Abstract
MicroRNAs (miRNAs) are a class of endogenous molecules that post-transcriptionally regulate target gene expression and play an important role in many developmental processes. Matrix extracellular phosphoglycoprotein (MEPE) is related to bone metabolism. We recently reported that MEPE protects cells from DNA damage-induced killing. The purpose of this study is to investigate whether miRNAs targeting MEPE play an important role in DNA damage response. We report in this study that miR-376a directly targets MEPE, and overexpression of miR-376a reduces the G2 arrest of the cells and sensitizes the cells to DNA damage-induced killing. These results indicate an association of MEPE gene inactivation with decreased survival after DNA damage and also provide useful information for miRNA-based drug development: a new target for sensitizing human tumor cells to radiotherapy or chemotherapy.
Key words: DNA damage, MEPE, microRNA, target
Introduction
Matrix extracellular phosphoglycoprotein/Osteoblast factor 45 (MEPE/OF45) was originally cloned from a human oncogenetic hypophosphatemia tumor1 and then identified in rat bone marrow-derived osteoblasts.2 The murine homologue of MEPE/OF45 was reported the following year.3 Since the identification of MEPE/OF45, its function related to bone metabolism has been widely investigated. Although we previously identified that MEPE/OF45, as a cofactor of CHK1, protects cells from DNA damage-induced killing in rat embryo fibroblast cells4 and in different types of human tumor cells,5 it is still unclear how miRNAs targeting MEPE in DNA damage response play the role.
MicroRNAs (miRNAs) are small noncoding single strand RNAs ranging from 18 to 25 nt, which play diversified roles in vertebrates, plants, and even viruses.6–11 They suppress gene expression at post-transcriptional level by partial complementary binding to the 3′-UTR of target mRNAs, resulting in mRNAs degradation and/or translational repression.12–14 Since its first discovery in C. elegans in 1993,6 more than 2000 miRNA sequences, either in precursor or mature form, have been identified in Homo sapiens and recorded in miRBase database to date.15 Bioinformatics and cloning studies have estimated that miRNAs may regulate 30% of all human genes, and each miRNA can control hundreds of gene targets.16 These human miRNAs play important roles in multiple biological processes, ranging from cell growth, survival, and differentiation, to embryonic development and cancer progression.6–16
Here, we predicted the candidate miRNAs targeting MEPE by TargetScan. We found that miR-376a directly targets MEPE, introduction of miR-376a expression in human cells resulted in decreased G2 phase arrest, and overexpression of miR-376a sensitizes cells following DNA damage. These findings could provide a rationale for the development of miRNA-based strategies for sensitizing tumor cells to radiotherapy or chemotherapy.
Materials and Methods
Cell culture, chemical treatment, and irradiation
The 293T cells, HeLa (human cervical cancer) cells and HepG2 (human liver cancer) cells were grown to 80% confluence in Dulbecco's modified Eagle's medium (DMEM) with 10% Calf Serum at 37°C in an atmosphere of 5% CO2 and 95% air. Irradiation of cells was done by exposing cells to Co60 (215cGy/min). Cells were either treated with camptothecin (CPT; kindly provided by Dr. Ya Wang, Emory University, Atlanta) or etoposide (Et; China National Medicines Guorui Pharmaceutical Co., Ltd.).
Plasmid construction and transfection
The 3′UTR of MEPE was PCR-amplified from human genomic DNA, DNA fragments from 3′UTR of MEPE that host the predicted complementary sites of miR-376a or the mutated sites were cloned downstream of Firefly luciferase reporter gene in pGL3-control plasmid (Promega). The following sets of primers were used to generate specific fragments: Mepe-3′UTR-forward, 5′-CCG CTC GAG CTG AAG ACC TCG TCA CCT-3′; Mepe-3′UTR-reverse, 5′-CG ACG CGT CAT AGA AGG CAT TTG TGA-3′; Mepe-3′UTR 376a mut-reverse, 5′-CG ACG CGT CTA CTC AAA TGT TAC CTA TTCCGTCGCT CTG TAA TGA TT-3′. The DNA sequences encoding the indicated miRNAs together with surrounding miRNAs precursor sequences (approximately 300 bp in total) were kindly provided by Dr. Xiao Yang (Beijing Institute of Biotechnology) and Dr. Xiaofei Zheng (Beijing Institute of Radiation Medicine). These miRNA sequences are subcloned into pcDNA3.1 vector (Invitrogen) with the digestion of Hind III and Xho I; the modified plasmids are used for miRNAs expression in future. The 293T cells were transfected with the plasmids by Lipofectamine 2000 (Invitrogen), according to the manufacturer's instructions.
Luciferase reporter assay for targeting MEPE 3 ′UTR
Luciferase reporter experiments were performed as described previously,17 a MEPE 3′UTR segment of 344 bp was inserted into the pGL3-control vector with simian virus 40 promoter by using the Bgl II and Mul I sites immediately downstream from the stop codon of luciferase. The 293T cells were seeded in 24-well plates and co-transfected by using Lipofectamine 2000 reagent (Invitrogen) according to the protocol of the manufacturer, with 50 ng of the Firefly luciferase report vectors including wild-type or mutant, 2 ng of the control vector containing Renilla luciferase pRL-TK vector (Promega), and 200 ng of miRNAs expression plasmids or control vector. The cells were lysed and Firefly and Renilla luciferase activities were consecutively measured by using dual-luciferase assays (Vigorous Biotechnology) 24 hours after the transfection. All experiments were performed in triplicate and data were normalized to the activity of the Firefly luciferase expressed from the same pGL3-control vector as an internal control.
Antibodies and western blot
The HeLa cells were seeded in 35 mm dishes and transfected with plasmids containing miR-129, miR-376a, miR-376b, miR-412, miR-425, miR-758, and control vector, and the cells were collected and counted after 24 hours. A total of 1×106 cells were lysed in 50 μL of RIPA lysis buffer (150 mM NaCl, 40 mM morpholinepropanesulfonic acid (MOPS, pH7.2), 1 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1%SDS, and 0.2 mM PMSF) for 30 minutes. The cell lyses were centrifuged at 12000 rpm for 10 minutes at 4°C, the supernatants were transferred to a new tube, and then the protein concentration was tested, 50 μg of proteins were loaded into 12% polyacrylamide gel (PAGE). Western blot analysis was performed using enhanced chemiluminescence. The MEPE/OF45 antibody was made by our laboratory,18 the PCNA, CHK1, and GAPDH antibodies were purchased from Santa Cruz Biotechnology, Inc.
Flow cytometry assay
The G2 checkpoint is detected by flow cytometry measurement.17 1×105 cells were plated in 35-mm dishes with 2 mL of growth medium. The cells were transfected with the plasmids containing miR-376a and control vector for 24 hours and then exposed to 6 Gy and returned to 37°C. At different times thereafter (0, 6, 12, 18, and 24 hours), cells were trypsinized and fixed in 70% ethanol. Cells were stained in the solution (62 μg/mL RNase A, 40 μg/mL propidium iodide, and 0.1% Triton X-100 in phosphate-buffered saline buffer) at room temperature for 1 hour. The distribution of cells in the cell cycle was measured in a flow cytometer (Cytomics FC500; Beckman Coulter).
Cell survival assay
Cell proliferation was measured by 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenylte-trazolium bromide (MTT) assay.19,20 The cells were seeded in 96-well plates at a density of 2×103 cells per well with 0.1 mL of growth medium. Twenty-four hours later, the cells were transfected with the plasmids containing pri-miR-376a and control vector for 24 hours and then exposed to 6 Gy and returned to 37°C, or treated with drugs (5 μM CPT or 100 μM Et) for 16 hours. At various time points (0, 12, 24, 36, and 48 hours), 20 μL of MTT solution (5 mg/mL) was added into the culture medium for 4 hours incubation. Then 150 μL dimethyl sulfoxide was added to each well to dissolve the crystals. After 10 minutes dissolution, the absorbance of each sample was recorded by a microplate reader (Model 680 with Microplate Manager Software; Bio-rad) at 490 nm. The experiment was performed in triplicate.
Statistical analysis
All the data were presented as mean±standard deviation (SD). Statistical significance was determined using Student's t-test. p<0.05 was considered as statistically significant difference.
Results and Discussion
Candidate miRNAs targeting MEPE
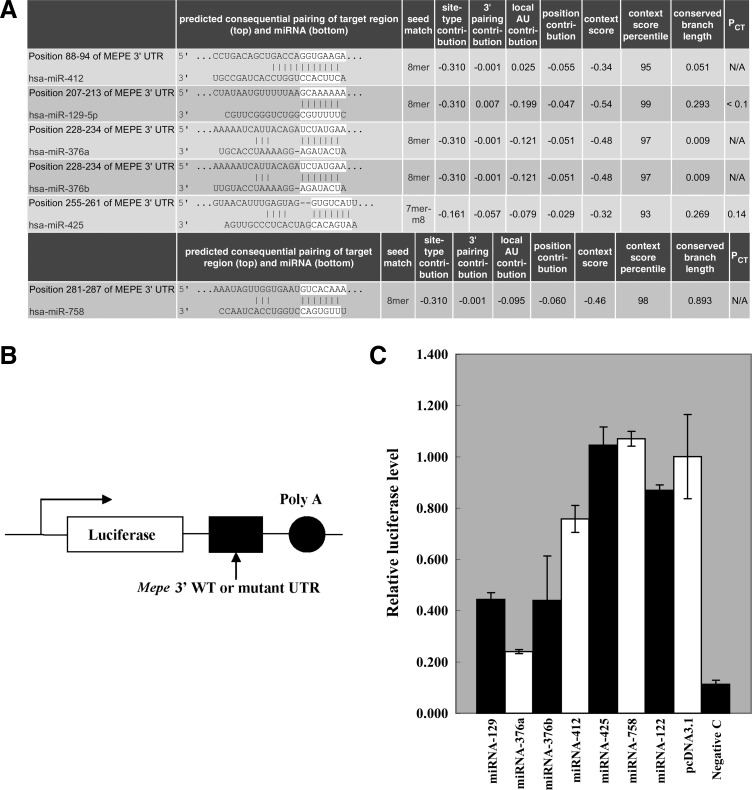
To investigate the role of miRNAs targeting MEPE in DNA damage response, first, TargetScan was used to predict the candidate miRNAs targeting MEPE, 36 miRNAs targeting MEPE were predicted (data not shown), only 6 of them were selected (Fig. 1A). To test whether these miRNAs target MEPE, the fragment of 3′UTR of human MEPE was cloned into pGL3-control dual luciferase reporter plasmid (Fig. 1B). miRNAs were cloned into pcDNA3.1. The 293T cells were co-transfected with MEPE 3′UTR Luciferase reporters, along with expression plasmids of miRNAs, respectively. Luciferase analysis revealed that only four miRNAs (hsa-miR-129, hsa-miR-376a, hsa-miR-376b, and has-miR-412) could significantly suppress the activity of wild-type 3′UTR reporter (Fig. 1C), hsa-miR-425 and hsa-miR-758 could not (Fig. 1C), so hsa-miR-129, hsa-miR-376a, hsa-miR-376b, and has-miR-412 may be the miRNAs targeting MEPE.
FIG. 1.
Candidate miRNAs targeting MEPE. (A) Predicted miRNAs targeting MEPE by TargetScan. (B) MEPE 3′UTR luciferase reporters. 3′ WT, wild-type 3′UTR. Mutant UTR (4 mutant sites in 3′UTR of MEPE). (C) 293T cells were co-transfected with MEPE 3′UTR luciferase reporters, along with expression plasmids of miRNAs and control vector. Renilla luciferase activity from the same construct were used to normalize and to generate relative activity of Firefly luciferase that was subject to the regulation of cloned Mepe 3′UTR. Relative luciferase level=(Sluc/Srenilla)/(Cluc/Crenilla). Luc, raw Firefly luciferase activity; Renilla, internal transfection control renilla activity; S, sample; C, control. The error bar represents the standard deviation (SD) from three independent experiments.
miRNA-376a directly targets MEPE gene
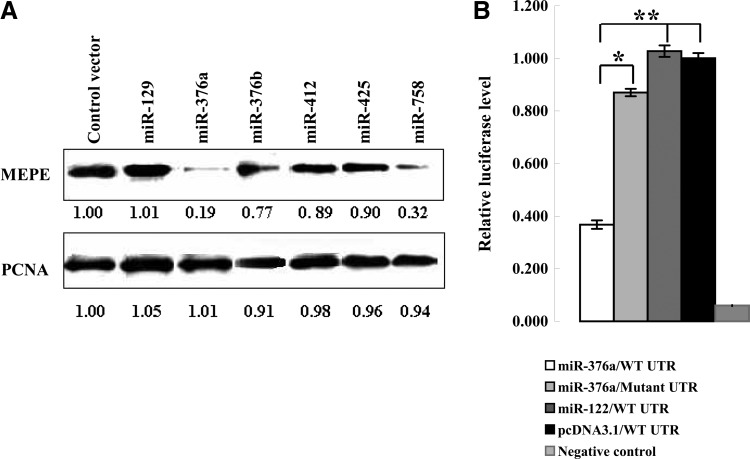
By western blot, the results show a significant reduction (about five-fold) of MEPE protein in HeLa cells with overexpression of miR-376a, and about four-fold reduction when miR-758 was overexpressed (Fig. 2A), other miRNAs could not suppress MEPE protein level. Because miR-758 could not significantly suppress the activity of wild-type 3′UTR reporter (Fig. 1C), our data strongly suggest that miR-376a targets MEPE. However, our RT-PCR analysis showed no difference in the mRNA levels of MEPE between the cells of miR-376a overexpressing and transfected with control vector (data not shown). This raised the possibility that miR-376a suppresses MEPE protein synthesis by a post-transcriptional repression mechanism, via its 3′UTR complementary sites.
FIG. 2.
Downregulation of MEPE by miR-376a is mediated by 3′UTR complementary sites. (A) MEPE protein levels in HeLa cells are significantly suppressed by miR-376a. PCNA was used as protein loading control. Protein was quantified by Image J. (B) 293T cells were co-transfected with Mepe 3′UTR or mutant 3′UTR (4 of 8 bases were mutated in complementary sites) luciferase reporters, along with expression plasmids of miR-376a and control vector. Student's t-test compared two data sets marked by stars in the panel. *p<0.05, **p<0.01.
To test this possibility, the fragment of 3′UTR of human MEPE containing wild-type or mutated (4 of 7 bases were mutated, CCGTCGC) miR-376a complementary sites (Fig. 1A) were cloned into pGL3-control dual luciferase reporter plasmid. Luciferase reporters were co-transfected with miR-376a and control vector, respectively. Luciferase analysis showed that the activity of wild-type 3′UTR reporter was significantly suppressed by miR-376a. Suppression by miR-376a depends on the wild-type miR-376a complementary sites, and was not longer observed in the reporter in which miR-376a complementary sites were mutated (Fig. 2B), miR-122 as irrelative control. Our data strongly suggested that MEPE is a direct target of miR-376a.
Overexpression of miR-376a in human cells reduces the G2 arrest
We previously reported that A1–5 and B4 were one pair of transformed rat embryo fibroblasts,21 the expression of MEPE was higher in A1–5 cells than in B4 cells,4 and A1–5 cells showed much stronger G2 checkpoint responses than its counterpart B4 cells.21 We also reported that wild-type hMEPE could increase the G2/M accumulation of cells after ionizing radiation (IR).5
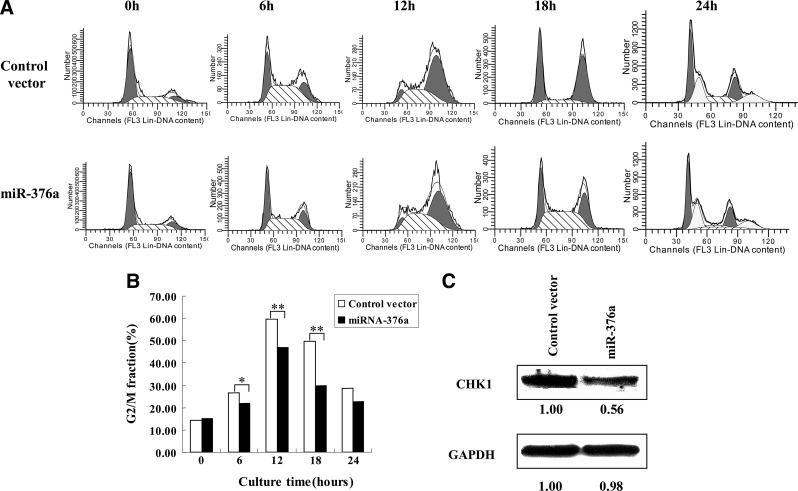
We were interested in determining whether IR-induced G2 accumulation was reduced in human cells after knocking down hMEPE. For this purpose, the impact of miR-376a in cell cycle in human cells was investigated. The construct of expressing miR376a was made and transfected into human HeLa cells using transient transfection for increasing miR-376a expression, pcDNA3.1 as control vector. After IR with dose of 6 Gy, G2 accumulation in these irradiated human cells was examined. Cell cycle analysis showed that G2 accumulation occurs at 6 hours after IR (Fig. 3), and 5%, 13%, 20%, and 6% increase in the G2 population of control cells relative to irradiated miR-376a-expressing cells at 6, 12 18, and 24 hours after IR (Fig. 3), indicating a decreased G2 checkpoint in irradiated miR-376a-expressing cells. These data suggest that irradiated miR-376a-expressing cells show a reduced G2 accumulation.
FIG. 3.
Overexpression of miR-376a in human cells reduces G2 arrest. HeLa cells were transfected with the plasmids containing miR-376a and control vector for 24 hours, and then exposed to 6 Gy and fixed in 70% ethanol. Cells were washed with PBS and stained with propidium iodide (PI). The distribution of cells through the cell cycle was measured by flow cytometry (A). The fraction of cells in the G2/M phase is plotted as a function of time after irradiation (B). The significant G2 phase reduction was observed in HeLa cells at 12 and 18 hours after irradiation. The data are processed with the ModFit LT program. Student's t-test compared two data sets marked by stars in the panel. *p<0.05, **p<0.01. (C) CHK1 protein levels in HeLa cells are suppressed by overexpressed miR-376a. GAPDH was used as protein loading control. Protein was quantified by Image J.
We previously reported that MEPE sensitized mammalian cells to radiation-induced killing by modulating the CHK1 pathway, and this kind of checkpoint response, G2-phase cell accumulation is CHK1 dependent.22 We need to know whether the CHK1 levels changed after upregulating miR376a. The results showed that the CHK1 levels decreased after overexpressing miR376a (Fig. 3C) by western blot, and that is why the cells showed a reduced G2 accumulation.
Overexpression of miR-376a sensitizes cells to DNA damage-induced killing
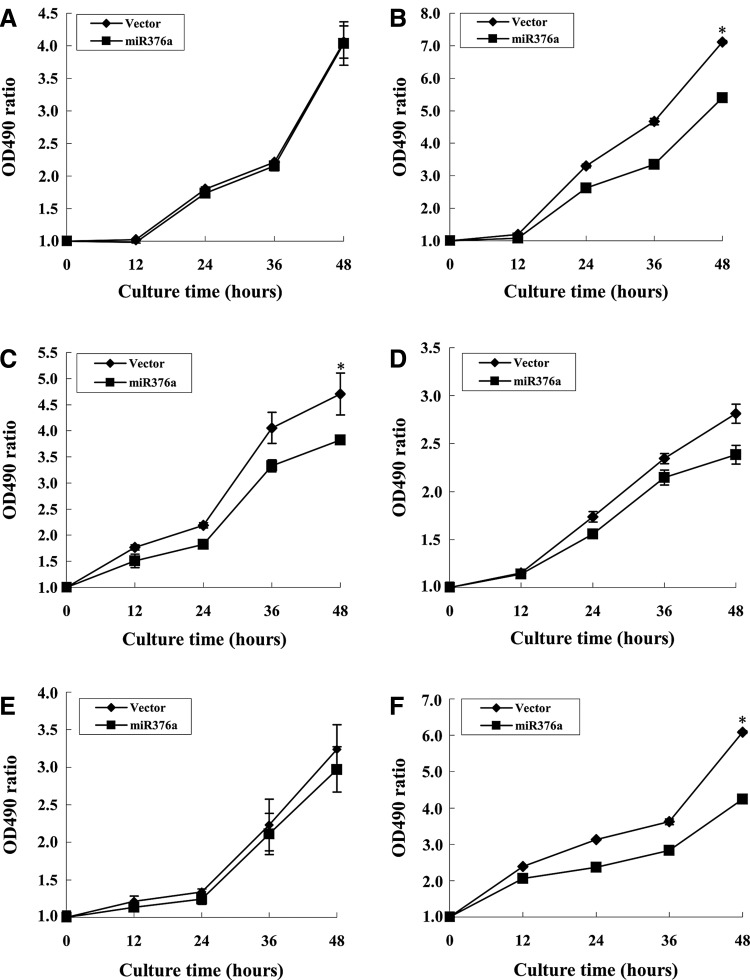
G2 checkpoint facilitates homologous recombination repair and therefore affects the radioresistance of cells.23–26 We previously reported that MEPE/OF45 protects mammalian cells from DNA damage-induced killing,4,5 suggesting that miR-376a-expressing human cells may be more radiosensitive than the cells transfected with control vector. To verify this hypothesis, the cell survivals of miR-376a-expressing cells (HeLa and HepG2) and the cells transfected with control vector were measured following DNA damage inducer treatment. As expected, miR-376a-expressing cells were sensitive to multiple DNA damage inducer including IR, CPT, and Et than the cells transfected with control vector (Fig. 4). These data suggest that overexpression of miR-376a sensitizes cells from DNA damage-induced killing.
FIG. 4.
Effects of miR-376a on the survival of HeLa and HepG2 cells. 2×103 cells were plated in 96-well plates for 24 hours, HeLa cells (A–D) and HepG2 cells (E–F) were transfected with the plasmids containing miR-376a (squares) and control vector (diamonds) for 24 hours, and then exposed to 6 Gy (B) or treated with 5 μM Camptothecin (C and F) or 100 μM Etoposide (D) for 16 hours, A and E depict untreated cells. The cell survival was determined using MTT assay as described under “Experimental Procedures.” Data shown are the averages from three independent experiments.
We previously reported that higher the level of MEPE/OF45 was in the cell line, more resistant the cell line was to IR or CPT.4 There were 293 cells with the lowest level of MEPE, and they were most sensitive to IR or CPT.5 In addition, we reported that knocking down MEPE could sensitize HeLa cells to DNA damage inducers.4 Because miR-376a can target MEPE and suppress MEPE protein level in human cells, we used HeLa to test the G2 checkpoint responses and the cell survivals, but not 293 cells.
Human chromosome 14 and syntenic regions of the distal end of mouse chromosome 12 harbor the miR-376 cluster of miRNA genes.27 Expression of miR-376 is detected in the placenta, developing embryos, and adult tissues.27,28 Research results show that in certain tissues the miR-376 cluster transcripts are subject to RNA editing of converting adenosine-to-inosine. Compared with normal miR-376, the edited miR-376 RNA silences specifically a different set of genes; in these targets, phosphoribosyl pyrophosphate synthetase 1, an enzyme involved in the uric-acid synthesis pathway, is repressed by the edited miR-376; this process contributes to tight and tissue-specific regulation of uric-acid levels.29 Enforced expression of miR-376a blocked erythroid differentiation by targeting Argonaute 2(Ago2) and Cyclin-Dependent Kinase 2(CDK2). The lentiviral vector-mediated transduction of miR-376a into CD34+ stem/progenitor cells (HPCs) delayed erythrocyte maturation in erythroid cultures and prevented erythroid colony formation in a semisolid medium.30 The re-expression of miR-376a and miR-376c lead to attenuation of melanoma proliferation and migration. These two miRNAs target IGF1R, a tyrosine kinase receptor implicated in melanoma tumorigenesis and metastasis.31
MEPE protecting human cells from IR- or CPT-induced killing mainly depends on its interaction with CHK1.4,5 The specific molecular mechanism for this protective role of MEPE could be linked to MEPE stabilizing CHK1 by reducing CHK1 degradation. Because CHK1 is an essential gene32,33 and MEPE is not,34 these results indicate that MEPE is a new cofactor of CHK1, which could be a new target with less side effects for sensitizing tumor cells to DNA damage inducers and could benefit cancer treatment. Here, we first report a new role of miR-376a that sensitized cells from DNA damage-induced killing by downregulation of MEPE, it provides that the miR-376a could be a new target for sensitizing tumor cells to radiotherapy or chemotherapy.
Conclusions
In summary, we have characterized the role of miR-376a in DNA damage response in human cervical cancer cells and human adenocarcinoma cells. We show that overexpressing miR-376a results in significant G2 phase reducing and sensitive cells from DNA damage-induced killing by downregulation of MEPE, and also provides useful information for miRNA-based drug development.
Acknowledgments
The authors thank Dr. Xiaofei Zheng from the Beijing Institute of Radiation Medicine and Dr. Xiao Yang from the Beijing Institute of Biotechnology for microRNAs plasmids. This work was supported by grants from the National Natural Sciences Foundation of China (31070760, 30770651, and 30670616) to B.H.
Disclosure Statement
No competing financial interests exist.
References
- 1.Rowe PS. de Zoysa PA. Dong R, et al. MEPE, a new gene expressed in bone marrow and tumors causing osteomalacia. Genomics. 2000;67:54. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- 2.Petersen DN. Tkalcevic GT. Mansolf AL, et al. Identification of osteoblast/osteocyte factor 45 (OF45), a bone-specific cDNA encoding an RGD-containing protein that is highly expressed in osteoblasts and osteocytes. J Biol Chem. 2000;275:36172. doi: 10.1074/jbc.M003622200. [DOI] [PubMed] [Google Scholar]
- 3.Argiro L. Desbarats M. Glorieux FH, et al. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342. doi: 10.1006/geno.2001.6553. [DOI] [PubMed] [Google Scholar]
- 4.Liu S. Wang H. Wang X, et al. MEPE/OF45 protects cells from DNA damage induced killing via stabilizing CHK1. Nucleic Acids Res. 2009;37:7447. doi: 10.1093/nar/gkp768. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 5.Zhang P. Wang H. Rowe PSN, et al. MEPE/OF45 as a new target for sensitizing human tumor cells to DNA damage inducers. Brit J Cancer. 2010;102:862. doi: 10.1038/sj.bjc.6605572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee RC. Feinbaum RL. Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. microRNAs: Tiny regulators with great potential. Cell. 2001;107:823. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 8.Ryan BM. Robles AI. Harris CC. Genetic variation in microRNA networks: The implications for cancer research. Nat Rev Cancer. 2010;10:389. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zisoulis DG. Kai ZS. Chang RK, et al. Autoregulation of microRNA biogenesis by let-7 and Argonaute. Nature. 2012;486:541. doi: 10.1038/nature11134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong YW. Ferland-McCollough D. Jackson TJ, et al. microRNAs in cancer management. Lancet Oncol. 2012;13:e249. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 11.Ebert MS. Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chitwood DH. Timmermans MC. Small RNAs are on the move. Nature. 2010;467:415. doi: 10.1038/nature09351. [DOI] [PubMed] [Google Scholar]
- 13.Chen CZ. MicroRNAs as oncogenes and tumor suppressors. N Engl J Med. 2005;353:1768. doi: 10.1056/NEJMp058190. [DOI] [PubMed] [Google Scholar]
- 14.Esquela-Kerscher A. Slack FJ. Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer. 2006;6:259. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 15.Kozomara A. Griffiths-Jones S. miRBase: Integrating microRNA annotation and deep-sequencing data. Nucleic Acids Res. 2011;39:D152. doi: 10.1093/nar/gkq1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim VN. Han J. Siomi MC. Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol. 2009;10:1. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y. Yu F. Gao N, et al. microRNA-143 protects cells from DNA damage induced killing by downregulating Fhit expression. Cancer Biother Radiopharm. 2011;26:365. doi: 10.1089/cbr.2010.0914. [DOI] [PubMed] [Google Scholar]
- 18.Liu S. Wei W. Gao N, et al. Study on the antibody preparation and the localization of MEPE/OF45 protein. J Chin Gereol. 2007;27:1017. [Google Scholar]
- 19.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 20.Ulukaya E. Ozdikicioglu F. Oral AY, et al. The MTT assay yields a relatively lower result of growth inhibition than the ATP assay depending on the chemotherapeutic drugs tested. Toxicol In Vitro. 2008;22:232. doi: 10.1016/j.tiv.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Hu B. Zhou XY. Wang X, et al. The radioresistance to killing of A1–5 cells derives from activation of the Chk1 pathway. J Biol Chem. 2001;276:17693. doi: 10.1074/jbc.M009340200. [DOI] [PubMed] [Google Scholar]
- 22.Wang X. Khadpe J. Hu B, et al. An over-activated ATR/CHK1 pathway is responsible for the prolonged G2 accumulation in irradiated AT cells. J Biol Chem. 2003;278:30869. doi: 10.1074/jbc.M301876200. [DOI] [PubMed] [Google Scholar]
- 23.Hu B. Han SY. Wang X, et al. Involvement of the Fhit gene in the ionizing radiation-activated ATR/CHK1 pathway. J Cell Physiol. 2005;202:518. doi: 10.1002/jcp.20139. [DOI] [PubMed] [Google Scholar]
- 24.Hu B. Wang H. Wang X, et al. Fhit and CHK1 have opposing effects on homologous recombination repair. Cancer Res. 2005;65:8613. doi: 10.1158/0008-5472.CAN-05-1966. [DOI] [PubMed] [Google Scholar]
- 25.Wang H. Wang X. Iliakis G, et al. Caffeine could not efficiently sensitize homologous recombination repair deficient cells to ionizing radiation-induced killing. Radiat Res. 2003;159:420. doi: 10.1667/0033-7587(2003)159[0420:ccnesh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Wang X. Wang H. Iliakis G, et al. Caffeine-induced radiosensitization is independent of non-homologous end joining of DNA double strand breaks. Radiat Res. 2003;159:426. doi: 10.1667/0033-7587(2003)159[0426:ciriio]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Seitz H. Royo H. Bortolin ML, et al. A Large Imprinted microRNA Gene Cluster at the Mouse Dlk1-Gtl2 Domain. Genome Res. 2004;14:1741. doi: 10.1101/gr.2743304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poy MN. Eliasson L. Krutzfeldt J, et al. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;432:226. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- 29.Kawahara Y. Zinshteyn B. Sethupathy P. Iizasa H. Hatzigeorgiou AG. Nishikura K. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang F. Yu J. Yang GH, et al. Regulation of erythroid differentiation by miR-376a and its targets. Cell Res. 2011;21:1196. doi: 10.1038/cr.2011.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zehavi L. Avraham R. Barzilai A, et al. Silencing of a large micro-RNA cluster on human chromosome 14q32 in melanoma: Biological effects of mir-376a and mir-376c on insulin growth factor 1 receptor. Mol Cancer. 2012;11:44. doi: 10.1186/1476-4598-11-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q. Guntuku S. Cui XS, et al. Chk1 is an essential kinase that is regulated by Atr and required for the G2/M DNA damage checkpoint. Genes Dev. 2000;14:1448. [PMC free article] [PubMed] [Google Scholar]
- 33.Lam MH. Liu Q. Elledge SJ, et al. Chk1 is haploinsufficient for multiple functions critical to tumor suppression. Cancer Cell. 2004;6:45. doi: 10.1016/j.ccr.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Gowen LC. Petersen DN. Mansolf AL, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]