Abstract
Epithelial–mesenchymal transition (EMT) plays an important role in tumor metastasis of human nonsmall cell lung cancer (NSCLC). The Wnt pathway is identified as a key regulator of normal tissue development, and its aberrant activation contributes to the process of EMT. The secreted frizzled-related protein 1 (sFRP1), a Wnt-signaling antagonist, is downregulated in many tumors, including lung cancer. However, the role of sFRP1 in EMT and tumor metastasis remains unclear. In this study, we found that sFRP1 was dramatically downregulated in transforming growth factor β1 (TGF-β1)-induced EMT in the A549 human lung cancer cell line. Restoration of sFRP1 could inhibit the TGF-β1-induced EMT phenotype and tumor metastasis of the A549 cell line both in vitro and in vivo through inhibition of the Wnt pathway. Furthermore, FH535, a reversible Wnt-signaling inhibitor, exerted a similar effect on the TGF-β1-induced EMT phenotype. These results indicate that sFRP1, an endogenous antagonist of the Wnt pathway, inhibits TGF-β1-induced EMT, and might be a potential biomarker for the treatment of NSCLC.
Key words: EMT, NSCLC, sFRP1, Wnt pathway
Introduction
Epithelial–mesenchymal transition (EMT) is a cellular mechanism recognized as a central feature of normal tissue development.1 Recent studies have revealed that EMT also occurs during the progression of epithelial tumors to increase the motility and invasiveness of cancer cells. A particular characteristic of EMT is downregulation of the epithelial marker E-cadherin and increase of mesenchymal markers such as vimentin, fibronectin, and N-cadherin.2 Although several cytokines are involved in EMT, transforming growth factor β1 (TGF-β1) has been identified as the most potent factor that can independently induce EMT in various types of cancer cells.3 The role of TGF-β1 in cancer metastasis is also confirmed by the fact that neutralizing antibodies for TGF-β1 could suppress cancer metastasis.4
Multiple signaling pathways could induce the process of EMT, including the Wnt/β-catenin pathway.5 The Wnt-signaling pathway regulates gene expression by stabilizing β-catenin, which translocates to the nucleus and forms complexes with T-cell factor transcription factors.6 β-catenin induces the transcription of several target genes, which are involved in cell survival, proliferation, and metastasis.7 The Wnt pathway is also shown to induce EMT and activate the nuclear translocation of snail through inhibition of GSK3β.8 Secreted frizzled-related protein 1 (sFRP1) acts as a Wnt pathway antagonist and exerts inhibitory effects in many different human malignancies, including colon cancer, breast cancer, and hepatocellular carcinoma.9,10 In the present study, we investigated the role of the Wnt/β-catenin pathway and sFRP1 on TGF-β1-induced EMT.
Materials and Methods
Cell culture and reagents
The human lung adenocarcinoma cell line A549 was cultured in the RPMI 1640 containing 10% fetal bovine serum (FBS; GIBCO), 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in an atmosphere of 5% CO2. Recombinant human TGF-β1 was purchased from R&D Systems and FH535 was purchased from Sigma.
Morphologic analysis
A549 cells were seeded into six-well plates (2×105 cells/well). After treated with TGF-β1 (10 ng/mL) for 2 days, cell morphological change of EMT was visualized via the Olympus microscope (Olympus America, Inc. at ×200 magnification.
Plasmid transfection
The expression plasmid of sFRP1 (pcDNA3.1/sFRP1) was a kind gift from Dr. Sekido (Nagoya University, Japan). The A549 cell line was transfected with pcDNA3.1/sFRP1 or pcDNA3.1 using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol and after selected with G418 (Sigma) for 2 weeks, A549 cell lines stably expressing sFRP1 (A549-sFRP1) and stably expressing pcDNA3.1 (A549-pcDNA3.1) were preserved in a medium containing 300 μg/mL of G418.
RNA isolation and real-time PCR
Total RNA was isolated from treated cells using the Trizol reagent (Invitrogen) and reversely transcribed into cDNA using a PrimeScript RT reagent Kit (Takara) following the manufacturer's instructions. Quantitative real-time PCR was performed by PRISM 7900 Sequence Detection System (Applied Biosystems). GAPDH was amplified as endogenous control. The sequences of primers were listed as follows.
E-cadherin: forward: 5′-CATTTCCCAACTCCTCTCCTGGC-3′, reverse: 5′-ATGGGCCTTTTTCATTTTCTGGG-3′;
CK-19: forward: 5′-CATCCAGGACCTGCGGGACAAGA-3′, reverse: 5′-AGCCAGACGGG
CATTGTCGATCT-3′;
Vimentin: forward: 5′-AGTTCAAGAACACCCGCACCAAC-3′, reverse: 5′-CAGGAAGCGCACCTTGTCGATGT-3′;
N-cadherin: forward: 5′-CTTCAGGCGTCTGTAGAGGCTTC-3′, reverse: 5′-TGCACATCCTTCGATAAGACTGC-3′;
sFRP: forward: 5′-GATGCTTAAGTGTGACAAGTTCCC-3′, reverse: 5′-TGGCCTCAGATTTCAACTCGT-3′;
GAPDH: forward: 5′-GCACCGTCAAGGCTGAGAAC-3′, reverse: 5′-TGGTGAAGACGCCAGTGGA-3′.
Wound-healing assay
Cells were seeded into six-well plates and cultured overnight to form a confluent monolayer. After scratched with a sterile pipette tip, cells were rinsed gently with phosphate-buffered saline to remove the detached cells and incubated with a medium containing 0.5% FBS. The imagination of wounded areas was taken at the indicated time points. The distances between the two edges of the scratched cells were measured and the healing rate was calculated using the following formula: healing rate=(the distance before healing-the distance after healing)/the distance before healing ×100%.
Migration and invasion assays
Cells were analyzed for invasion/migration using Transwell migration chambers (8-μm pore size; Costar) according to the manufacturer's protocol. For the A549 cell line, 50,000 cells were placed in the upper chambers in serum-free media, and the lower chambers were filled with RPMI 1640%+10% FBS. After incubation for 24 hours at 37°C, nonmigrating and noninvasive cells were wiped off the top surface of the membrane. Membranes were fixed with methanol for 10 minutes and stained with 0.5% Crystal Violet for 5 minutes. The number of cells that migrated to the bottom of the filter was manually counted under an inverted microscope.
Western blotting
Western blotting was performed as described previously.11 Briefly, equal amounts of cellular protein were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred to the nitrocellulose membrane. After blocked by 5% skim milk, the membrane was incubated with primary antibodies to E-cadherin (1:1000; BD Biosciences), N-cadherin (1:1000; BD Biosciences), Vimentin (1:1000; BD Biosciences), sFRP1 (1:300; Abcam), GAPDH (1:1000; Bioworld), and GSK3β (1:1000; Bioworld) overnight at 4°C. Then, the membrane was probed with the HRP-conjugated secondary antibody (1:10000; Bioworld). Proteins were visualized with ECL reagents (Cell signaling) according to the manufacturer's instructions.
Luciferase assay
Topflash, Fopflash, and pcDNA3-S33Yβ-catenin were kind gifts of Dr. Hong Jiang (University of Louisville). A549 cells (4×104 cells/well) were transfected with Topflash (or Fopflash), pcDNA3.1/sFRP1 (or pcDNA3.1), pcDNA3-S33Y β-catenin, and pRL-SV40 for 24 hours, and luciferase activities were determined by the dual-luciferase reporter assay systems (Promega) according to the manufacturer's instructions. All data points were the averages of at least four independent transfections.
Animal experiments
A549-sFRP1 and A549-pcDNA cells were treated with TGF-β1 (10 ng/mL) for 2 days and 2×106 cells were injected into the lateral tail vein of nude mice. After 8 weeks, lung tissues were embedded for the HE staining. Blood from mice was isolated and red blood cells were lysed. RNA from the remaining cells was extracted for real-time PCR. The presence of circulating tumor cells was assessed as a function of human-specific GAPDH (hGAPDH) expression relative to mouse-specific GAPDH (mGAPDH). All animal work was performed following the Animal Experimentation Ethics Committee of the Jinling Hospital.
Statistical analysis
The data presented in the figures are expressed as mean±SEM and determined by SPSS12.0 statistical analytical software (SPSS). Comparisons between groups were conducted by the Student's t-test and p<0.05 was considered statistically significant.
Results
A549 cell line treated with TGF-β1 displays mesenchymal-like features and increased metastatic potential
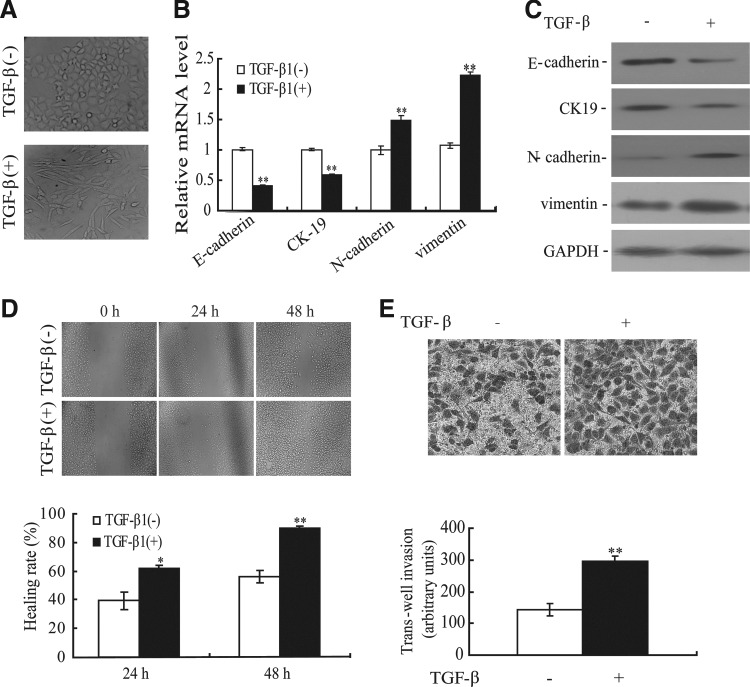
To address the effect of TGF-β1 on EMT, we first detected the morphological change of the A549 cell line at 48 hours after TGF-β1 treatment. As shown in Figure 1A, TGF-β1 (10 ng/mL) treatment promoted the change in the cell phenotype established by a loss of cell to cell contact and elongated, fibroblastoid morphology, while an untreated A549 cell line maintained uniform cobblestone morphology with adherens and tight junctions. The morphological changes of the A549 cell line undergoing EMT were also accompanied by a shift in specific molecular changes from an epithelial to a mesenchymal repertoire. As shown in Figure 1B and C, compared with untreated cells, decreased E-cadherin and CK-19 and increased vimentin and N-cadherin were observed in the A549 cell line induced by TGF-β1 at both mRNA and protein levels. To further determine the effect of TGF-β1 on migratory and invasive ability in the A549 cell line, we evaluated the change of migratory ability using wound-healing assays. The healing rate of TGF-β1-induced A549 cell line increased 1.56-fold and 1.60-fold at 24 and 48 hours, respectively, compared with that of untreated cells (Fig. 1D). In addition, TGF-β1 could enhance the ability of the A549 cell line to invade through the transwell membrane (Fig. 1E). Taken together, these data demonstrated that the A549 cell line exhibits phenotypes consistent with EMT after TGF-β1 induction.
FIG. 1.
Transforming growth factor (TGF)-β1-induced EMT and increased migration ability in the A549 cell line. (A) The A549 cell line treated with TGF-β1 displayed EMT phenotypic changes, including a loss of cell to cell contact and elongated, fibroblastoid morphology. Magnification, ×200. The expression of epithelial and mesenchymal markers was determined by real-time PCR (B) and Western blotting (C) in the A549 cell line induced by TGF-β1. **p<0.01. (D) Wound-healing assays were performed in the TGF-β1-treated A549 cell line at 24 and 48 hours. *p<0.05, **p<0.01. (E) Transwell assays were performed in the TGF-β1-treated A549 cell line. **p<0.01.
sFRP1 inhibits TGF-β1-induced Wnt pathway in A549 cell line
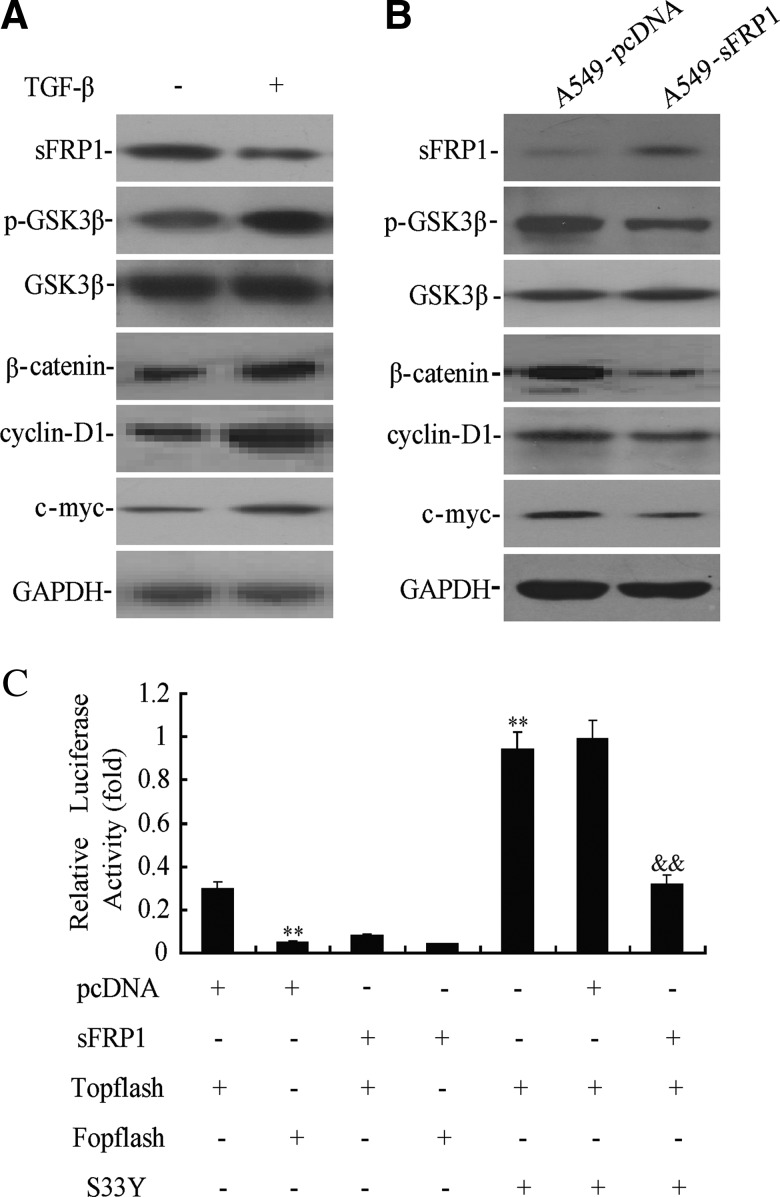
Emerging evidence has shown that the Wnt pathway plays an important role in EMT induction. As shown in Figure 2A, TGF-β1 downregulated the expression of sFRP1 and simultaneously inactivated GSK3β by phosphorylation at serine-9 in the A549 cell line. In addition, TGF-β1 increased the expression of β-catenin and its downstream targets c-myc and cyclin D1 (Fig. 2A). These data suggest that TGF-β1 might induce EMT through activation of the Wnt/β-catenin pathway.
FIG. 2.
Secreted frizzled-related protein 1 (sFRP1) restoration suppressed the Wnt pathway. (A) The expression of sFRP1, GSK3β, β-catenin, cyclinD-1, and c-myc was determined by Western blotting in the A549 cell line induced by TGF-β1 (10 ng/mL). (B) The expression of GSK3β, β-catenin, cyclinD-1, and c-myc was determined by Western blotting in A549 cell line stably transfected with sFRP1 treated with TGF-β1 (10 ng/mL). (C) Luciferase reporter assays in the A549 cell line cotransfected with sFRP1 and pTopflash. **p<0.01 versus pcDNA+Topflash, &&pcDNA+Topflash+S33Y.
To investigate the role of sFRP1 on TGF-β1-induced EMT, the Wnt/β-catenin pathway was detected in A549-sFRP1 and A549-pcDNA cells treated with TGF-β1. As shown in Figure 2B, decreased p-GSK3β and β-catenin was observed in TGF-β1-induced A549-sFRP1 cell line. In addition, the protein levels of cycline D1 and c-myc were downregulated in TGF-β1-induced A549-sFRP1 cell line. Furthermore, we examined whether restoration of sFRP1 could downregulate the transcriptional activity of β-catenin. As shown in Figure 2C, sFRP1 could significantly suppress the transcriptional activity of β-catenin (p<0.01), even in the presence of pcDNA-S33Y β-catenin, while pcDNA-S33Y β-catenin could significantly induce the transcriptional activity of β-catenin (p<0.01).
Restoration of sFRP1 inhibits TGF-β1-induced EMT phenotype in A549 cell line
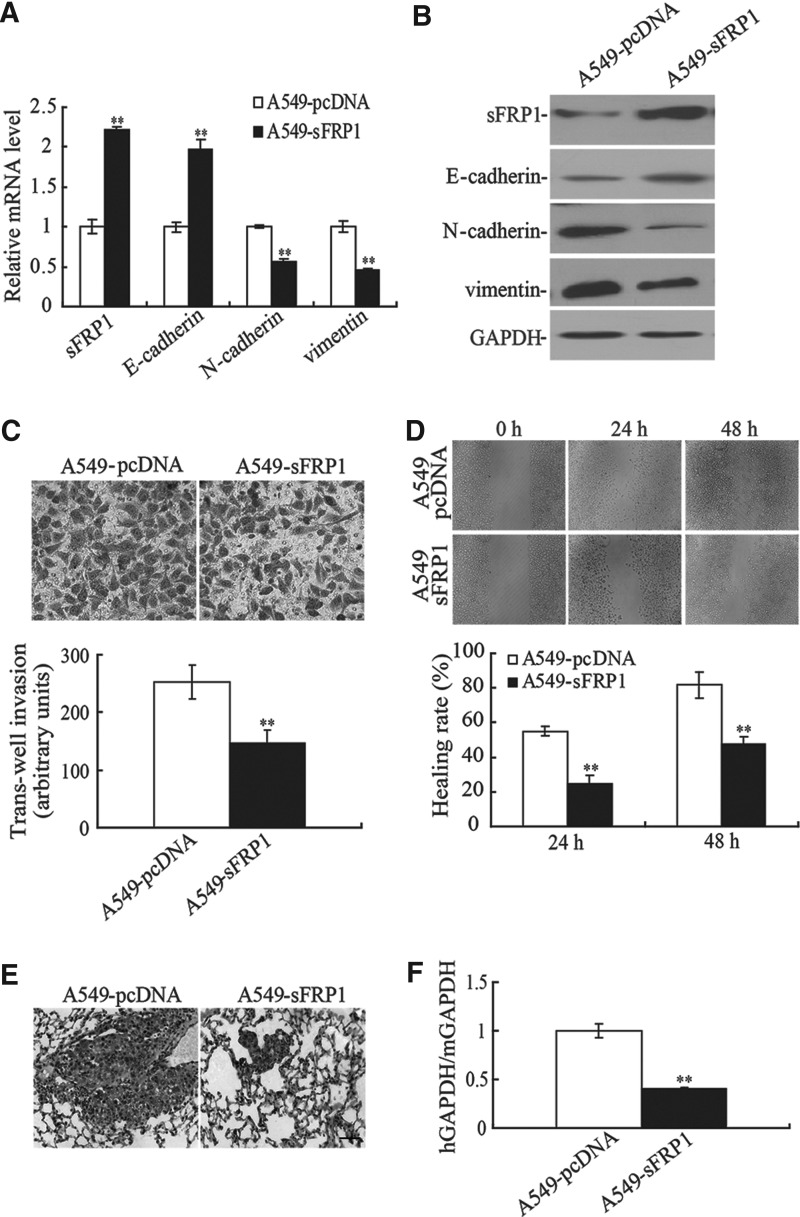
Given that sFRP1 was decreased during TGF-β1-induced EMT, we asked whether ectopic expression of sFRP1 could reverse the EMT phenotype by inhibiting the Wnt pathway in the A549 cell line. As shown in Figure 3A and B, overexpression of sFRP1 inhibited the expression of N-cadherin and vimentin and upregulated the expression of E-cadherin. In addition, the A549-sFRP1 cell line showed a 1.73-fold decrease in the number of cells invading through the transwell membrane compared with the A549-pcDNA cell line after TGF-β1 induction (Fig. 3C). The results of wound-healing assay also indicated that a significant decrease in cell migration was observed in the A549-sFRP1 cell line compared with the A549-pcDNA cell line at 24 and 48 hours (p<0.01, Fig. 3D). As EMT played a critical role in tumor metastasis, we then examined the inhibitory role of sFRP1 on the metastatic potential of the A549 cell line in vivo. As shown in Figure 3E, compared with the A549-sFRP1 cell-injected nude mice, H&E staining showed that numerous multinucleate huge cells, newly formed vessels, and scattered lymphocytes were seen in the lung sections and markedly more space in pulmonary alveolus was occupied by tumor cells in A549-pcDNA cell-injected nude mice. The number of circulating tumor cells was also significantly decreased in A549-sFRP1 cell-injected nude mice (p<0.01, Fig. 3F), suggesting that sFRP1 played an important role in the reversal of EMT phenotype and that the ectopic expression of sFRP1 could inhibit the TGF-β1-induced EMT in the A549 cell line.
FIG. 3.
Ectopic of sFRP1 suppressed EMT and the invasive ability of TGF-β1-induced A549 cell line. The levels of epithelial and mesenchymal markers were detected by real-time PCR (A) and Western blotting (B) in TGF-β1 (10 ng/mL)-induced A549/sFRP1 cell line. **p<0.01. (C) The invasive ability of TGF-β1-induced A549/sFRP1 cell line was determined by transwell assay. **p<0.01. (D) The migratory potential of TGF-β1 (10 ng/mL)-induced A549/sFRP1 cell line was detected by wound-healing assay. **p<0.01. (E) Eight weeks after tail vein injection of TGF-β1 (10 ng/mL)-treated A549-pcDNA or A549-sFRP1 cells, HE staining was performed to determine the metastasis ability. Bar=50 μm. (F) The relative concentration of circulating tumor cells in blood was determined by real-time PCR. **p<0.01.
FH535, a small-molecule inhibitor of Wnt pathway, could suppress TGF-β1-induced EMT in A549 cell line
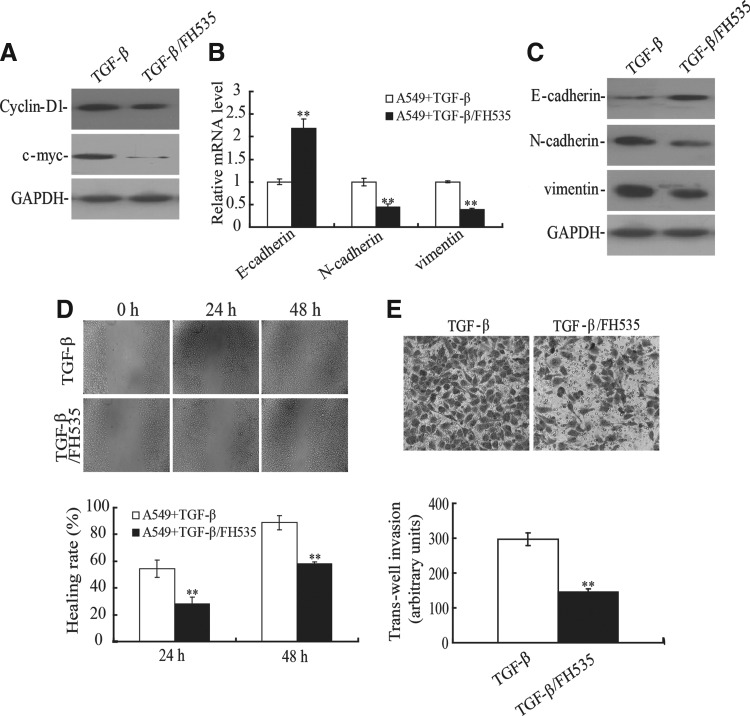
To further investigate the role of the Wnt pathway in TGF-β1-induced EMT, FH535, a reversible Wnt pathway inhibitor, was used. As expected, FH535 could inhibit the expression of cycline-D1 and c-myc, which were downstream targets of β-catenin (Fig. 4A). Next, we tested whether FH535 could affect the EMT markers in TGF-β1-treated A549 cell line. As shown in Figure 4B, the mRNA level of E-cadherin was upregulated after treatment with TGF-β1 and FH535, whereas the levels of N-cadherin and vimentin were significantly downregulated compared with TGF-β1-treated A549 cell line. Similar results were obtained at the protein level (Fig. 4C). In addition, the migratory and invasive potential of the A549 cell line was determined using the wounding-healing assay and transwell chamber assay. The migratory ability of the A549 cell line treated with TGF-β1 and FH535 was significant reduced compared with cells treated with TGF-β1 alone (Fig. 4D). Furthermore, the number of cells migrating to the basal side of the membrane was significantly decreased compared with cells treated with TGF-β1 alone (p<0.01, Fig. 4E). These results demonstrated that FH535 was able to inhibit TGF-β1-induced EMT in the A549 cell line, suggesting that the Wnt/β-catenin pathway was critical for induction of EMT by TGF-β1.
FIG. 4.
FH535 reversed the phenotype of TGF-β1-induced EMT in A549 cell line. (A) The A549 cell line was treated with FH535 (20 μM) and TGF-β1 (10 ng/mL) for 72 hours. The expression of cyclin-D1 and c-myc was determined by Western blotting. The mRNA (B) and protein (C) levels of E-cadherin, N-cadherin, and vimentin were determined by real-time PCR and Western blotting, respectively. **p<0.01. (D) The migration capacity of A549 cells treated with TGF-β1 and FH535 was determined by wound-healing assays. **p<0.01. (E) The migration capacity of A549 cells treated with TGF-β1 and FH535 was detected by transwell assay. **p<0.01.
Discussion
In this study, we confirmed that the A549 cell line exhibited EMT characteristics, including an elongated fibroblastoid shape, switch of EMT marker proteins, and increased migratory and invasive potential after induction with TGF-β1. We also demonstrated that sFRP1 could inhibit TGF-β1-induced EMT through suppressing the Wnt pathway in the A549 cell line. Additionally, FH535, a reversible Wnt signaling inhibitor, exerted a similar effect on TGF-β1-induced EMT, which indicated the critical role of Wnt signaling in the process of EMT, and sFRP1 was a potential target for the treatment of nonsmall cell lung cancer (NSCLC).
The process of EMT is involved in tumor migration, invasion, and dissemination, thus facilitating tumor progression.12 TGF-β1 has been recognized as a regulator of EMT in advanced-stage human cancers and the most widely used inducer of EMT for in vitro studies.13 In this study, we confirmed that TGF-β1 could induce EMT characteristics in the A549 cell line, which was consistent with previous studies.14,15 Accumulating evidence has indicated that the Wnt signaling pathway not only plays an important role in normal mammary development, but also regulates EMT in cancer progression.16 β-catenin is the central molecule that activates various downstream effectors responsible for cell proliferation, dedifferentiation, inhibition of apoptosis, and tumor progression. Activation of the Wnt pathway induces EMT in numerous models, including mammary epithelial and carcinoma cell lines.17,18 Activation of the Wnt pathway increases the expression of EMT regulator Snail and vimentin in breast cancer cells.19 Activation of the Wnt/β-catenin pathway also enhances the motility of glioblastoma cells by activation of ZEB1 in vitro.20 Similarly, in prostate cancer, inhibition of Wnt signaling leads to the reversal of EMT induced by HIF-1α.21 Increased expression of β-catenin is often correlated with poor prognosis in hepatocellular cancer.22 Importantly, nuclear β-catenin has been shown to induce EMT and is used as a mesenchymal marker.23 Herein we showed that the Wnt pathway was activated in the process of TGF-β1-induced EMT phenotype, including the increased expression of phosphorylated GSK3β and β-catenin.
FRP1 acts as a Wnt signaling antagonist and plays an important role in the control of cellular proliferation by inhibiting Wnt activity. We found that TGF-β1 could downregulate the expression of SFRP1 in A549 cells and SFRP1 restoration decreases the expression of β-catenin and GSK3β phosphorylation as well as the expression of c-myc and cyclin D1. Meanwhile, our study also showed that overexpression of sFRP1 inhibited human lung cancer cell invasion and migration in vivo and in vitro. All these results suggested that sFRP1 might be able to reverse the EMT process by inhibition of the Wnt signaling pathway in A549 cells. It is generally considered that sFRP1 is frequently silenced by promoter CpG island hypermethylation in numerous solid and nonsolid tumors and establishes tumor suppressive roles in cancer cell lines and xenografts.24,25 sFRP1 promoter methylation has been recognized as strong prognostic markers of poor outcome in primary cancers of the kidney, blood, breast, and lung.26–28 Additionally, recent research documented that microRNA34a could also significantly regulate the expression levels of sFRP1.29 Further studies are required to determine the relationship between the epigenetic modulation of sFRP1 and EMT. Furthermore, FH535, a potent Wnt inhibitor, could reverse the phenotype of TGF-β1-induced EMT, including alteration of marker proteins and migratory potential in A549 cells. All these results suggested that the Wnt pathway played a critical role in the process of EMT, and SFRP1 was a potential target for the treatment of NSCLC.
In summary, our results have demonstrated that sFRP1 was downregulated in TGF-β1-induced EMT, and restoration of sFRP1 exhibited the antitumor activity associated with EMT reversal. These findings may provide new insights into understanding the mechanism of lung cancer progression, which may offer a new biomarker for lung cancer treatment.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 81172106, 81172335) and the National Natural Science Foundation of Jiangsu Province of China (BK2012371).
Disclosure Statement
The authors are not employed by any commercial companies that may influence the study performed here.
References
- 1.Thiery JP. Acloque H. Huang RY, et al. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 2.Liu LK. Jiang XY. Zhou XX, et al. Upregulation of vimentin and aberrant expression of E-cadherin/beta-catenin complex in oral squamous cell carcinomas: Correlation with the clinicopathological features and patient outcome. Mod Pathol. 2010;23:213. doi: 10.1038/modpathol.2009.160. [DOI] [PubMed] [Google Scholar]
- 3.Ikushima H. Miyazono K. TGFbeta signalling: A complex web in cancer progression. Nat Rev Cancer. 2010;10:415. doi: 10.1038/nrc2853. [DOI] [PubMed] [Google Scholar]
- 4.Biswas S. Guix M. Rinehart C, et al. Inhibition of TGF-beta with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J Clin Invest. 2007;117:1305. doi: 10.1172/JCI30740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiery JP. Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131. doi: 10.1038/nrm1835. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Cadigan KM. Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 8.Ko H. Kim HS. Kim NH, et al. Nuclear localization signals of the E-cadherin transcriptional repressor Snail. Cells Tissues Organs. 2007;185:66. doi: 10.1159/000101305. [DOI] [PubMed] [Google Scholar]
- 9.Kaur P. Mani S. Cros MP, et al. Epigenetic silencing of sFRP1 activates the canonical Wnt pathway and contributes to increased cell growth and proliferation in hepatocellular carcinoma. Tumour Biol. 2012;33:325. doi: 10.1007/s13277-012-0331-5. [DOI] [PubMed] [Google Scholar]
- 10.Zhou XL. Qin XR. Zhang XD, et al. Downregulation of Dickkopf-1 is responsible for high proliferation of breast cancer cells via losing control of Wnt/beta-catenin signaling. Acta Pharmacol Sin. 2010;31:202. doi: 10.1038/aps.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X. Chen C. Ye D, et al. Diammonium glycyrrhizinate upregulates PGC-1alpha and protects against Abeta1–42-induced neurotoxicity. PLoS One. 2012;7:e35823. doi: 10.1371/journal.pone.0035823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber MA. Kraut N. Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Muraoka-Cook RS. Shin I. Yi JY, et al. Activated type I TGFbeta receptor kinase enhances the survival of mammary epithelial cells and accelerates tumor progression. Oncogene. 2006;25:3408. doi: 10.1038/sj.onc.1208964. [DOI] [PubMed] [Google Scholar]
- 14.Ha B. Kim EK. Kim JH, et al. Human peroxiredoxin 1 modulates TGF-beta1-induced epithelial-mesenchymal transition through its peroxidase activity. Biochem Biophys Res Commun. 2012;421:33. doi: 10.1016/j.bbrc.2012.03.103. [DOI] [PubMed] [Google Scholar]
- 15.Masaki S. Masutani H. Yoshihara E, et al. Deficiency of thioredoxin binding protein-2 (TBP-2) enhances TGF-beta signaling and promotes epithelial to mesenchymal transition. PLoS One. 2012;7:e39900. doi: 10.1371/journal.pone.0039900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alfieri CM. Cheek J. Chakraborty S, et al. Wnt signaling in heart valve development and osteogenic gene induction. Dev Biol. 2010;338:127. doi: 10.1016/j.ydbio.2009.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian X. Liu Z. Niu B, et al. E-cadherin/beta-catenin complex and the epithelial barrier. J Biomed Biotechnol. 2011;2011:567305. doi: 10.1155/2011/567305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sylvie J. Ellen C. Kris V. The role of Wnt in cell signaling and cell adhesion during early vertebrate development. Front Biosci. 2011;16:2352. doi: 10.2741/3858. [DOI] [PubMed] [Google Scholar]
- 19.Yook JI. Li XY. Ota I, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nat Cell Biol. 2006;8:1398. doi: 10.1038/ncb1508. [DOI] [PubMed] [Google Scholar]
- 20.Kahlert UD. Maciaczyk D. Doostkam S, et al. Activation of canonical WNT/beta-catenin signaling enhances in vitro motility of glioblastoma cells by activation of ZEB1 and other activators of epithelial-to-mesenchymal transition. Cancer Lett. 2012;325:42. doi: 10.1016/j.canlet.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 21.Zhao JH. Luo Y. Jiang YG, et al. Knockdown of beta-Catenin through shRNA cause a reversal of EMT and metastatic phenotypes induced by HIF-1alpha. Cancer Invest. 2011;29:377. doi: 10.3109/07357907.2010.512595. [DOI] [PubMed] [Google Scholar]
- 22.Xia H. Ooi LL. Hui KM. MiR-214 Targets beta-catenin pathway to suppress invasion, stem-like traits and recurrence of human hepatocellular carcinoma. PLoS One. 2012;7:e44206. doi: 10.1371/journal.pone.0044206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavert N. Ben-Ze'ev A. Epithelial-mesenchymal transition and the invasive potential of tumors. Trends Mol Med. 2008;14:199. doi: 10.1016/j.molmed.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka J. Watanabe T. Kanazawa T, et al. Silencing of secreted frizzled-related protein genes in MSI colorectal carcinogenesis. Hepatogastroenterology. 2008;55:1265. [PubMed] [Google Scholar]
- 25.Morris MR. Ricketts C. Gentle D, et al. Identification of candidate tumour suppressor genes frequently methylated in renal cell carcinoma. Oncogene. 2010;29:2104. doi: 10.1038/onc.2009.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urakami S. Shiina H. Enokida H, et al. Combination analysis of hypermethylated Wnt-antagonist family genes as a novel epigenetic biomarker panel for bladder cancer detection. Clin Cancer Res. 2006;12:2109. doi: 10.1158/1078-0432.CCR-05-2468. [DOI] [PubMed] [Google Scholar]
- 27.Fukui T. Kondo M. Ito G, et al. Transcriptional silencing of secreted frizzled related protein 1 (SFRP 1) by promoter hypermethylation in non-small-cell lung cancer. Oncogene. 2005;24:6323. doi: 10.1038/sj.onc.1208777. [DOI] [PubMed] [Google Scholar]
- 28.Sturgeon SR. Balasubramanian R. Schairer C, et al. Detection of promoter methylation of tumor suppressor genes in serum DNA of breast cancer cases and benign breast disease controls. Epigenetics. 2012;7:11. doi: 10.4161/epi.22220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J. Tang W. Du P, et al. Identifying MicroRNA-mRNA regulatory network in colorectal cancer by a combination of expression profile and bioinformatics analysis. BMC Syst Biol. 2012;6:68. doi: 10.1186/1752-0509-6-68. [DOI] [PMC free article] [PubMed] [Google Scholar]