 million people die of active TB annually. M. tuberculosis is one of the most successful intracellular pathogens, which can resist a variety of antimicrobial mechanisms, and even modulate the immunological niches of monocytes and macrophages (Huang and others 2010). The M. tuberculosis PE_PGRS family has been widely proposed as a molecular mantra mediating the evasion of host killing. This family is featured by the conserved N-terminal and variable C-terminal. The PE_PGRS protein family, with functions largely unknown, exclusively presents among pathogenic mycobacteria and is associated with the mycobacterial cell wall (Banu and others 2002; Singh and others 2008).
Several members of the PE_PGRS family have been heterologously expressed and functionally characterized. The PE_PGRS family can be functionally classified into 3 groups: (1) some cell wall-associated PE_PGRS proteins can change the cell structure such as the shape and size, as well as colony formation (Delogu and others 2004); (2) the PE_PGRS family contains proteins such as lipase necessary for M. tuberculosis multiplication and persistence within macrophages (Deb and others 2006; Talarico and others 2007); (3) some PE_PGRS proteins are well-established antigens, and actively engaged in the interaction with the host cells (Brennan and others 2001). Partially activated macrophages after M. tuberculosis infection seek to suppress the intracellular persistence and replication of the bacteria. However, virulent M. tuberculosis outwits the macrophages somehow (Shinnick and others 1995; Fenton and Vermeulen 1996; Behar and others 2010). Investigation of mechanisms underlying the interactions between macrophages and M. tuberculosis is crucial to understand the pathogenesis of M. tuberculosis and find better countermeasures.
The PE_PGRS 17 (Rv0978c) displays distinct antigenic attributes and is dependent on the pathological conditions (Bansal and others 2010). The characteristics of PE_PGRS 17 include the following 3 aspects: (1) PE_PGRS 17 is upregulated in M. tuberculosis within mouse macrophages, and upregulated more than 8-fold in the human brain microvascular endothelial cell (Schnappinger and others 2003; Jain and others 2006); (2) among all the proteins of the PE family, PE_PGRS 17 exhibits very high antigenicity (Narayana and others 2007); (3) PE_PGRS 17 is reactive to sera from tuberculosis patients and demonstrates much higher titration than other PE antigens (Narayana and others 2007).
The rapid growth and readily expression of heterogenous genes from pathogenic mycobacteria enable the nonpathogenic Mycobacterium smegmatis a good surrogate for the pathogenic mycobacteria. We constructed the recombinant M. smegmatis strain expressing the PE_PGRS 17 protein, and investigated whether PE_PGRS17 can promote the survival of recombinant M. smegmatis in the differentiated human macrophage-like cell line U937. The cytokine responses such as the tumor necrosis factor (TNF)-α and interleukin-10 (IL-10) secreted by U937 were also assayed.
Materials and Methods
Strains and vectors
The sterilized MTB H37Rv strain was provided by Chongqing Pulmonary Hospital. The pMD19-T Simple Vector was purchased from TakaRa BIO Co., Ltd. The human monocytic U937 cell was purchased from the Conservation Center in Wuhan University, China. The strains of Escherichia coli and M. smegmatis mc2 155, and pNIT (MYC) plasmid were preserved in the Institute of Modern Biopharmaceuticals.
Methods
Mycobacterial culture and recombinant DNA manipulations
M. smegmatis mc2 155 (Msmeg) and the derivative strains were cultured at 37°C in the Middlebrook 7H9 broth with 0.05% Tween-80, and plated in 7H10 supplemented with 0.5% bovine serum albumin and 0.15% sodium chloride (Pandey and others 2009). E. coli was grown in Luria-Bertani media according to the protocol instruction. Kanamycin was added to the required concentration at 20 μg/mL.
Gene amplification, plasmids construction, and recombinant M. smegmatis
The open reading frame of PE_PGRS 17 (Rv0978c) was amplified by polymerase chain reaction (PCR) using genomic DNA of the sterilized MTB H37Rv strain as previously described. The primer sequence of Rv0978c was 5′-GGGAATTCATGTCGTTTGTCAACGTG-3′ (forward) and 5′-TGGGATCCGCTGATTACCGACAC-3′ (reverse) with EcoRI and BamHI sites (underscored), respectively. The purified PCR fragments were cloned into the shuttle expression vector pNIT (MYC) pretreated with the same enzymes to yield pNIT (MYC)-Rv0978c. The plasmids pNIT (MYC) and pNIT (MYC)-Rv0978c were introduced into M. smegmatis by electroporation (Snapper and others 1990), and colonies were selected on 7H10 agar plates, and subsequently cultured in 7H9 liquid media. The success of the gene transformed in recombinant M. smegmatis was confirmed by sequencing.
Expression of recombinant plasmid in M. smegmatis
The recombinant M. smegmatis strains were cultured in the 7H9 liquid medium supplemented with 0.05% Tween-80, 0.5% bovine serum albumin, and 0.15% sodium chloride at 37°C. When OD600 reaches 0.8–1.0, the inducer ɛ-caprolactam was added to a final concentration of 28 mM and incubating for 24 h (Pandey and others 2009). An aliquot of 2 mL was centrifuged with 12,000 r/min for 1 min and the cell pellet was washed twice with prechilled phosphate-buffered saline (PBS) and resuspended in 200 μL prechilled PBS for ultrasonication. About 36 μL of the 20× sodium dodecyl sulfate (SDS)-loading buffer was subsequently added to 60 μL cell suspension with 4 μL β - mercaptoethanol. The cell suspension was boiled at 100°C for 10 min, followed by centrifugation with 12,000 r/min for 10 min. The proteins were then analyzed by SDS-polyacrylamide gel electrophoresis and Western Blot.
Cell cultures
The human monocytic U937 cells were cultured in the RPMI-1640 (Hyclone) medium containing 2 mM L-glutamine, 10 mM HEPES, supplemented with 10% fetal calf serum (Hyclone), 100 U/mL of penicillin, and 100 U/mL of streptomycin. U937 cells can be converted from a nonadherent, weak phagocytic form to an adherent, active phagocytic state after the stimulation of phorbol esters and other agents. In our study, monocytic U937 cells were differentiated into macrophages by incubating with Phorbol Myristate Acetate (Sigma) at the final concentration of 100 ng/mL for 48 h before infection. The cell line of U937 is an established model to study the interaction between the macrophage and intracellular pathogens (Bosque and others 1998; Wei and others 2000; Song and others 2003).
Measurement of the viability of the infected macrophages by lactate dehydrogenase (LDH) release assay
After differentiation into macrophages, U937 cells containing 2×106 monolayers were infected with recombinant M. smegmatis strains at a multiplicity of infection (MOI) of 10:1 bacteria/cell, and incubated for 4 h at 37°C under 5% CO2. After the duration allowed for phagocytosis, cells were washed 3 times with sterile PBS, and then hygromycin was added to a final concentration of 100 μg/mL to remove extracellular bacteria. After 2 h, cells were incubated again with a fresh RPMI-1640 medium plus 10% fetal calf serum for 6, 24, and 48 h. Each sample has 3 repeats in this experiment, and similar results were obtained in 3 independent experiments. Macrophages infected with M. smeg-pNIT (MYC) were set as control. Up to indicated incubating time, the macrophages were harvested by scraping, and the LDH activity in the culture supernatants was assayed with CytoTox 96®Non-Radioactive Cytotoxicity Assay (Promega). The percentage of LDH release was calculated according to the formula: the percentage of cell death=100×experimental LDH release (OD490)/maximal LDH release (OD490). A value of maximal LDH release was obtained from the culture supernatants of macrophages without infection lysed with 1% (v/v) Triton X-100.
Measurement of the intracellular survival rate of recombinant M. smegmatis by CFU assay
The U937 cells were infected by M. smegmatis transformants as well as the control strains at an MOI 10:1 bacteria/cell for 4 h at 37°C in 5% CO2. After 4-h incubation, the media were discarded, and the cells were washed 3 times with PBS, and treated with hygromycin. After 2 h, cells were incubated again with the fresh RPMI−1640 medium plus 10% fetal calf serum. At different time points postinfection (24, 48, 72 h), the infected macrophages were washed by the sterile PBS buffer for 3 times at each time point. The sediments were collected through centrifugation at 250 g, 4 min. Cells were lysed by adding 1 mL of 1% TritonX-100 for 10 min, and then diluted serially (10-fold in PBS). About 10-μL aliquots from each dilution were plated on the Middlebrook 7H10 plate. The plates were incubated at 37°C, and the CFU were determined after 72 h.
Evaluation of the expression of TNF-α and IL-10 by ELISA
Culture supernatants were harvested 6, 12, 24, and 30 h after infection of U937 cells with Msmeg-pNIT (MYC) or Msmeg-pNIT (MYC)-Rv0978c, respectively. The concentrations of TNF-α and IL-10 in the culture supernatants were determined by ELISA kits (eBioscience).
Measurement of enzyme activities involved in the signaling of TNF-α and IL-10 secretion
U937 cells were pretreated with 20 mM U0126 (a MEK1/2 inhibitor; Sigma) and with 10 mM SB202190 (a p38 inhibitor; Sigma) for 1 h, respectively, and then infected by recombinant M. smegmatis. The concentrations of the inhibitors were optimized to give the best MEK1/2 and p38 activities inhibition. After 4-h incubation, the media were discarded, and the cells were washed 4 times with PBS, and subsequently treated with hygromycin at the indicatied concentration. Cells were then incubated again with a fresh RPMI -1640 medium plus 10% fetal calf serum.
After 24 h, the macrophage cells were harvested by scraping. The RNA-Solv reagent (Invitrogen) was added to each sample. RNA was extracted according to the manufacturer's instructions and then treated with DNAase I (Takara). The expression levels of the cytokine genes for TNF-α and IL-10 were detected by one-step semi-quantitative PCR (Tiangen). The sequences of TNF-α are 5′-CGCTCCCCAAG AAGACAG-3′ (forward) and 5′-TGAAGAGGACCTGGG AGT-3′ (reverse) with a size of 375 bp; For IL-10, 5′-GCAC AGCTCCAAGAGAAAGGCATCT-3′ (forward) and 5′-GAA TCCCTCCGAGACACT-3′ (reverse) with a product of 625 bp; For β-actin, 5′-CGGCTCCGGCATGTGCAA-3′ (forward) and 5′-ATGTCACGCACGATTTCC-3′ (reverse) and the product is 600 bp.
Culture supernatants of the infected U937 cells were harvested at 24 h after infection, and ELISA assays were performed to determine the changes of cytokine concentrations to pinpoint the particular MAPK involved.
Statistical analysis
Significance analysis was performed by SPSS software (Statistical Package for the Social Sciences, version 16.0; SPSS, Inc.). Statistical significance was defined as a P value<0.05.
Results
The expression of PE_PGRS17 in M. smegmatis
We generated 2 recombinant M. smegmatis strains—Msmeg-pNIT (MYC) and Msmeg-pNIT (MYC)-Rv0978c— to investigate the effects of PE_PGRS17 on the responses of U937 cells during infection. The recombinant strain was engineered to express the PE_PGRS17 protein from a recombinant pNIT (MYC)-Rv0978c vector, while the control strain contained the vector only. Total proteins of recombinant M. smegmatis were gained after ɛ-caprolactam induction for 24 h. The presence of the expected 36-kDa protein in the recombinant M. smegmatis total cell lysates was confirmed by Western blotting with the anti-MYC antibody, while absence in the M. smegmatis control strain (Fig. 1). The data showed that the PE_PGRS17 protein from M. tuberculosis was successfully expressed in M. smegmatis, and thus can be used for further analysis.
FIG. 1.
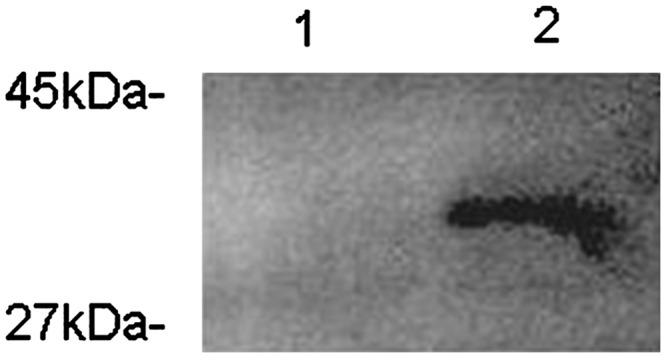
Expression of PE_PGRS17 in recombinant Mycobacterium smegmatis revealed by Western Blotting. The 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis exhibited a 36-kDa protein, the sum of a 31-kDa expected protein, and 5-kDa MYC tag expressed by recombinant M. smegmatis strains induced by ɛ-caprolactam. Lane 1: the protein of PE_PGRS 17 expressed in recombinant M. smegmatis strains induced by ɛ-caprolactam for 24 h. Lane 2: recombinant M. smegmatis strains containing the vector only.
The survival of M. smegmatis expressing PE_PGRS 17 in U937 cell
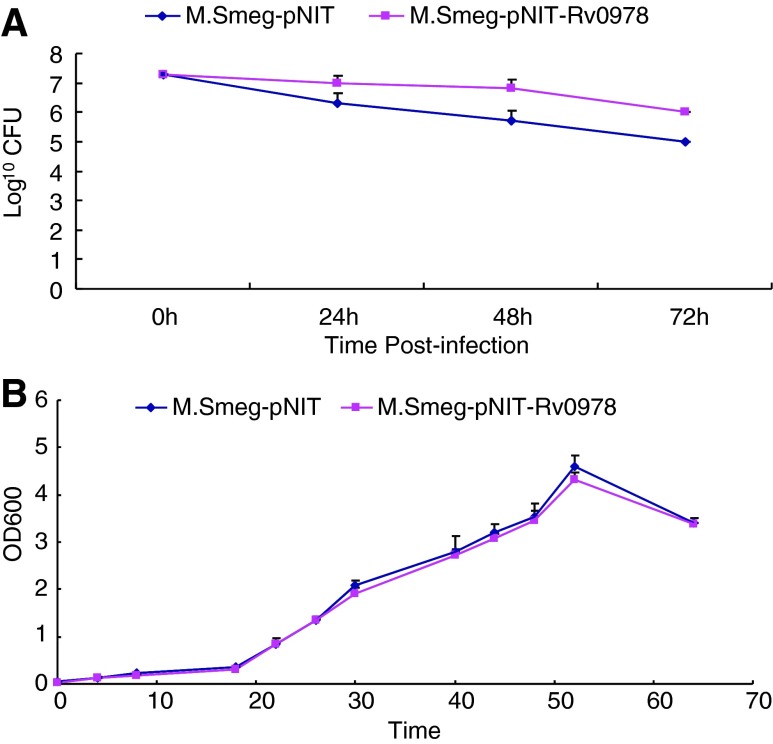
To test whether PE_PGRS 17 can promote the survival of the pathogen within host macrophages, U937 cells were infected with 2 engineered recombinant M. smegmatis strains at the MOI of 10:1, respectively. At 24, 48, and 72 h after infection, CFU assays showed that compared with the strain containing the vector only, M. smegmatis expressing the PE_PGRS 17 protein presented with greater numbers of bacteria residing within macrophages for 3 days (Fig. 2A). Whether this is due to the enhanced replication or augmented survival of the bacterium within macrophages remains to be determined. No in vitro growth kinetic difference can be found for both recombinant strains (Fig. 2B).
FIG. 2.
Survival of recombinant M. smegmatis strains after infection of U937 at an MOI of 10:1. (A) U937 cells were infected with recombinant M. smegmatis strains containing vector only (♦), PE_PGRS 17 (▪) at the MOI of 10. At 24, 48, and 72 h after infection, the macrophages were washed and lysed. Lysates containing the live bacteria were diluted gradually and then plated on 7H10 agar plates to determine CFU. (B) Growth of 2 recombinant M. Smegmatis strains containing vector only (♦), PE_PGRS 17 (▪) at 37°C in 7H9 liquid media were assayed by OD600 values. Similar results were obtained in three independent experiments.
Viability of U937 cell after infection with M. smegmatis expressing PE_PGRS 17
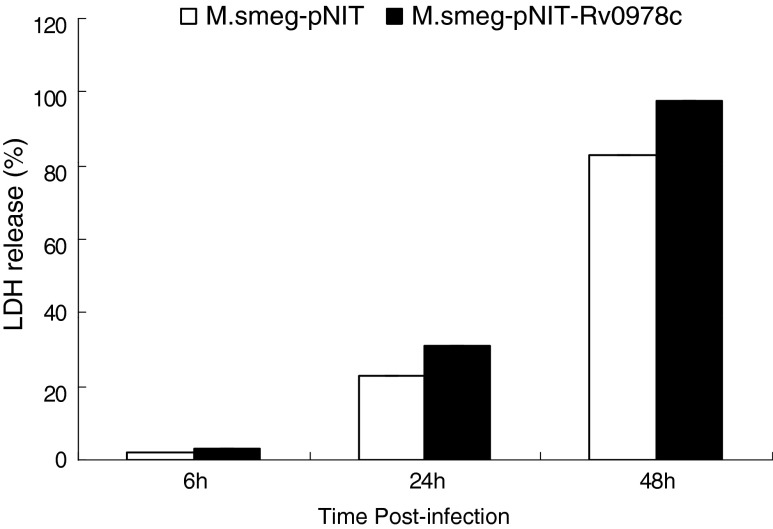
To determine whether PE_PGRS17 can affect cell death, U937 cells were infected with recombinant Msmeg-pNIT (MYC) and Msmeg-pNIT (MYC)-Rv0978c at the MOI of 10:1, respectively. LDH assay at OD490 was performed at 6, 24, and 48 h after infection. The results showed that for 6-h infection, both groups of macrophages released comparable low amounts of LDH. At 24 and 48 h, macrophages infected by M. smegmatis containing PE_PGRS 17 showed higher LDH release amounts than the control group with appreciable difference: 22.7% and 31.1% for 24 h, 82.7% and 92.7% for 48 h. In each time point, the former value represented the control group and the latter one was for the experimental group, indicating that PE_PGRS17 could induce cell death at the MOI of 10 (Fig. 3).
FIG. 3.
Death of U937 macrophages infected with recombinant M. smegmatis. U937 macrophages were infected with Msmeg-pNIT (MYC)-Rv0978c (black bars) and M. smeg-pNIT (MYC) (white bars) at an MOI of 10, respectively. At 6-, 24-, and 48-h postinfection, culture supernatants were harvested. The release of LDH was estimated by testing its activity in the culture supernatants. The results showed that recombinant M. smegmatis containing PE_PGRS 17 increased U937 macrophages death at the MOI 10:1 in a time-dependant manner. Each treatment was repeated three times.
Cytokines secretion in U937 cells infected with M. smegmatis expressing PE_PGRS 17
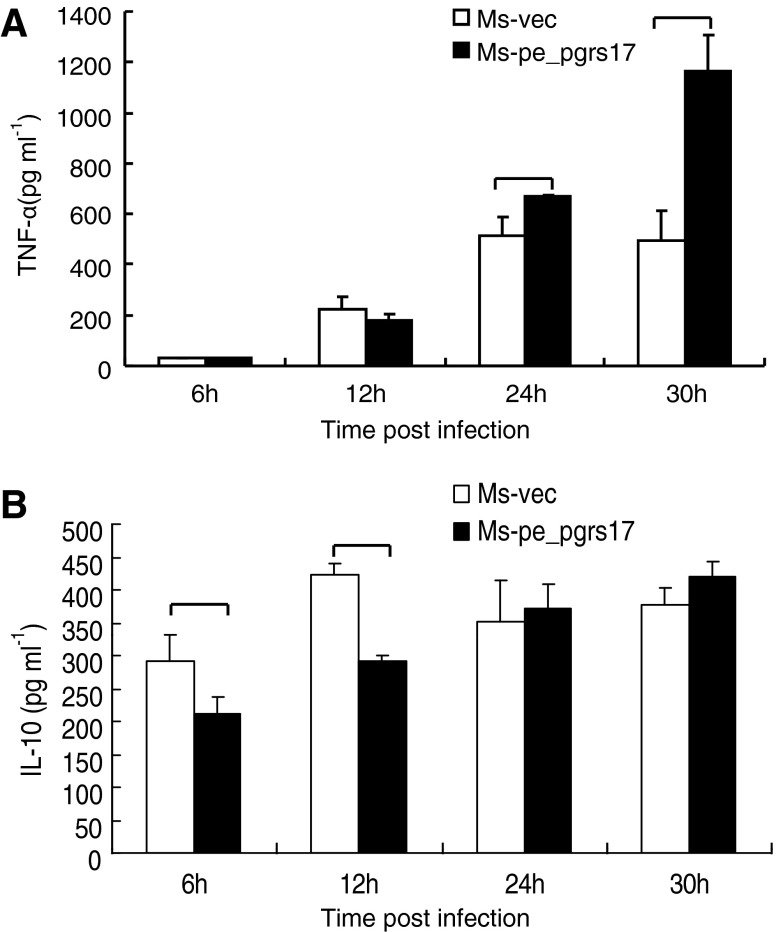
To explore the role of PE_PGRS 17 in the virulence of M. tuberculosis, we investigated the effect of PE_PGRS 17 on the U937 cytokine production. U937 cells were infected with Msmeg-pNIT (MYC) and Msmeg-pNIT (MYC)-Rv0978c, and the concentrations of cytokines in the culture supernatants were determined at 6, 12, 24, and 30 h by ELISA assay. We found that the infected U937 cells can produce TNF-α and IL-10 (Fig. 4). Previous studies (Post and others 2001; Roach and Schorey 2002; Lee and Schorey 2005) showed that soluble TNF-α can be detrimental to mycobacteria by contributing to destructive inflammation and cell death. In our results, the secretion of TNF-α by macrophages infected with M. smegmatis expressing PE_PGRS 17 was higher than the control group (Fig. 4A). In addition to TNF-α, no noticeable difference as to the production of IL-10 was tested by macrophages infected with M. smegmatis expressing PE_PGRS17 and the control (Fig. 4B). Taken together, these results suggested that PE_PGRS17 might interfere with the cytokine production.
FIG. 4.
Secretion of the tumor necrosis factor (TNF)-α and interleukin-10 (IL-10) in U937 infected with recombinant M. smegmatis strains measured by ELISA. (A, B) U937 were infected at an MOI of 10:1 with M. smegmatis containing vector only (□), PE_PGRS 17 (▪) and incubated for different time duration. Culture supernatants were collected at indicated intervals and used to quantify the production of TNF-α and IL-10 by the ELISA kit. (A) The secretion of TNF-α is significantly higher by macrophages infected with M. smegmatis expressing PE_PGRS 17 than the control group at 24 h and 30 h post-infection. (B) The release of IL-10 in macrophages is much higher by the control group at 6 h after infection. Each treatment was repeated three times.
ERK might mediate the effect of PE_PGRS17 on TNF-α release
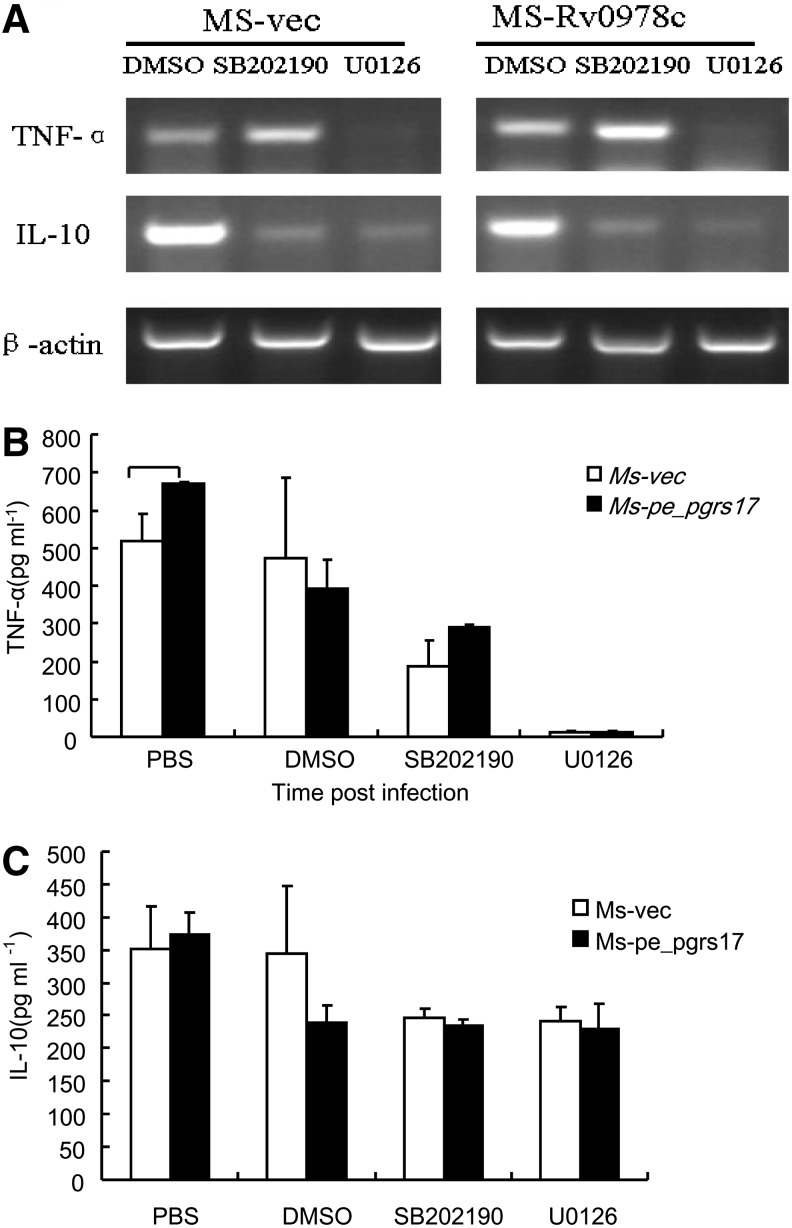
To study the signaling pathway underlying the effect of PE_PGRS17 on TNF-α release, we first investigate whether the activation of mitogen-activated protein kinases (MAPKs) might be affected in macrophages infected with Msmeg-pNIT (MYC)-Rv0978c. Reverse transcription-PCR (RT-PCR) and ELISA assays were performed to assess the levels of TNF-α and IL-10 in the infected macrophages with the addition of the inhibitors of p38 and MEK1/2. Both ELISA and semiquantitative PCR assays showed that the secretion of TNF-α was significantly inhibited (Fig. 5). These results indicated that PE_PGRS17-promoted TNF-α release was mediated by ERK.
FIG. 5.
The secretion of TNF-α and IL-10 in U937 cells infected with recombinant M. smegmatis strains measured by ELISA and reverse transcription-polymerase chain reaction (RT-PCR). (A) U937 cells were infected at an MOI of 10:1 with M. smegmatis containing vector only (□), PE_PGRS 17 (▪) and incubated for 24 h. Culture sediments were used to extract RNA, followed by semiquantitative RT-PCR to demonstrate the transcriptional levels of TNF-α and IL-10. (B, C) Culture supernatants were collected at 24 h after infection and used to quantify the production of TNF-α and IL-10 by the ELISA kit.
Discussion
PE_PGRS proteins represent a unique M. tuberculosis family consisting of 61 members (Brennan and Delogu 2002). The PE_PGRS family has a highly conserved N-terminal domain (PE) containing 110 amino acid residues. The C-terminal of the family is a PGRS domain encoded by a polymorphic GC-rich repeated sequence, with variable sizes, sequences, and repeat copy numbers (Tian and Jian-ping 2010). A highly polymorphic PGRS domain might be engaged in the antigenic variation and immune evasion. The PE_PGRS family members exclusively present in the mycobacterium, mostly within pathogenic mycobacteria (Singh and others 2008), such as M. tuberculosis, Mycobacterium bovis, Mycobacterium ulcerans, and Mycobacterium marinum. It is tempting to speculate that PE_PGRS proteins may have a role in the pathogenesis and persistence of mycobacterial disease and that there may be a specific role for this family in influencing host cell responses to infection (Tian and Jian-ping 2010). While some members have been studied, the function of most PE_PGRS proteins remains unknown. Therefore, selective expression of specific PE_PGRS proteins in vitro can facilitate the understanding of their roles in M. tuberculosis. In this study, the recombinant M. smegmatis strain expressing PE_PGRS 17 instead of M. smegmatis strains containing the vector only showed enhanced survival within U937 macrophages in vitro. It is fascinating to expect a similar role for PE_PGRS 17 during M. tuberculosis infection. The variable transcriptional levels of PE-PGRS 17 with exposure to hypoxia, DNA damaging agents, and clinical isolates (www.ncbi.nlm.nih.gov/geoprofiles?term=PE-PGRS17) also suggested its important roles. Intensive study of this family might provide novel insights into TB pathogenesis and inform new strategies for better prevention and therapy.
TNF-α exerts a key role in the innate and adaptive immunity against tuberculosis (Basu and others 2006), such as the induction of apoptosis and activation of a series of antimicrobial responses (Stenger 2005). Elevated levels of TNF-α during avirulent mycobacteria infection presumably can facilitate the hosts to eliminate the invading bacteria, resulting in poor survival of nonpathogenic M. smegmatis within macrophages. On the contrary, virulent mycobacteria can thwart the clearance effort mounted by the host and maintain a favorable niche within the host (Roach and others 2002). However, a delicate timing and balance with other cytokines are crucial for the proper function of TNF-α. In our study, significant greater amounts of TNF-α were released from U937 macrophage cultures infected with M. smegmatis expressing PE_PGRS 17 compared with the control. The increased secretion of TNF-α is accompanied by greater levels of macrophage necrosis infected with M. smegmatis expressing PE_PGRS 17 after 24 h of infection, through measurement of the level of released LDH, an assessment of cytotoxicity and cell necrosis. We also found that the survival rate of M. smegmatis expressing PE_PGRS 17 is higher than the control groups during the detected time length. The induction of macrophages necrosis might be imposed by high levels of TNF-α, and could promote the bacteria spreading to infect fresh cells for new niches. This might underlie the superior survival of virulent mycobacteria within the host in contrast with the avirulent mycobacteria. However, the role the death of infected macrophages invested during M.tuberculosis infection remains controversial to date. It might be stage and niche specific. Understanding the strategy M.tuberculosis employed to regulate TNF-α induction within the host is important to address this dispute.
IL-10 is a cytokine with pleiotropic effects in immunoregulation and inflammation, and also important in anti-inflammatory function during M. tuberculosis infection. IL-10 is primarily produced by monocytes and, to a lesser extent, lymphocytes and is normally secreted at a later stage in the immune response toward a pathogen compared with other cytokines (Redpath and others 2001). IL-10 can downregulate the production of many cytokines, including interferon gamma (IFN-γ) and TNF-α. Being an immunosuppressive cytokine, IL-10 is supposed to favor the intracellular survival of M.tuberculosis. However, the cases regarding IL-10-mediated immunoregulatory function of macrophages are quite controversial. Dheenadhayalan and others (2006) reported that the increased production of IL-10 promoted human macrophage necrosis and poor survival of intracellular bacteria. On the other hand, Haruaki Tomioka and coworkers reported that IL-10 was not effective in downregulating the antibacterial functions of macrophages both in vitro and in vivo (Sano and others 1999). In our study, we found the levels of IL-10 were significantly lower in macrophages infected with recombinant strains expressing PE_PGRS 17 at 6- and 12-h postinfection (Fig. 4B). However, the level of macrophage necrosis between the 2 groups at the indicated time was the same. In-depth appreciation of the discrepancy between macrophage necrosis and enhanced M. smegmatis survival needs further investigation, particularly the signaling events mediating the 2 outcomes
Briefly, our data suggest that the expression of the PE_PGRS 17 might provide the nonpathogenic M. smegmatis with some novel properties, including affecting the viability of macrophages and increasing the expression levels of TNF-α. Further studies in mice with this recombinant or the PE_PGRS 17 deletion mutants of M. tuberculosis will shed more light on this important molecule. Further studies on how this gene is regulated within the host, their expression level, and receptors are warranted to clarify the underlying mechanism.
Acknowledgment
This work was funded by the National Natural Science Foundation (grant No. 81071316, 81271882), National megaprojects for key infectious diseases (No.2008ZX10003-006, 2012ZX10003-003), New century excellent talents in universities (NCET-11-0703), Excellent PhD thesis fellowship of Southwest university (Grant Nos. kb2009010 and ky2011003), the Fundamental Research Funds for the Central Universities (Grant No. XDJK2012D011, XDJK2012D007, XDJK2013D003, XDJK2011C020), and the Natural Science Foundation Project of CQ CSTC(Grant No. CSTC, 2010BB5002).
Author Disclosure Statement
No competing financial interests exist.
References
- Bansal K. Elluru SR. Narayana Y. Chaturvedi R. Patil SA. Kaveri SV. Bayry J. Balaji KN. PE_PGRS antigens of Mycobacterium tuberculosis induce maturation and activation of human dendritic cells. J Immunol. 2010;184(7):3495–3504. doi: 10.4049/jimmunol.0903299. [DOI] [PubMed] [Google Scholar]
- Banu S. Honore N. Saint-Joanis B. Philpott D. Prevost MC. Cole ST. Are the PE-PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol Microbiol. 2002;44(1):9–19. doi: 10.1046/j.1365-2958.2002.02813.x. [DOI] [PubMed] [Google Scholar]
- Basu S. Pathak SK. Banerjee A. Pathak S. Bhattacharyya A. Yang Z. Talarico S. Kundu M. Basu J. Execution of Macrophage Apoptosis by PE_PGRS33 of Mycobacterium tuberculosis is mediated by toll-like receptor 2-dependent release of tumor necrosis factor. J f Biol Chem. 2006;282(2):1039–1050. doi: 10.1074/jbc.M604379200. [DOI] [PubMed] [Google Scholar]
- Behar SM. Divangahi M. Remold HG. Evasion of innate immunity by Mycobacterium tuberculosis: is death an exit strategy? Nat Rev Microbiol. 2010;8(9):668–674. doi: 10.1038/nrmicro2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosque F. Milon G. Valderrama L. Saravia NG. Permissiveness of human monocytes and monocyte-derived macrophages to infection by promastigotes of Leishmania (Viannia) panamensis. J Parasitol. 1998;84(6):1250–1256. [PubMed] [Google Scholar]
- Brennan MJ. Delogu G. The PE multigene family: a “molecular mantra” for mycobacteria. Trends Microbiol. 2002;10(5):246–249. doi: 10.1016/s0966-842x(02)02335-1. [DOI] [PubMed] [Google Scholar]
- Brennan MJ. Delogu G. Chen Y. Bardarov S. Kriakov J. Alavi M. Jacobs WR., Jr. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect Immun. 2001;69(12):7326–7333. doi: 10.1128/IAI.69.12.7326-7333.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deb C. Daniel J. Sirakova TD. Abomoelak B. Dubey VS. Kolattukudy PE. A novel lipase belonging to the hormone-sensitive lipase family induced under starvation to utilize stored triacylglycerol in Mycobacterium tuberculosis. J Biol Chem. 2006;281(7):3866–3875. doi: 10.1074/jbc.M505556200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delogu G. Pusceddu C. Bua A. Fadda G. Brennan MJ. Zanetti S. Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol Microbiol. 2004;52(3):725–733. doi: 10.1111/j.1365-2958.2004.04007.x. [DOI] [PubMed] [Google Scholar]
- Dheenadhayalan V. Delogu G. Brennan MJ. Expression of the PE_PGRS 33 protein in Mycobacterium smegmatis triggers necrosis in macrophages and enhanced mycobacterial survival. Microbes Infect. 2006;8(1):262–272. doi: 10.1016/j.micinf.2005.06.021. [DOI] [PubMed] [Google Scholar]
- Fenton MJ. Vermeulen MW. Immunopathology of tuberculosis: roles of macrophages and monocytes. Infect Immun. 1996;64(3):683–690. doi: 10.1128/iai.64.3.683-690.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. Wang Y. Bai Y. Wang ZG. Yang L. Zhao D. Expression of PE_PGRS 62 protein in Mycobacterium smegmatis decrease mRNA expression of proinflammatory cytokines IL-1beta, IL-6 in macrophages. Mol Cell Biochem. 2010;340(1–2):223–229. doi: 10.1007/s11010-010-0421-x. [DOI] [PubMed] [Google Scholar]
- Jain SK. Paul-Satyaseela M. Lamichhane G. Kim KS. Bishai WR. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J Infect Dis. 2006;193(9):1287–1295. doi: 10.1086/502631. [DOI] [PubMed] [Google Scholar]
- Lee SB. Schorey JS. Activation and mitogen-activated protein kinase regulation of transcription factors Ets and NF-kappaB in Mycobacterium-infected macrophages and role of these factors in tumor necrosis factor alpha and nitric oxide synthase 2 promoter function. Infect Immun. 2005;73(10):6499–6507. doi: 10.1128/IAI.73.10.6499-6507.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayana Y. Joshi B. Katoch VM. Mishra KC. Balaji KN. Differential B-cell responses are induced by Mycobacterium tuberculosis PE antigens Rv1169c, Rv0978c, and Rv1818c. Clin Vaccine Immunol. 2007;14(10):1334–1341. doi: 10.1128/CVI.00181-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey A. Raman S. Proff R. Joshi S. Kang C. Rubin E. Husson R. Sassetti C. Nitrile-inducible gene expression in mycobacteria. Tuberculosis. 2009;89(1):12–16. doi: 10.1016/j.tube.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Post FA. Manca C. Neyrolles O. Ryffel B. Young DB. Kaplan G. Mycobacterium tuberculosis 19-kilodalton lipoprotein inhibits Mycobacterium smegmatis-induced cytokine production by human macrophages in vitro. Infect Immun. 2001;69(3):1433–1439. doi: 10.1128/IAI.69.3.1433-1439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviglione MC. Snider DE., Jr. Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995;273(3):220–226. [PubMed] [Google Scholar]
- Redpath S. Ghazal P. Gascoigne NR. Hijacking and exploitation of IL-10 by intracellular pathogens. Trends Microbiol. 2001;9(2):86–92. doi: 10.1016/s0966-842x(00)01919-3. [DOI] [PubMed] [Google Scholar]
- Roach DR. Bean AG. Demangel C. France MP. Briscoe H. Britton WJ. TNF regulates chemokine induction essential for cell recruitment, granuloma formation, and clearance of mycobacterial infection. J Immunol. 2002;168(9):4620–4627. doi: 10.4049/jimmunol.168.9.4620. [DOI] [PubMed] [Google Scholar]
- Roach SK. Schorey JS. Differential regulation of the mitogen-activated protein kinases by pathogenic and nonpathogenic mycobacteria. Infect Immun. 2002;70(6):3040–3052. doi: 10.1128/IAI.70.6.3040-3052.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano C. Sato K. Shimizu T. Kajitani H. Kawauchi H. Tomioka H. The modulating effects of proinflammatory cytokines interferon-gamma (IFN-gamma) and tumour necrosis factor-alpha (TNF-alpha), and immunoregulating cytokines IL-10 and transforming growth factor-beta (TGF-beta), on anti-microbial activity of murine peritoneal macrophages against Mycobacterium avium-intracellulare complex. Clin Exp Immunol. 1999;115(3):435–442. doi: 10.1046/j.1365-2249.1999.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnappinger D. Ehrt S. Voskuil MI. Liu Y. Mangan JA. Monahan IM. Dolganov G. Efron B. Butcher PD. Nathan C. Schoolnik GK. Transcriptional Adaptation of Mycobacterium tuberculosis within Macrophages: Insights into the Phagosomal Environment. J Exp Med. 2003;198(5):693–704. doi: 10.1084/jem.20030846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick TM. King CH. Quinn FD. Molecular biology, virulence, and pathogenicity of mycobacteria. Am J Med Sci. 1995;309(2):92–98. doi: 10.1097/00000441-199502000-00007. [DOI] [PubMed] [Google Scholar]
- Singh PP. Parra M. Cadieux N. Brennan MJ. A comparative study of host response to three Mycobacterium tuberculosis PE_PGRS proteins. Microbiology. 2008;154(Pt 11):3469–3479. doi: 10.1099/mic.0.2008/019968-0. [DOI] [PubMed] [Google Scholar]
- Snapper SB. Melton RE. Mustafa S. Kieser T. Jacobs WR., Jr. Isolation and characterization of efficient plasmid transformation mutants of Mycobacterium smegmatis. Mol Microbiol. 1990;4(11):1911–1919. doi: 10.1111/j.1365-2958.1990.tb02040.x. [DOI] [PubMed] [Google Scholar]
- Song CH. Lee JS. Kim HJ. Park JK. Paik TH. Jo EK. Interleukin-8 is differentially expressed by human-derived monocytic cell line U937 infected with Mycobacterium tuberculosis H37Rv and Mycobacterium marinum. Infect Immun. 2003;71(10):5480–5487. doi: 10.1128/IAI.71.10.5480-5487.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenger S. Immunological control of tuberculosis: role of tumour necrosis factor and more. Ann Rheum Dis. 2005;64(Suppl.4):iv24–iv28. doi: 10.1136/ard.2005.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talarico S. Cave MD. Foxman B. Marrs CF. Zhang L. Bates JH. Yang Z. Association of Mycobacterium tuberculosis PE PGRS33 polymorphism with clinical and epidemiological characteristics. Tuberculosis (Edinb) 2007;87(4):338–346. doi: 10.1016/j.tube.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C. Jian-ping X. Roles of PE_PGRS family in Mycobacterium tuberculosis pathogenesis and novel measures against tuberculosis. Microbial Pathogenesis. 2010;49(6):311–314. doi: 10.1016/j.micpath.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Wei J. Dahl JL. Moulder JW. Roberts EA. O'Gaora P. Young DB. Friedman RL. Identification of a Mycobacterium tuberculosis gene that enhances mycobacterial survival in macrophages. J Bacteriol. 2000;182(2):377–384. doi: 10.1128/jb.182.2.377-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]