Abstract
OBJECTIVES
Diabetes mellitus (DM) has been associated with an increased risk of colorectal cancer (CRC). The American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008 recommend that clinicians be aware of an increased CRC risk in patients with smoking and obesity, but do not highlight the increase in CRC risk in patients with DM. To provide an updated quantitative assessment of the association of DM with colon cancer (CC) and rectal cancer (RC), we conducted a meta-analysis of case–control and cohort studies. We also evaluated whether the association varied by sex, and assessed potential confounders including obesity, smoking, and exercise.
METHODS
We identified studies by searching the EMBASE and MEDLINE databases (from inception through 31 December 2009) and by searching bibliographies of relevant articles. Summary relative risks (RRs) with 95% confidence intervals (CIs) were calculated with fixed- and random-effects models. Several subgroup analyses were performed to explore potential study heterogeneity and bias.
RESULTS
DM was associated with an increased risk of CC (summary RR 1.38, 95% CI 1.26–1.51; n = 14 studies) and RC (summary RR 1.20, 95% CI 1.09–1.31; n = 12 studies). The association remained when we limited the meta-analysis to studies that either controlled for smoking and obesity, or for smoking, obesity, and physical exercise. DM was associated with an increased risk of CC for both men (summary RR 1.43, 95% CI 1.30–1.57; n = 11 studies) and women (summary RR 1.35, 95% CI 1.14–1.53; n = 10 studies). For RC, there was a significant association between DM and cancer risk for men (summary RR 1.22, 95% CI 1.07–1.40; n = 8 studies), but not for women (summary RR 1.09, 95% CI = 0.99–1.19; n = 8 studies).
CONCLUSIONS
These data suggest that DM is an independent risk factor for colon and rectal cancer. Although these findings are based on observational epidemiological studies that have inherent limitations due to diagnostic bias and confounding, subgroup analyses confirmed the consistency of our findings across study type and population. This information can inform risk models and specialty society CRC screening guidelines.
INTRODUCTION
Approximately 76,000 men and 72,000 women were diagnosed with cancer of the colon and rectum in the United States in 2009, and 49,900 died from this disease (1). Colorectal cancer (CRC) is the fourth most common cancer in the United States (2), and fourth in men and third in women worldwide (3). Understanding the risk factors for this disease is integral to the development of effective strategies for the prevention of CRC. The risk of developing CRC is influenced by both genetic and acquired risk factors. Acquired risk factors associated with CRC identified in prior studies include (4) the following: (i) dietary factors, such as low intake of fruit, vegetables, or fiber, and high intake of red meat, saturated fat, caffeine, or alcohol (5–9); (ii) lifestyle factors, such as lack of exercise, smoking, and obesity (10–12); (iii) side effects of some medical or surgical interventions, such as pelvic irradiation, cholecystectomy, and ureterocolic anastomosis (13–16); (iv) comorbid medical conditions such as Barrett’s esophagus, human immunodeficiency virus infection, diabetes mellitus (DM), acromegaly, and inflammatory bowel disease (17–21). These conditions may either directly modify risk (e.g., diet) or may serve as personal markers of altered risk through shared genetic or environmental exposures, separate from any direct mechanistic link (e.g., Barrett’s esophagus).
In 1910, Maynard (22) provided one of the earliest reports on the association between DM and cancer. Recently, a series of studies and meta-analyses confirmed that the risk for several solid and hematological malignancies (including liver, pancreas, colorectal, kidney, bladder, endometrial and breast cancers, and non-Hodgkin’s lymphoma) is elevated in diabetic patients (23). In 1984, Williams et al. (24) published a retrospective analysis of three patient populations and documented a statistically significant, over 2-fold increase in the prevalence of overt DM in CRC patients compared with age-matched controls. The search for a pathological explanation for this connection has led to the so-called hyperinsulinemia hypothesis. Giovannucci (25) hypothesized that insulin, an important growth factor, may at high serum concentrations increase the risk of CRC by promoting growth of colon tumors, and acting as a cell mitogen. Other proposed explanations for the increased risk include decreased bowel transit time and elevated fecal concentrations of bile acids (26,27). Yang et al. (28) showed that insulin treatment may further elevate risk for CRC among patients with type 2 DM.
Although there is some heterogeneity in the published literature regarding the association between DM and CRC, a meta-analysis by Larsson et al. (19) in 2005 showed a relationship between DM and increased risk of CRC in both women and men by combining relative risk (RR) of colon cancer (CC) and rectal cancer (RC) (summary RR of CRC incidence 1.30, 95 % confidence interval (CI) 1.20–1.40). They also did subgroup analyses and identified elevated summary RRs of CC (summary RR 1.43, 95 % CI 1.28–1.60; n=7 studies) and RC (summary RR 1.33, 95 % CI 1.14–1.54; n=7 studies). CRC has been historically considered together; however, it is increasingly being recognized that differences in etiology and risk may exist between right CCs, left CCs, and RCs (29,30). Since the previous meta-analysis, several new studies have published separate data for CC and RC.
The American College of Gastroenterology Guidelines for Colorectal Cancer Screening 2008 recommend that clinicians be aware of an increased risk of CRC in cigarette smokers and obese patients (31), but do not highlight the increased risk in patients with DM. Obesity and smoking are associated with the incidence of both type 2 DM and CRC (12,32–34); thus, they also could be important positive confounders of the association between DM and CRC. The previous meta-analysis by Larsson et al. (19) did not specifically evaluate for these confounders.
To provide an updated quantitative assessment of the association of DM with CC and RC risk, we conducted a meta-analysis of case–control and cohort studies. We evaluated whether the association varied by sex and study design, and calculated summary RRs separately for CC (n=14 studies) and RC (n=12 studies). We also provide the first meta-analysis to quantitatively assess the effect of certain potentially important confounding variables including obesity, smoking, and physical exercise.
METHODS
Search strategy
We identified studies by a literature search of the EMBASE and MEDLINE databases (from inception through 31 December 2009) for English-language studies with the following medical subject heading terms and/or text words: “diabetes mellitus,” “diabetes,” “colorectal cancer,” “colorectal neoplasm,” “colon cancer,” “colon neoplasm,” “rectal cancer,” “rectal neoplasm,” and “risk factor.” We also reviewed reference lists of the identified publications for additional pertinent studies.
Inclusion and exclusion criteria
Only reports fulfilling the following inclusion criteria were included in the meta-analysis. First, studies were included only if they reported an estimate of RR of colon and/or RC in individuals with DM compared with individuals without DM, with a corresponding measure of uncertainty (i.e., 95 % CI, standard error, variance, or P value). Studies reporting only RR for CRC were excluded. Studies were also excluded if the estimates were not adjusted by age. Second, we included case–control studies or cohort studies published as original articles; cross-sectional, ecological, and prevalence studies were excluded. Third, only studies that included distinct cohorts of patients were included. For multiple reports on the same population or subpopulation, we considered the estimates from the most recent report or the one containing the most cases.
Data extraction
The data extracted included publication data (the first author’s last name, year of publication, and country of the population studied), study design, number of cases, number of exposed and unexposed subjects (cohort studies), number of controls and the source of the controls (case–control studies), follow-up period (for cohort studies), type of DM (type 1 or 2), risk estimates with their corresponding CIs, and variables controlled for by matching or in the multivariable models. Abstractions of the data elements and the assessment of methodological quality (see below) were conducted separately by two authors (HY and CS); discordant results were resolved by consensus.
Assessment of methodological quality
We assessed the methodological “quality” of included studies based on the Newcastle-Ottawa Scale (35) for quality of case–control studies and cohort studies in meta-analysis; for this assessment, we used the Newcastle-Ottawa Scale star system (range, 0 to 9 stars). In the current study, we considered a study awarded seven or more stars as a high-quality study, because standard validated criteria for important end points have not been established. The mean value for the six case–control studies and eight cohort studies assessed was 5.3 stars and 6.2 stars, respectively. A table containing the rankings for each study is shown in the Supplementary data online.
Statistical analysis
Summary RR estimates were calculated using both the fixed-effects inverse variance weighting method (36) and the random-effects method (37). Statistical heterogeneity between studies was evaluated with Cochran’s Q-test and the I2-statistic (38). Publication bias was assessed by constructing a funnel plot and using Egger and Begg tests (39,40). All statistical analyses were carried out with STATA, version 11.0 (Stata Corp, College Station, TX). P values that were less than 0.05 were considered statistically significant. All statistical tests were two-sided.
As it is increasingly being recognized that differences in etiology and risk may exist between right CCs, left CCs, and RCs (29,30), we calculated summary RRs separately for CC and RC. Too few studies provided separate data for the right and left CCs to calculate separate summary RRs for these.
In order to explore potential heterogeneity and evaluate different forms of possible bias, several subgroup analyses were performed. These included subgroup meta-analyses based on study design (case–control vs. cohort), sex, type of RR estimate (odds ratio, incidence rate ratio, standardized incidence ratio, and hazard ratio), and whether or not the studies adjusted for certain important potential confounders (obesity, smoking, or physical exercise).
RESULTS
Study characteristics
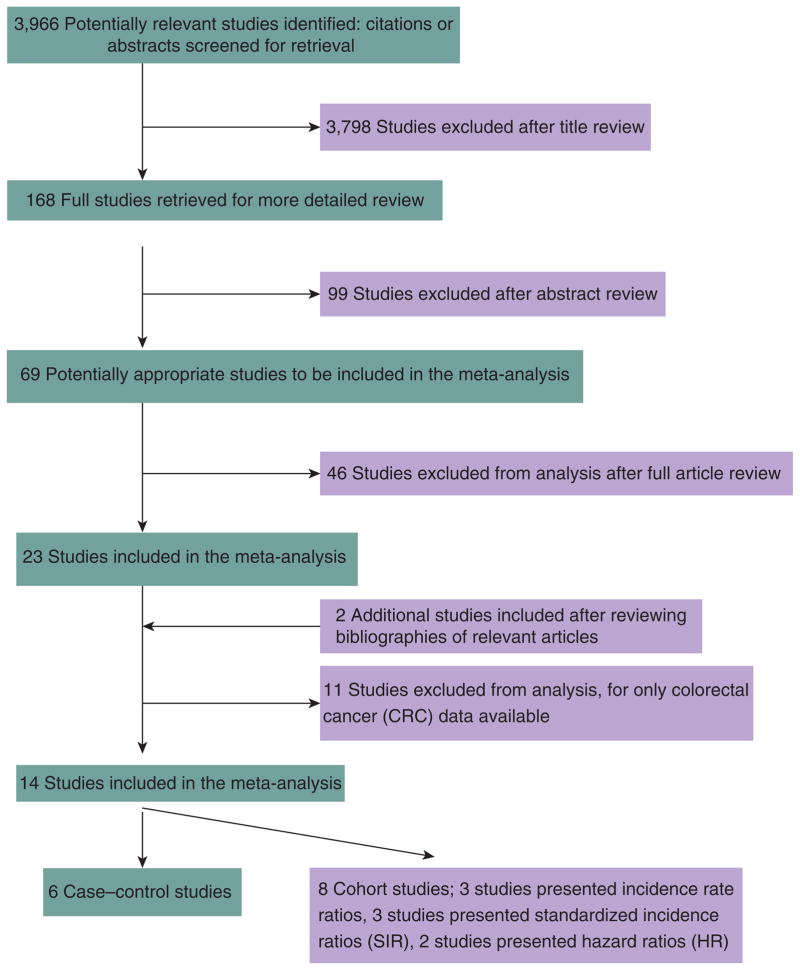
Detailed search steps are described in Figure 1. Briefly, from the initial literature search we identified and screened 3,966 abstracts. Sixty-nine articles were considered of interest and full text was retrieved for detailed evaluation. References cited by all 69 studies were reviewed and two additional studies were identified, for a total of 71 articles for full review. Fifty-seven of the seventy-one articles did not meet the study inclusion and were excluded. Fourteen independent studies met our predefined inclusion criteria. Of these 14 studies, 6 were case–control studies (41–46) and 8 were cohort studies (Table 1) (47–54). Three of the cohort studies calculated incidence rate ratios as the measure of RR (47–49). Of the remaining five cohort studies, three presented standardized incidence ratios (50–52) and two presented hazard ratios (53,54). In terms of the geographical settings of the studies, seven studies were conducted in the United States and Canada, five in Europe, and two in Japan. Excluded studies (26,55–64) that reported only RR for CRC are listed in Table 2. None of the cohort studies specified whether the circumstances and methods for diagnosing CC were the same for patients with DM and without DM. Only one study (by Hu et al. (49)) controlled for smoking, body mass index (BMI), and exercise, and also had follow-up >10 years, therefore no additional subgroup analyses were performed.
Figure 1.
Flowchart of meta-analysis.
Table 1.
Characteristics of studies of diabetes mellitus and colon and/or rectal cancer
| Author, year (ref. no.), country | No. of case patients by cancer subsites | No. of control subjects (source of controls) | Type of diabetes | RR (95% CI), sex, cancer site | Controlled variables |
|---|---|---|---|---|---|
| Case–control studies of diabetes and colon and/or rectal cancer incidence | |||||
| Rousseau et al., 2006 (41), Canada | 435 CC 234 RC |
509 (population controls matched by age) | Type 1 and 2 (self-reported) | 1.2 (0.7–1.8), m, CC 0.9 (0.5–1.6), m, RC |
Age, BMI, cigarette, calothene, marital status, family income, education |
| Vinikoor et al., 2009 (42), U.S.A | 606 CC | 971 (population controls matched) | Type 1 and 2 (self-reported) | 1.4 (0.93–2.12), m + w, CC | Age, BMI, family history of CRC, calcium, calorie, sex |
| Kuriki et al., 2007 (43), Japan | 382 CC 304 RC |
47,768 (cancer-free controls) | Type 1 and 2 (self-reported) | 1.32 (0.95–1.84), m, CC 0.94 (0.52–1.71), w, CC 1.29 (0.87–1.92), m, RC 1.47 (0.76–2.85), w, RC |
Age, BMI, cigarette, exercise, alcohol, family history of CRC, family history of cancer, fruit/vegetable, calothene |
| Yang et al., 2005 (44), USA | 6,862 CC 3,585 RC |
104,429 (population controls matched) | Type 2 (medical records) | 1.45 (1.25–1.70), m + w, CC 1.34 (1.08–1.68), m + w, RC |
Age |
| Le Marchand et al., 1997 (45), USA | 825 CC 350 RC |
1,192 (population controls matched by age, sex, and ethnicity) | Type 1 and 2 (self-reported) | 0.9 (0.4–1.8), m, PCC 1.9 (1.1–3.5), m, DCC 1.3 (0.6–2.7), w, PCC 3.0 (1.2–7.1), w, DCC 0.7 (0.3–1.6), m, RC 1.7 (0.7–4.4), w, RC |
Age, BMI, cigarette, exercise, family history of CRC, calcium, calorie, fiber, egg |
| La Vecchia et al., 1997 (46), Italy | 1,225 CC 728 RC |
4,154 (hospital patients without cancer and gastrointestinal disease) | Type 1 and 2 (self-reported) | 1.2 (0.8–1.6), m + w, CC 1.5 (1.1–2.2), m + w, RC |
Age, BMI, exercise, alcohol, family history of CRC, greasy food, calorie, fiber, education, area of residence sex |
| Author, year (ref. no.), country, (follow-up period) | Study population | RR (95% CI), sex, cancer site (number of cases) | Controlled variables | ||
| Cohort studies of DM and colon and/or rectal cancer incidence based on incidence rate ratios | |||||
| Limburg et al., 2005 (47), USA (1986–1999) | Exposed group: 1,900 women with self-reported DM (type 2) Comparison group: 33, 072 women without self-reported DM | 1.9 (1.3 to 2.6), w, PCC (402) 1.1 (0.6 to 1.8), w, DCC (259) 0.8 (0.4 to 1.6), w, RC (196) |
Age, BMI, vitamin, calcium, calorie | ||
| Larrson et al., 2005 (48), Sweden (1997–2004) | Exposed group: 3,847 men with self-reported DM (type 2) Comparison group: 41,703 men without self-reported DM | 1.53 (1.02–2.29), m, CC (190) 1.79 (1.18–2.73), m, RC (156) |
Age, BMI, cigarette, exercise, family history of CRC, fruit/vegetable, vitamin, NSAIDs, dairy food, meat, education | ||
| Hu et al., 1999 (49), USA (1976–1994) | Exposed group: 7,069 women with self-reported DM (Type 2) Comparison group: 111, 003 women without self-reported DM | 1.49 (1.09–2.06), w, CC (607) 1.11 (0.56–2.21), w, RC (176) |
Age, BMI, cigarette, exercise, alcohol, family history of CRC, vitamin, NSAIDs, meat, hormonal therapy, menopausal status | ||
| Cohort studies of diabetes and colon and/or rectal cancer based on standardized incidence ratio (50–52) and hazard ratio (53,54) | |||||
| Limburg et al., 2006 (50), USA (1971–1994) | Exposed group: 1975 Rochester residents with DM Comparison group: Rochester population | 1.96 (1.16–3.10), m, PCC (18) 1.17 (0.58–2.09), w, PCC (11) |
Age | ||
| Weiderpass et al., 1997 (51), Sweden (1965–1989) | Exposed group: 153,852 patients with discharge diagnosis of DM (type 1 and 2) Comparison group: Swedish population | 1.66 (1.42–1.93), m, C/AC (172) 1.48 (1.28–1.69), w, C/AC (210) 1.19 (0.87–1.59), m, TC (46) 1.31 (1.00–1.67), w, TC (63) 1.20 (0.71–1.90), m, DC (18) 1.38 (0.88–2.08), w, DC (23) 1.30 (1.09–1.54), m, SC (134) 1.30 (1.09–1.53), w, SC (140) 1.36 (1.21–1.52), m, RC (294) 1.1 (0.95–1.26), w, RC (198) |
Age | ||
| Wideroff et al., 1997 (52), Denmark (1977–1989) | 109,581 Individuals with discharge diagnosis of DM (type 1 and 2) Comparison group: Danish population |
1.3 (1.1–1.4), m, CC (413) 1.1 (1.0–1.2), w, CC (442) 1.1 (0.9–1.2), m, RC (235) 1.0 (0.9–1.2), w, RC (167) |
Age | ||
| Inoue et al., 2006 (53), Japan (1994–2003) | 97,771 General Japanese persons | 1.36 (1.00–1.85), m, CC (491) 0.83 (0.42–1.61), w, CC (303) 0.80 (0.47–1.36), m, RC (243) 1.65 (0.80–3.39), w, RC (153) |
Age, BMI, cigarette, exercise, alcohol, caffeine, fruit/vegetable, history of cerebrovascular disease/ischemic heart disease | ||
| Bowers et al., 2006 (54), Finland (1985–2002) | 28,983 Finish male smokers | 1.09 (0.66–1.80), m, CC (10) 1.23 (0.65–2.32), m, RC (96) |
Age, BMI, cigarette, HDL level | ||
AC, ascending colon; BMI, body mass index; C, cecum; CC, colon cancer; CI, confidence interval; CRC, colorectal cancer; DC, descending colon; DCC: distal colon cancer; DM, diabetes mellitus; HDL, high-density lipoprotein; m, men; NSAIDs, non-steroidal anti-inflammatory drugs; PCC, proximal colon cancer; RC, rectal cancer; RR, relative risk; SC, sigmoid colon; TC, transverse colon; w, women.
Table 2.
Characterstics of excluded studies that contain only RR of colorectal cancer
| Author, year (ref. no.), country | Study design | RR (95% CI), sex |
|---|---|---|
| Levi et al., 2002 (55), Switzerkand | Case–control study | 1.3 (0.63–2.68), m 3.56 (1.05–12.11), w |
| Kune et al., 2007 (56), Australia | Case–control study | 1.28 (0.67–2.47), m 0.75 (0.35–1.61), w |
| Seow et al., 2006 (57), Singapore | Cohort study based on incidence rate | 1.5 (1.2–2.1), m 1.4 (1.0–1.9), w |
| Jee et al., 2005 (58), Korea | Cohort study based on incidence rate | 1.11 (1.0–2.4), m 1.17 (0.98–1.4), w |
| Khaw et al., 2004 (59), UK | Cohort study based on incidence rate | 3.37 (1.17–9.72), m 1.71 (0.23–12.64), w |
| Nielsen and Vatten, 2001 (60), Norway | Cohort study based on incidence rate | 0.66 (0.35–1.24), m 1.55 (1.04–2.31), w |
| Schoen et al., 1999 (61), USA | Cohort study based on incidence rate | 1.6 (0.8–3.1), m 1.1 (0.5–2.6), w |
| Will et al., 1998 (26), USA | Cohort study based on incidence rate | 1.3 (1.03–1.65), m 1.16 (0.87–1.53), w |
| Steenland et al., 1995 (62), USA | Cohort study based on incidence rate | 1.43 (0.61–3.31), m 1.4 (0.64–3.1), w |
| Zendehdel et al., 2003 (63), Sweden | Cohort study based on standardized incidence ratio | 1.1 (0.6–1.7), m + w |
| Stürmer et al., 2006 (64), USA | Cohort study based on hazard ratio | 1.5 (1.1–2.0), m |
CI, confidence interval; m, men; RR, relative risk; w, women.
CRC incidence
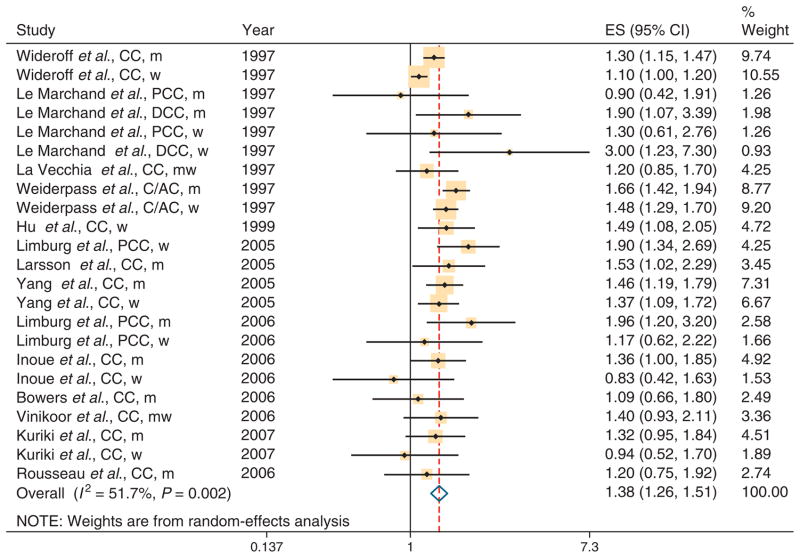
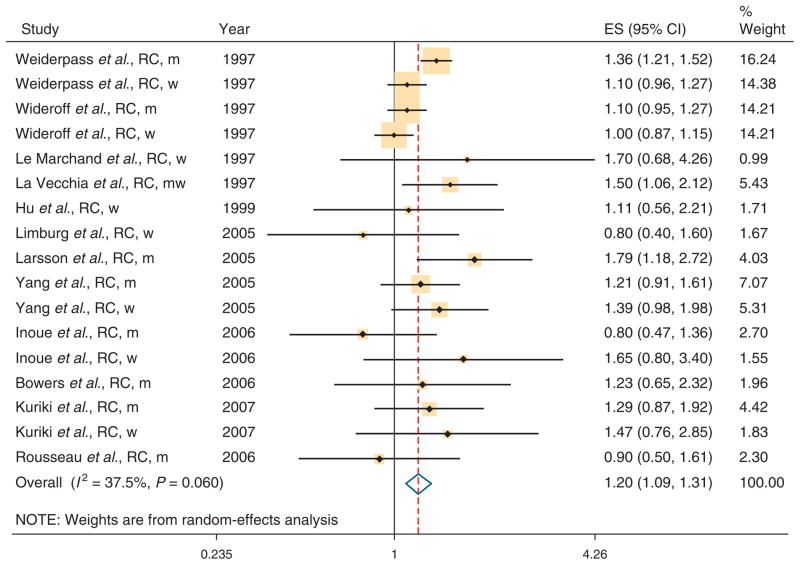
Individual study results and the overall summary results are shown separately for CC and RC in Figure 2 and Figure 3, respectively. The summary RR was 1.38 (95 % CI 1.26–1.51) for the 14 studies with data on CC and 1.20 (95 % CI 1.09–1.31) for the 12 studies with data on RC. There was evidence of heterogeneity for both CC (Q=45.57, P value for heterogeneity=0.002, I2=51.7%) and RC (Q=25.58, P value for heterogeneity=0.06, I2=37.5%). Bowers et al. (54) included only smokers and excluding this study had little impact on our results. Table 3 shows the results of subgroup meta-analyses by study design, sex, and whether studies adjusted for potential confounders (obesity, smoking, and physical exercise). The association between DM and CC incidence was similar in case–control studies (summary RR 1.36, 95% CI 1.22–1.52; n=11 studies) and cohort studies (summary RR 1.40, 95 % CI 1.23–1.58; n=12). The association between DM and RC incidence was higher in case–control studies (summary RR 1.31, 95 % CI 1.12–1.53; n=7) than in cohort studies (summary RR 1.16, 95 % CI 1.02–1.31; n=10). The results for CC were similar in men (summary RR 1.43, 95 % CI 1.30–1.57; n=11) and women (summary RR 1.35, 95% CI 1.14 – 1.53; n=10). For RC, there was a significant association between DM and cancer risk for men (summary RR 1.22, 95 % CI 1.07 – 1.40; n=8), but not for women (summary RR 1.09, 95% CI=0.99–1.19; n=8 studies).
Figure 2.
Association between diabetes and colon cancer (CC) incidence in six case–control and eight cohort studies. AC, ascending colon; C, cecum; CI, confidence interval; DCC, distal colon cancer; ES, effect size; m, men; PCC, proximal colon cancer; w, women.
Figure 3.
Association between diabetes and rectal cancer (RC) incidence in six case–control and eight cohort studies. CI, confidence interval; m, men; w, women.
Table 3.
Summary RR estimates and 95% CIs for case–control and cohort studies of the association of diabetes with colon cancer and rectal cancer incidence by study design, sex, and confounders (obesity, smoking, and physical exercise)
| Subgroup | No. of studies | Summary RR (95% CI), fixed-effect model | Q | P-heterogeneity | I 2-statistic % | Summary RR (95% CI), random-effect model |
|---|---|---|---|---|---|---|
| Colon cancer | ||||||
| All studies | 23 | 1.32 (1.26 – 1.39) | 45.57 | < 0.01 | 51.7 | 1.38 (1.26 – 1.51) |
| Study design | ||||||
| Case–control | 11 | 1.36 (1.22 – 1.52) | 8.26 | 0.60 | 0.0 | 1.36 (1.22 – 1.52) |
| Cohort | 12 | 1.31 (1.24 – 1.38) | 36.90 | < 0.01 | 70.2 | 1.40 (1.23 – 1.58) |
| Sex | ||||||
| Men | 11 | 1.42 (1.32 – 1.53) | 12.10 | 0.28 | 17.3 | 1.43 (1.30 – 1.57) |
| Women | 10 | 1.25 (1.17 – 1.34) | 26.71 | < 0.01 | 66.3 | 1.35 (1.14 – 1.53) |
| BMI/smoking adjusted | ||||||
| Yes | 12 | 1.34 (1.17 – 1.52) | 10.67 | 0.47 | 0.0 | 1.34 (1.17 – 1.52) |
| No | 11 | 1.32 (1.25 – 1.39) | 34.88 | < 0.01 | 71.3 | 1.41 (1.25 – 1.58) |
| BMI/smoking/physical exercise adjusted | ||||||
| Yes | 10 | 1.37 (1.19 – 1.58) | 9.71 | 0.37 | 7.3 | 1.37 (1.18 – 1.59) |
| No | 13 | 1.31 (1.25 – 1.39) | 35.57 | < 0.01 | 66.3 | 1.38 (1.24 – 1.54) |
| Rectal cancer | ||||||
| All studies | 17 | 1.18 (1.12 – 1.26) | 25.58 | 0.06 | 37.5 | 1.20 (1.09 – 1.31) |
| Study design | ||||||
| Case–control | 7 | 1.31 (1.12 – 1.53) | 3.02 | 0.81 | 0.0 | 1.31 (1.12 – 1.53) |
| Cohort | 10 | 1.16 (1.09 – 1.24) | 20.67 | 0.01 | 56.5 | 1.16 (1.02 – 1.31) |
| Sex | ||||||
| Men | 8 | 1.25 (1.15 – 1.35) | 11.96 | 0.10 | 41.5 | 1.22 (1.07 – 1.40) |
| Women | 8 | 1.09 (0.99 – 1.19) | 6.95 | 0.43 | 0.0 | 1.09 (0.99 – 1.19) |
| BMI/smoking adjusted | ||||||
| Yes | 9 | 1.28 (1.06 – 1.54) | 8.06 | 0.95 | 0.8 | 1.28 (1.06 – 1.54) |
| No | 8 | 1.17 (1.10 – 1.25) | 16.80 | 0.19 | 58.3 | 1.18 (1.05 – 1.31) |
| BMI/smoking/physical exercise adjusted | ||||||
| Yes | 7 | 1.34 (1.09 – 1.65) | 6.43 | 0.38 | 6.7 | 1.34 (1.08 – 1.67) |
| No | 10 | 1.17 (1.10 – 1.25) | 17.61 | 0.04 | 48.9 | 1.17 (1.05 – 1.29) |
BMI, body mass index; CI, confidence interval; RR, relative risk.
Subgroup meta-analysis by methodological quality of the studies as ranked by the Newcastle-Ottawa Scale scale revealed a similar significant positive association in both the high-quality studies (summary RR=1.44 95% CI 1.24–1.66 n=9 studies for CC, summary RR=1.30 95% CI 1.10–1.54 n=6 studies for RC) and the low-quality studies (summary RR=1.35 95% CI 1.21–1.51; n=14 studies for CC, summary RR=1.17 95% CI 1.04–1.31; n=11 studies for RC).
Smoking and obesity are potentially the most important known confounders of the positive association between DM and CRC risk. In the meta-analysis that was restricted to the seven publications that controlled for these variables (41,43,45,48,49,53,54), the positive association of diabetes with CC (summary RR 1.34, 95% CI 1.17–1.52; n=12 studies) and RC (summary RR 1.28, 95% CI=1.06–1.54; n=9 studies) remained. In the meta-analysis that was restricted to the five publications that controlled for smoking, obesity, and physical activity (43,45,48,49,53), the positive association of DM with CC (summary RR 1.37, 95% CI 1.18–1.59; n=10 studies) and RC (summary RR 1.34 95% CI 1.08–1.67; n=7 studies) also remained.
Publication bias
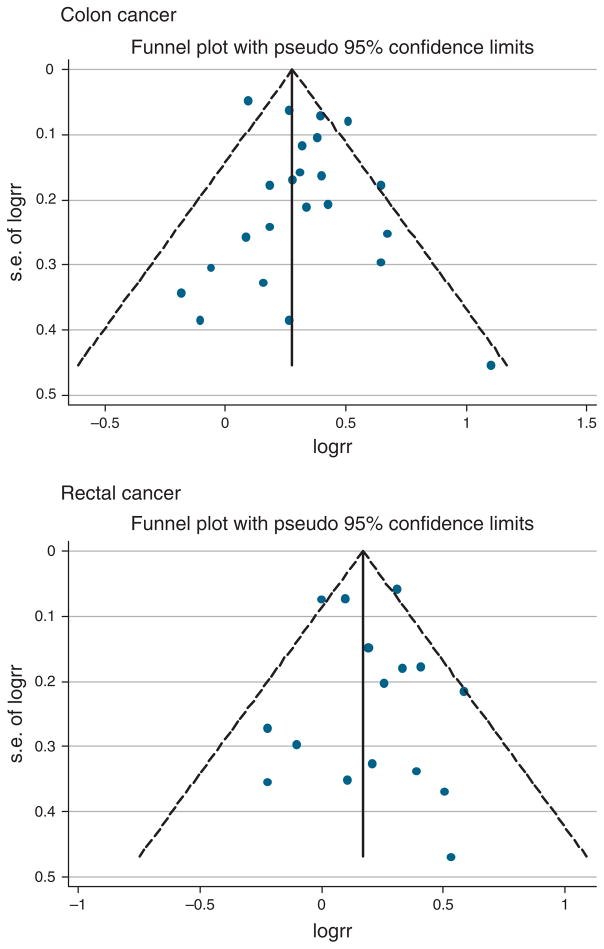
Visual inspection of the Begg funnel plot for both CC and RC did not show the asymmetry typically associated with publication bias (Figure 4). Evidence of publication bias was also not seen with the Egger or Begg tests (Egger P=0.27 and 0.64 for CC and RC, respectively).
Figure 4.
Funnel plot with pseudo 95% confidence limits.
DISCUSSION
This meta-analysis of observational studies indicates that DM is associated with increased risks of CC and RC. To our knowledge, this meta-analysis is the first to show a statistically significant association between DM and risks of CC and RC separately after controlling for obesity, smoking, and physical exercise, which are important potential confounders. Many of the odd ratios we identified are close to 1.0; however, the very low P values show that these are unlikely due to chance (P<0.0001 for CC and P<0.0001 for RC).
The association of DM and cancer risk was stronger for CC than for RC (summary RR 1.38, 95 % CI 1.26 – 1.51 vs. summary RR 1.20, 95 % CI 1.09–1.31, respectively). This difference may be because the proximal colon, distal colon, and rectum have different embryological origins (65). Previous studies have found subsite variations in susceptibility to carcinogens and neoplastic transformation (66,67). Molecular biological studies also indicate that tumor suppressor genes and point mutations and genetic instability differ by subsite of the colorectum (68–71).
Type 2 DM and CC and RC share similar risk factors, including smoking and obesity (12,32–34). Thus, the increased risk of CC and RC associated with a history of DM could be the result of confounding by these risk factors. A meta-analysis by Botteri et al. (34) on smoking and CRC showed that the pooled risk estimate for ever vs. never smokers was 1.25 (95 % CI 1.14 – 1.37), and a meta-analysis by Moghaddam et al. (12) showed that the estimated RR of CRC was 1.19 (95 % CI 1.11–1.29) comparing obese (BMI>30kg/m2) with normal weight people (BMI<25kg/m2).
The positive association of DM with CC and RC risk did not decrease when the meta-analysis was limited to studies that controlled for smoking and BMI (summary RR for colon 1.34, 95 % CI 1.17–1.52, and summary RR for rectum 1.28, 95% CI 1.06–1.54). This suggests that the confounding effect of obesity and smoking is relatively weak, and that DM appears to be an independent risk factor for CC and RC.
Statistical heterogeneity was seen in several of our analyses, and much of this is likely due to differences in study design, study population, definition of diabetes, and statistical methods (e.g., type of RR estimate). Statistical heterogeneity was lower in our analyses of studies which adjusted for BMI, smoking, and physical activity than in the analyses of studies that did not adjust for these potential confounders. This suggests that the lack of these adjustments in some studies accounted for much of the heterogeneity observed.
Data from several sources suggest that the association between DM and the risk of CRC is biologically plausible. Type 2 DM is associated with hyperinsulinemia. Mechanistically, insulin stimulates cell proliferation through two pathways. One pathway involves direct binding of insulin to insulin or insulin-like growth factor-1 (IGF-1) receptors, and the other pathway is via inhibition of IGF-binding proteins and the resultant increase in IGF-1 availability to the IGF receptor (72). The IGF system is a potent growth regulator closely linked with carcinogenesis (73). Although not consistent across all studies, several prospective observational studies have shown an association between elevated IGF-1 levels and the risks of CRC or advanced adenoma (74–78). These data support the notion that IGF-1 has a role in the biological pathway of colorectal neoplasia, beginning at the adenoma stage.
Several limitations of this study must be considered. All of the studies that assess the association between DM and CRC are observational studies, as an intervention study addressing this question would not be ethical or feasible. Rating the scales for strength of evidence rank the quality of evidence derived from observational studies as low, especially as compared with randomized trials (79). However, there are several indications that despite the inherent limitations of observational studies, our finding that DM is associated with an increased risk of CRC may be a real association. These include comparable results for different study designs (case–control and cohort), a lack of heterogeneity among studies that adjusted for important confounders, and consistency of the results within the population-based studies, which increases the ability to generalize study results to similar large populations. These results, however, should still be viewed with caution, as the magnitude of effect found was modest; such moderate effects may be due to unmeasured confounding that persists across studies.
Most of the studies reviewed did not distinguish between type 1 and type 2 DM. Type 1 accounts for 5–10% of all cases of diabetes and type 2 accounts for 90–95 % of all DM cases (80). DM duration and insulin requirement are different in type 1 and type 2 DM, and this may affect tissue exposure to insulin in different ways. If hyperinsulinemia has a role in promoting cancer initiation and progression, it might be expected that duration of DM would affect cancer RRs. Indeed, six studies used in this meta-analysis showed that CRC risk was higher among diabetics with longer duration of disease (42,45,47,49,51,52).
Because almost one-third of diabetics is undiagnosed (81), some degree of non-differential misclassification of exposure to diabetes is likely to have occurred in the studies, included in this meta-analysis. Although the American Diabetes Association and the World Health Organization now share identical diagnostic criteria for type 2 DM, diagnostic criteria have changed over time (82). The prevalence of DM can change as a function of diagnostic criteria used (83). The studies used in this meta-analysis did not specify which criterion for DM was used, and in most studies the DM was self-reported. Such non-differential misclassification of exposure would tend to underestimate the true relationship between DM and CRC.
Although some of the studies included in this meta-analysis controlled for obesity, smoking, or lack of physical activity, we were unable to control for other important potential confounders of the relationship between DM and CRC, including low intake of fruits and vegetables and high alcohol intake (84,85). These confounders are more prevalent in individuals of lower socioeconomic status (86). The lack of adjustment for socioeconomic status, diet, and alcohol consumption could contribute to a non-causal association between DM and CRC. However, alcohol intake and fruit and vegetable intake are likely correlated with obesity, smoking, and exercise. Because of these relationships, studies that adjusted for obesity, smoking, and exercise probably also, at least partially, adjusted for these other related factors. In addition, our finding that adjustment for obesity, smoking, and exercise had little impact on the relationship between DM and CRC suggests that further adjustment for alcohol and fruit and vegetable intake is unlikely to have a major impact on this relationship.
There are ethnic differences in the incidence of CRC in the United States. The age-adjusted incidence rates for CRC (2000–2006) are highest for blacks (both males and females), followed by non-Hispanic whites, Asian/Pacific Islanders, Hispanics, and American Indians/Alaska natives (SEER database) (1). This may distort the true relationship between DM and CRC, if these differences are not taken into account.
Another potential bias is detection bias. A person with DM is likely to have many more contacts with the health-care system than a person without DM. This might have provided more opportunities for them to have a sigmoidoscopy, colonoscopy, or fecal blood test ordered. Although the extent of this bias is unknown, it is possible that modest increases in cancer incidence could be explained solely by more intense screening in the DM group.
In this meta-analysis, no evidence of publication bias was seen in the funnel plot or in Begg or Egger tests (Egger P=0.27 and 0.64 for CC and RC, respectively). However, it should be noted that several factors other than publication bias can affect the outcome of these statistical tests, and their validity and interpretation has been debated (87).
Finally, it is widely accepted that the majority of CRCs develop slowly through polypoid growth, and endoscopists have traditionally focused on finding and removing polypoid adenomas–growths that protrude from the mucosa – during screening colonoscopy. Reports from Japan in the 1980s and 1990s suggested that nonpolypoid (flat and depressed) colorectal neoplasms were common and ominous (88,89). The likelihood that nonpolypoid (flat and depressed) colorectal neoplasms harbor serious pathology (in situ or submucosal carcinoma) was more than five times higher than the rate polypoid lesions after adjusting for polyp size (90). It is only recently that the existence of nonpolypoid (flat and depressed) colorectal neoplasm has been shown to contribute to the development of CRC (91). As a result, many endoscopists may not be aware of the subtle features of these lesions. Furthermore, one study indicated that diabetic patients (irrespective of insulin use, diabetic control, or diabetic neuropathy) have a significantly poorer response to a colonoscopy bowel cleansing preparations than do nondiabetic patients (92). A recent study also suggests that optimal bowel preparation was significantly poorer in DM patients with autonomous neuropathy than in DM patients without autonomous neuropathy and controls (93). The diagnostic accuracy of the colonoscopy depends on the quality of the colon cleansing. If patients with DM are less likely to have precancerous polyps removed on colonoscopy, this may increase their risk of subsequent cancer over long periods of time. Conversely, if poor preparations lead to decreased cancer detection on short-term studies, this could underestimate the true relationship between DM and CRC.
Our results have important clinical and public health implications. The results from this meta-analysis suggest that DM is an independent risk factor of CC and RC. Although observational studies cannot exclude confounding as an explanation, especially when effect sizes are modest to moderate, the consistency of the evidence suggests a potential role for hyperinsulinemia or factors related to insulin resistance in colorectal carcinogenesis. Other data suggest that chronic insulin therapy is associated with increased colorectal adenoma risk among type 2 DM patients. These results may suggest a need for more intensive CRC screening program in patients with type 2 DM, especially those who receive chronic insulin therapy (94).
In the United States, about 23.6 million people or 7.8% of adults have DM (95), and by 2050, the number of people in the United States with diagnosed DM is estimated to grow to 48.3 million (80). Furthermore, as a recent study indicated, persons with DM and CRC may be at increased risk for CRC recurrence, non-response to chemo and radiotherapy treatment, and treatment-related complications (96). CRC deaths may be reduced through CRC screening programs. We encourage the American College of Gastroenterology to review CRC screening guidelines to determine whether diabetic status should be included as a risk factor that warrants changes in screening frequency or intensity. We also recommend that future studies on the association between DM and CRC focus on plausible causal mechanisms or mediating factors, such as obesity, smoking, physical activity, diagnostic bias, duration of diabetes, and antidiabetic therapy.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
Diabetes mellitus (DM) has been associated with an increased risk of colorectal cancer (CRC).
The American College of Gastroenterology (ACG) Guidelines for Colorectal Cancer Screening 2008 recommend that clinicians be aware of an increased CRC risk in patients with smoking and obesity, but do not highlight the increase in CRC risk in patients with DM.
Obesity and smoking are associated with the incidence of both type 2 DM and CRC; thus, they also could be important positive confounders of the association between DM and CRC.
WHAT IS NEW HERE
We assess the effect of certain potentially important confounding variables, including obesity, smoking, and physical exercise.
Our data suggest that diabetes mellitus is an independent risk factor for colon and rectal cancer.
Acknowledgments
Financial support: None.
Footnotes
CONFLICT OF INTEREST
Guarantor of the article: Hiroki Yuhara, MD, MPH.
Specific author contributions: H.Y. and C.S. participated in study design and drafted the manuscript. C.S. helped to establish protocols for study selection, and methods for the statistical analysis of data and assisted in quality control procedures and publication writing. S.E.C. assisted in publication writing. H.Y. and S.E.C. performed the statistical analysis. S.E.C., P.A.B., Y.T., and D.A.C. critically revised the manuscript. All authors read and approved the final manuscript.
Potential competing interests: None.
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
References
- 1.U.S National Cancer Institute. SEER Stat Fact Sheets. http://seer.cancer.gov/csr/1975_2006/results_single/sect_01_table.01.pdf.
- 2.American Cancer Society. Cancer Facts & Figures. 2009. [Google Scholar]
- 3.World Health Organization. International Agency for Research on Cancer Higher blood vitamin D levels are associated with significantly decreased colon cancer risk in European populations. Jan, 2010. Press release No. 19822. [Google Scholar]
- 4.Lin OS. Acquired risk factors for colorectal cancer. Methods Mol Biol. 2009;472:361–72. doi: 10.1007/978-1-60327-492-0_16. [DOI] [PubMed] [Google Scholar]
- 5.Park Y, Subar AF, Kipnis V, et al. Fruit and vegetable intakes and risk of colorectal cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2007;166:170–80. doi: 10.1093/aje/kwm067. [DOI] [PubMed] [Google Scholar]
- 6.Huxley RR, Ansary-Moghaddam A, Clifton P, et al. The impact of dietary and lifestyle risk factors on risk of colorectal cancer: a quantitative overview of the epidemiological evidence. Int J Cancer. 2009;125:171–80. doi: 10.1002/ijc.24343. [DOI] [PubMed] [Google Scholar]
- 7.Lin J, Zhang SM, Cook NR, et al. Dietary intakes of fruit, vegetables, and fiber, and risk of colorectal cancer in a prospective cohort of women (United States) Cancer Causes Control. 2005;16:225–33. doi: 10.1007/s10552-004-4025-1. [DOI] [PubMed] [Google Scholar]
- 8.Cho E, Smith-Warner SA, Ritz J, et al. Alcohol intake and colorectal cancer: a pooled analysis of 8 cohortstudies. Ann Intern Med. 2004;140:603–13. doi: 10.7326/0003-4819-140-8-200404200-00007. [DOI] [PubMed] [Google Scholar]
- 9.Giovannucci E. Meta-analysis of coffee consumption and risk of colorectal cancer. Am J Epidemiol. 1998;147:1043–52. doi: 10.1093/oxfordjournals.aje.a009398. [DOI] [PubMed] [Google Scholar]
- 10.Howard RA, Freedman DM, Park Y, et al. Physical activity, sedentary behavior, and the risk of colon and rectal cancer in the NIH-AARP Diet and Health Study. Cancer Causes Control. 2008;19:939–53. doi: 10.1007/s10552-008-9159-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer. 2009;124:2406–15. doi: 10.1002/ijc.24191. [DOI] [PubMed] [Google Scholar]
- 12.Moghaddam AA, Woodward M, Huxley R. Obesity and risk of colorectal cancer: a meta-analysis of 31 studies with 70,000 events. Cancer Epidemiol Biomarkers Prev. 2007;16:2533–47. doi: 10.1158/1055-9965.EPI-07-0708. [DOI] [PubMed] [Google Scholar]
- 13.Sandler RS, Sandler DP. Radiation-induced cancers of the colon and rectum: assessing the risk. Gastroenterology. 1983;84:51–7. [PubMed] [Google Scholar]
- 14.Reid FD, Mercer PM, harrison M, et al. Cholecystectomy as a risk factor for colorectal cancer: a meta-analysis. Scand J Gastroenterol. 1996;31:160–9. doi: 10.3109/00365529609031981. [DOI] [PubMed] [Google Scholar]
- 15.Bollschweiler E, Feussner H, Huber F, et al. Is cholecystectomy a risk factor for colorectal cancer? A meta-analysis Langenbecks Arch Chir. 1993;378:304–12. doi: 10.1007/BF00183969. [DOI] [PubMed] [Google Scholar]
- 16.Giannini O, Friedli A, Schärli AF. Sigmoid adenocarcinoma complicating ureterosigmoidostomy. Pediatr Surg Int. 1998;14:124–6. doi: 10.1007/s003830050458. [DOI] [PubMed] [Google Scholar]
- 17.Sontag SJ, Schnell TG, Chejfec G, et al. Barrett’s oesophagus and colonic tumours. Lancet. 1985;1:946–9. doi: 10.1016/s0140-6736(85)91725-8. [DOI] [PubMed] [Google Scholar]
- 18.Patel P, Hanson DL, Sullivan PS, et al. Adult and Adolescent Spectrum of Disease Project and HIV Outpatient Study Investigators. Incidence of types of cancer among HIV-infected persons compared with the general population in the United States, 1992–2003. Ann Intern Med. 2008;148:728–36. doi: 10.7326/0003-4819-148-10-200805200-00005. [DOI] [PubMed] [Google Scholar]
- 19.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: a meta-analysis. J Natl Cancer Inst. 2005;97:1679–87. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 20.Delhougne B, Deneux C, Abs R, et al. The prevalence of colonic polyps in acromegaly: A colonoscopic and pathological study in 103 patients. J Clin Endocrinol Metabol. 1995;80:3223–3226. doi: 10.1210/jcem.80.11.7593429. [DOI] [PubMed] [Google Scholar]
- 21.Xie J, Itzkowitz SH. Cancer in inflammatory bowel disease. World J Gastroenterol. 2008;21(14):378–89. doi: 10.3748/wjg.14.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maynard GD. A statistical study in cancer death-rates. Biometrika. 1910;7:276–304. [Google Scholar]
- 23.Vigneri P, Frasca F, Sciacca L, et al. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. doi: 10.1677/ERC-09-0087. [DOI] [PubMed] [Google Scholar]
- 24.Williams JC, Walsh DA, Jackson JF. Colon carcinoma and diabetes mellitus. Cancer. 1984;54:3070–1. doi: 10.1002/1097-0142(19841215)54:12<3070::aid-cncr2820541243>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E. Insulin and colon cancer. Cancer Causes Control. 1995;6:164–79. doi: 10.1007/BF00052777. [DOI] [PubMed] [Google Scholar]
- 26.Will JC, Galuska DA, Vinicor F, et al. Colorectal cancer: another complication of diabetes mellitus? Am J Epidemiol. 1998;147:816–25. doi: 10.1093/oxfordjournals.aje.a009534. [DOI] [PubMed] [Google Scholar]
- 27.Bernstein H, Bernstein C, Payne CM, et al. Bile acids as carcinogens in human gastrointestinal cancers. Mutat Res. 2005;589:47–65. doi: 10.1016/j.mrrev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Yang YX, Hennessy S, Lewis JD. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients. Gastroenterology. 2004;127:1044–50. doi: 10.1053/j.gastro.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88:261–6. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 30.Konishi K, Fujii T, Boku N, et al. Clinicopathological differences between colonic and rectal carcinomas: are they based on the same mechanism of carcinogenesis? Gut. 1999;45:818–21. doi: 10.1136/gut.45.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening [corrected] Am J Gastroenterol. 2009;104:739–750. doi: 10.1038/ajg.2009.104. [DOI] [PubMed] [Google Scholar]
- 32.Keller U. From obesity to diabetes. Int J Vitam Nutr Res. 2006;76:172–7. doi: 10.1024/0300-9831.76.4.172. [DOI] [PubMed] [Google Scholar]
- 33.Willi C, Bodenmann P, Ghali WA, et al. Active smoking and the risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2007;298:2654–64. doi: 10.1001/jama.298.22.2654. [DOI] [PubMed] [Google Scholar]
- 34.Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300:2765–78. doi: 10.1001/jama.2008.839. [DOI] [PubMed] [Google Scholar]
- 35.The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 36.Greenland S. Meta-analysis. In: Rothman K, Greenland S, editors. Modern Epidemiology. 2. Philadelphia: Lippincott Raven; 1998. pp. 643–73. [Google Scholar]
- 37.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–101. [PubMed] [Google Scholar]
- 41.Rousseau MC, Parent ME, Pollak MN, et al. Diabetes mellitus and cancer risk in a population-based case-control study among men from Montreal, Canada. Int J Cancer. 2006;118:2105–9. doi: 10.1002/ijc.21600. [DOI] [PubMed] [Google Scholar]
- 42.Vinikoor LC, Long MD, Keku TO, et al. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:1239–42. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuriki K, Hirose K, Tajima K. Diabetes and cancer risk for all and specific sites among Japanese men and women. Eur J Cancer Prev. 2007;16:83–9. doi: 10.1097/01.cej.0000228404.37858.40. [DOI] [PubMed] [Google Scholar]
- 44.Yang YX, Hennessy S, Lewis JD. Type 2 diabetes mellitus and the risk of colorectal cancer. Clin Gastroenterol Hepatol. 2005;3:587–94. doi: 10.1016/s1542-3565(05)00152-7. [DOI] [PubMed] [Google Scholar]
- 45.Le Marchand L, Wilkens LR, Kolonel LN, et al. Associations of sedentary lifestyle, obesity, smoking, alcohol use, and diabetes with the risk of colorectal cancer. Cancer Res. 1997;57:4787–94. [PubMed] [Google Scholar]
- 46.La Vecchia C, Negri E, Decarli A, et al. Diabetes mellitus and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 1997;6:1007–10. [PubMed] [Google Scholar]
- 47.Limburg PJ, Anderson KE, Johnson TW, et al. Diabetes mellitus and subsite-specific colorectal cancer risks in the Iowa Women’s Health Study. Cancer Epidemiol Biomarkers Prev. 2005;14:133–7. [PubMed] [Google Scholar]
- 48.Larsson SC, Giovannucci E, Wolk A. Diabetes and colorectal cancer incidence in the cohort of Swedish men. Diabetes Care. 2005;28:1805–7. doi: 10.2337/diacare.28.7.1805. [DOI] [PubMed] [Google Scholar]
- 49.Hu FB, Manson JE, Liu S, et al. Prospective study of adult onset diabetes mellitus (type 2) and risk of colorectal cancer in women. J Natl Cancer Inst. 1999;91:542–7. doi: 10.1093/jnci/91.6.542. [DOI] [PubMed] [Google Scholar]
- 50.Limburg PJ, Vierkant RA, Fredericksen ZS, et al. Clinically confirmed type 2 diabetes mellitus and colorectal cancer risk: a population-based, retrospective cohort study. Am J Gastroenterol. 2006;101:1872–9. doi: 10.1111/j.1572-0241.2006.00725.x. [DOI] [PubMed] [Google Scholar]
- 51.Weiderpass E, Gri G, Nyrén O, et al. Diabetes mellitus and risk of large bowel cancer. J Natl Cancer Inst. 1997;89:660–1. doi: 10.1093/jnci/89.9.660. [DOI] [PubMed] [Google Scholar]
- 52.Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst. 1997;89:1360–5. doi: 10.1093/jnci/89.18.1360. [DOI] [PubMed] [Google Scholar]
- 53.Inoue M, Iwasaki M, Otani T, et al. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch Intern Med. 2006;166:1871–7. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 54.Bowers K, Albanes D, Limburg P, et al. A prospective study of anthropometric and clinical measurements associated with insulin resistance syndrome and colorectal cancer in male smokers. Am J Epidemiol. 2006;164:652–64. doi: 10.1093/aje/kwj253. [DOI] [PubMed] [Google Scholar]
- 55.Levi F, Pasche C, Lucchini F, et al. Diabetes mellitus, family history, and colorectal cancer. J Epidemiol Community Health. 2002;56:479–80. doi: 10.1136/jech.56.6.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations and medications: case control results from the Melbourne Colorectal Cancer Study. Int J Epidemiol. 2007;36:951–7. doi: 10.1093/ije/dym193. [DOI] [PubMed] [Google Scholar]
- 57.Seow A, Yuan JM, Koh WP, et al. Diabetes mellitus and risk of colorectal cancer in the Singapore Chinese Health Study. J Natl Cancer Inst. 2006;98:135–8. doi: 10.1093/jnci/djj015. [DOI] [PubMed] [Google Scholar]
- 58.Jee SH, Ohrr H, Sull JW, et al. Fasting serum glucose level and cancer risk in Korean men and women. JAMA. 2005;293:194–202. doi: 10.1001/jama.293.2.194. [DOI] [PubMed] [Google Scholar]
- 59.Khaw KT, Wareham N, Bingham S, et al. Preliminary communication: glycated hemoglobin, diabetes, and incident colorectal cancer in men and women: a prospective analysis from the European prospective investigation into cancer-Norfolk study. Cancer Epidemiol Biomarkers Prev. 2004;13:915–9. [PubMed] [Google Scholar]
- 60.Nilsen TI, Vatten LJ. Prospective study of colorectal cancer risk and physical activity, diabetes, blood glucose and BMI: exploring the hyperinsulinaemia hypothesis. Br J Cancer. 2001;84:417–22. doi: 10.1054/bjoc.2000.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schoen RE, Tangen CM, Kuller LH, et al. Increased blood glucose and insulin, body size, and incident colorectal cancer. J Natl Cancer Inst. 1999;91:1147–54. doi: 10.1093/jnci/91.13.1147. [DOI] [PubMed] [Google Scholar]
- 62.Steenland K, Nowlin S, Palu S. Cancer incidence in the National Health and Nutrition Survey I. Follow-up data: diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol Biomarkers Prev. 1995;4:807–11. [PubMed] [Google Scholar]
- 63.Zendehdel K, Nyrén O, Ostenson CG, et al. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst. 2003;95:1797–800. doi: 10.1093/jnci/djg105. [DOI] [PubMed] [Google Scholar]
- 64.Stürmer T, Buring JE, Lee IM, et al. Metabolic abnormalities and risk for colorectal cancer in the physicians’ health study. Cancer Epidemiol Biomarkers Prev. 2006;15:2391–7. doi: 10.1158/1055-9965.EPI-06-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779–88. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 66.Yang H, Stuart GR, Glickman BW, et al. Modulation of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine-induced mutation in the cecum and colon of big blue rats by conjugated linoleic acid and 1,2-dithiole-3-thione. Nutr Cancer. 2001;39:259–66. doi: 10.1207/S15327914nc392_16. [DOI] [PubMed] [Google Scholar]
- 67.Stuart GR, de Boer JG, Haesevoets R, et al. Mutations induced by 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in cecum and proximal and distal colon of lac I transgenic rats. Mutagenesis. 2001;16:431–7. doi: 10.1093/mutage/16.5.431. [DOI] [PubMed] [Google Scholar]
- 68.Zhou CZ, Peng ZH, Zhang F, et al. Loss of heterozygosity on long arm of chromosome 22 in sporadic colorectal carcinoma. World J Gastroenterol. 2002;8:668–73. doi: 10.3748/wjg.v8.i4.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wiencke JK, Zheng S, Lafuente A, et al. Aberrant methylation of p16INK4a in anatomic and gender-specific subtypes of sporadic colorectal cancer. Cancer Epidemiol Biomarkers Prev. 1999;8:501–6. [PubMed] [Google Scholar]
- 70.Gervaz P, Bouzourene H, Cerottini JP, et al. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis Colon Rectum. 2001;44:364–72. doi: 10.1007/BF02234734. [DOI] [PubMed] [Google Scholar]
- 71.Lindblom A. Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol. 2001;13:63–9. doi: 10.1097/00001622-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 72.Sandhu MS, Dunger DB, Giovannucci EL. Insulin, insulin-like growth factor-I (IGF-I), IGF binding proteins, their biologic interactions, and colorectal cancer. J Natl Cancer Inst. 2002;94:972–80. doi: 10.1093/jnci/94.13.972. [DOI] [PubMed] [Google Scholar]
- 73.LeRoith D, Baserga R, Helman L, et al. Insulin-like growth factors and cancer. Ann Intern Med. 1995;122:54–9. doi: 10.7326/0003-4819-122-1-199501010-00009. [DOI] [PubMed] [Google Scholar]
- 74.Schoen RE, Weissfeld JL, Kuller LH, et al. Insulin-like growth factor-I and insulin are associated with the presence and advancement of adenomatous polyps. Gastroenterology. 2005;129:4644–75. doi: 10.1016/j.gastro.2005.05.051. [DOI] [PubMed] [Google Scholar]
- 75.Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst. 2000;92:1592–600. doi: 10.1093/jnci/92.19.1592. [DOI] [PubMed] [Google Scholar]
- 76.Giovannucci E. Insulin, insulin-like growth factors and colon cancer: a review of the evidence. J Nutr. 2001;131:3109S–20S. doi: 10.1093/jn/131.11.3109S. [DOI] [PubMed] [Google Scholar]
- 77.Palmqvist R, Hallmans G, Rinaldi S, et al. Plasma insulin-like growth factor 1, insulin-like growth factor binding protein 3, and risk of colorectal cancer: a prospective study in northern Sweden. Gut. 2002;50:642–6. doi: 10.1136/gut.50.5.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 79.Guyatt GH, Oxman AD, Kunz R, et al. GRADE Working Group. What is “quality of evidence” and why is it important to clinicians? Br Med J. 2008;336:995–8. doi: 10.1136/bmj.39490.551019.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2005;28 (suppl 1):S37–42. doi: 10.2337/diacare.28.suppl_1.s37. [DOI] [PubMed] [Google Scholar]
- 82.Botas P, Delgado E, Castano G, et al. Comparison of the diagnostic criteria for diabetes mellitus, WHO-1985, ADA-1997 and WHO-1999 in the adult population of Asturias (Spain) Diabet Med. 2003;20:904–8. doi: 10.1046/j.1464-5491.2003.01006.x. [DOI] [PubMed] [Google Scholar]
- 83.van Dam RM, Rimm EB, Willett WC, et al. Dietary patterns and risk for type 2 diabetes mellitus in US men. Ann Intern Med. 2002;136:201–9. doi: 10.7326/0003-4819-136-3-200202050-00008. [DOI] [PubMed] [Google Scholar]
- 84.Chiolero A, Wietlisbach V, Ruffieux C, et al. Clustering of risk behaviors with cigarette consumption: a population-based survey. Prev Med. 2006;42:348–53. doi: 10.1016/j.ypmed.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 85.Healton CG, Vallone D, McCausland KL, et al. Smoking, obesity, and their co-occurrence in the United States: cross sectional analysis. Br Med J. 2006;333:25–6. doi: 10.1136/bmj.38840.608704.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bagla N, Schofield JB. Rectosigmoid tumours: should we continue sitting on the fence? Colorectal Dis. 2007;9:606–8. doi: 10.1111/j.1463-1318.2007.01329.x. [DOI] [PubMed] [Google Scholar]
- 87.Thornton A, Lee P. Publication bias in meta-analysis: its causes and consequences. J Clin Epidemiol. 2000;53:207–16. doi: 10.1016/s0895-4356(99)00161-4. [DOI] [PubMed] [Google Scholar]
- 88.Muto T, Kamiya J, Sawada T, et al. Small “flat adenoma” of the large bowel with special reference to its clinicopathologic features. Dis Colon Rectum. 1985;28:847–51. doi: 10.1007/BF02555490. [DOI] [PubMed] [Google Scholar]
- 89.Minamoto T, Sawaguchi K, Ohta T, et al. Superficial-type adenomas and adenocarcinomas of the colon and rectum: a comparative morphological study. Gastroenterology. 1994;106:1436–43. doi: 10.1016/0016-5085(94)90395-6. [DOI] [PubMed] [Google Scholar]
- 90.Soetikno RM, Kaltenbach T, Rouse RV, et al. Prevalence of nonpolypoid (fiat and depressed) colorectal neoplasms in asymptomatic and symptomatic adults. JAMA. 2008;299:1027–35. doi: 10.1001/jama.299.9.1027. [DOI] [PubMed] [Google Scholar]
- 91.Kudo S, Lambert R, Allen JI, et al. Nonpolypoid neoplastic lesions of the colorectal mucosa. Gastrointest Endosc. 2008;68 (Suppl 4):S3–47. doi: 10.1016/j.gie.2008.07.052. [DOI] [PubMed] [Google Scholar]
- 92.Taylor C, Schubert ML. Decreased efficacy of polyethylene glycol lavage solution (golytely) in the preparation of diabetic patients for outpatient colonoscopy: a prospective and blinded study. Am J Gastroenterol. 2001;96:710–4. doi: 10.1111/j.1572-0241.2001.03610.x. [DOI] [PubMed] [Google Scholar]
- 93.Ozturk NA, Gokturk HS, Demir M, et al. The effect of autonomous neuropathy on bowel preparation in type 2 diabetes mellitus. Int J Colorectal Dis. 2009;24:1407–12. doi: 10.1007/s00384-009-0757-4. [DOI] [PubMed] [Google Scholar]
- 94.Chung YW, Han DS, Park KH, et al. Insulin therapy and colorectal adenoma risk among patients with Type 2 diabetes mellitus: a case-control study in Korea. Dis Colon Rectum. 2008;51:593–7. doi: 10.1007/s10350-007-9184-1. [DOI] [PubMed] [Google Scholar]
- 95.Prevalence of Diagnosed and Undiagnosed Diabetes in the United States, All Ages, 2007 National Institute of Diabetes and Digestive and Kidney Disease.
- 96.Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci. 2009 doi: 10.1007/s10620-009-0944-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.