Abstract
Objective. The Medical Outcomes Short Form-36 Survey (SF-36) has been widely used as a measure of health-related quality of life (HRQOL) in different populations. SLE patients have consistently reported lower scores compared with the general population. The objective of our study was to identify predictors of HRQOL using SF-36 among patients with SLE enrolled in a 2-year randomized controlled trial (RCT).
Methods. We analysed 200 SLE patients enrolled in the Lupus Atherosclerosis Prevention Study (LAPS), an RCT of atorvastatin vs placebo, who completed SF-36 at qualifying, 12- and 24-month (final) visits.
Results. At baseline, mean SF-36 domain scores were lower than those of age- and gender-matched population norms. There was no statistical difference reported between Physical Component Summary (PCS), Mental Component Summary and eight domain scores in the atorvastatin vs placebo group at 2 years. In multiple regression analyses, African American patients reported significantly lower scores in Physical Functioning compared with Caucasians. The presence of FM was significantly associated with lower scores in physical functioning, role physical, bodily pain, general health, vitality, social functioning and lower overall mean PCS scores. The Physician’s Global Assessment of disease activity was associated with multiple SF-36 domains in univariate analysis.
Conclusion. This longitudinal study confirmed lower scores reported across all SF-36 domains. No one explanatory variable was independently associated with all domain scores. FM was independently associated with poorer HRQOL in most domains, underscoring the need for effective treatments for FM in SLE.
Keywords: SLE, SF-36, HRQOL, fibromyalgia, statins, disease activity, PCS, MCS, disease activity indices, spydergram
Introduction
In SLE, health-related quality of life (HRQOL), disease activity and organ damage are all important outcomes. However, disease activity and disease damage do not correlate highly with HRQOL in SLE. Thus HRQOL represents a distinct domain of outcome [1–5]. The Medical Outcomes Study Short Form-36 (SF-36) is the most widely used, validated and reliable instrument to assess self-reported HRQOL [6]. It consists of 36 questions, individually combined into eight domains, with higher scores reflecting better perceived HRQOL [7]. SLE patients have consistently reported low scores in most, if not all, SF-36 domains [4, 5, 8] compared with age- and gender-matched populations, as well as patients with chronic diseases such as congestive heart failure [9], RA [1, 5] or AIDS [10]. Several studies of HRQOL in SLE have reported that demographic, socio-economic and disease-related factors contribute to its overall impact on physical and psychological well-being [4, 11–18]. The primary objective of the study was to identify predictors of HRQOL using SF-36 among patients with SLE enrolled in this 2-year randomized controlled trial (RCT) comparing the use of a statin with placebo.
Materials and methods
Patient selection
Two hundred SLE patients enrolled in the Lupus Atherosclerosis Prevention Study (LAPS) were evaluated by one rheumatologist of Johns Hopkins University School of Medicine from 2002 to 2005. Patients were examined quarterly for 2 years during the trial of atorvastatin 40 mg nightly vs placebo. All patients gave informed consent. The LAPS was approved by the Johns Hopkins University School of Medicine institutional review board (Clinicaltrials.gov NCT 00120887).
Clinical evaluation
Demographic, socio-economic, clinical and immunological data from each patient were obtained and recorded as part of the Hopkins Lupus Cohort database. The presence of FM was also ascertained, based on the ACR definition, as chronic widespread pain and pain in 11 of 18 specific tender point (TP) sites on digital palpation.
Measures of disease activity and health-related quality of life assessment
Disease activity was assessed using the SELENA-SLEDAI (Safety of Estrogens in Lupus Erythematosus National Assessment–Systemic Lupus Erythematosus Disease Activity Index) and Physician's Global Assessment (PGA) by a visual analogue scale from 0 to 3 [19, 20]. The SELENA-SLEDAI, a reliable and validated measure of disease activity, includes 24 descriptors in nine organ systems. The total score falls between 0 and 105, with higher scores representing increased disease activity [20]. SELENA-SLEDAI was also used to determine changes in SLE activity from baseline, 12 and 24 months.
Every patient at baseline, 12 and 24 months completed the SF-36 [7], which includes two summary scores, the Physical Component Summary (PCS) and the Mental Component Summary (MCS), and eight domains: physical function (PF), role physical (RP), bodily pain (BP), general health (GH), vitality (VT), social functioning (SF), role emotional (RE) and mental health (MH), all scored from 0 to 100, with higher scores indicating better health status. Five domains are scored positively in the PCS (PF, RP, BP, GH and VT) and the other three negatively; and in the MCS, VT, SF, RE and MH are weighted positively and the remaining four negatively. The two component scores are standardized to have a mean of 50 with a s.d. of 10 [7].
Statistical analysis
A univariate analysis was performed to determine associations with PCS, MCS and domain scores of the SF-36. Data are expressed as means. P-values are reported, with P < 0.05 being accepted as statistically significant. Multivariate regression analyses estimated the effects of demographic and disease activity variables on summary and domain scores of SF-36. Paired t-test and analysis of covariance (ANCOVA) were used to examine the effect of the statin vs placebo on reported HRQOL.
Results
Two hundred SLE patients were enrolled in the LAPS; 90% were female, 61% Caucasian, 34% African American, 2% Hispanic and 2% Asian, with a mean age of 44.3 ± 11.4 years. Of 200 patients, 93% (n = 186) completed the SF-36 questionnaire at qualifying, 12-month and 24-month visits and were included in the analyses.
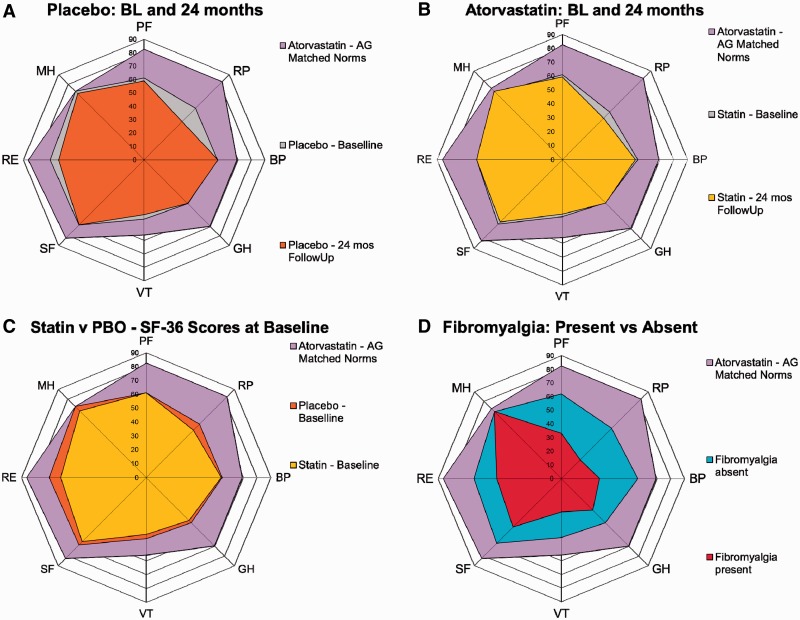
The SF-36 summary component and domain scores at baseline, 12- and 24-month visits, including means, are shown in Table 1, compared with an age- and gender-matched population specific to this protocol population. In both groups, scores in all eight domains were much lower compared with age- and gender-matched normative values at baseline, 12 and 24 months. At baseline, the domains with the lowest scores were VT, followed by GH and RP; at 12 months, VT, followed by RP and GH; and at 24 months, VT, RP and GH. Overall, there was a marked reduction of SF-36 scores compared with age- and gender-matched US norms (Table 1). Atorvastatin did not have any apparent effect on HRQOL (Table 1). There were no significant changes in either group (atorvastatin and placebo) over 2 years of follow-up. Data are presented as spydergrams in Fig. 1, with benchmark comparisons to normative values [16].
Table 1.
Atorvastatin and SF-36 domains showing mean at baseline and follow-up
| Domain | Age- and gender-matched norms | Group | Mean at baselinea | Mean at 12 months | Mean at 24 monthsa | P-value for changeb | P-value for difference in changesc |
|---|---|---|---|---|---|---|---|
| PF | 82.5 | Statin | 60.7 | 57.7 | 58.7 | 0.79 | 0.72 |
| Placebo | 60.2 | 59.3 | 59.3 | 0.41 | |||
| RP | 82.3 | Statin | 48.3 | 44.8 | 41.1 | 0.21 | 0.32 |
| Placebo | 52.8 | 46.1 | 40.9 | 0.0008 | |||
| BP | 69.1 | Statin | 54.1 | 53.3 | 52.3 | 0.72 | 0.61 |
| Placebo | 54.7 | 53.5 | 55.8 | 0.92 | |||
| GH | 69.6 | Statin | 44.8 | 45.6 | 42.9 | 0.70 | 0.81 |
| Placebo | 45.6 | 45.4 | 46.2 | 0.86 | |||
| VT | 55.9 | Statin | 41.0 | 39.5 | 38.8 | 0.42 | 0.90 |
| Placebo | 43.3 | 42.1 | 41.3 | 0.11 | |||
| SF | 82.4 | Statin | 64.9 | 64.2 | 63.3 | 0.60 | 0.42 |
| Placebo | 67.5 | 66.5 | 68.8 | 0.91 | |||
| RE | 86.3 | Statin | 59.5 | 58.0 | 61.9 | 0.83 | 0.70 |
| Placebo | 67.7 | 58.0 | 63.1 | 0.24 | |||
| MH | 72.3 | Statin | 67.5 | 67.7 | 69.2 | 0.51 | 0.41 |
| Placebo | 71.0 | 69.1 | 70.3 | 0.17 | |||
| MCS | Statin | 42.7 | 43.2 | 43.9 | 0.40 | 0.80 | |
| Placebo | 46.0 | 43.4 | 45.2 | 0.54 | |||
| PCS | Statin | 39.1 | 38.4 | 37.0 | 0.17 | 0.99 | |
| Placebo | 38.8 | 39.0 | 37.4 | 0.16 |
aData illustrated as spydergrams in Fig. 1. bBased on a paired t-test; change between 24 months and baseline. cBased on an ANCOVA model.
Fig. 1.
Spydergrams of SF-36 domain scores at baseline and end point.
Gridlines represent 10 points = 2× minimum clinically important difference (MCID). Outer polygon (lavender): age- and gender-matched US norms specific for this protocol population. (A) Placebo + SOC. Innermost polygon (orange): reported HRQOL at 24 months in placebo + SOC; intermediate polygon (grey): reported HRQOL at baseline in placebo, indicating small levels of deterioration in VT domain and worsening in RP and RE domains that exceeds MCID for deterioration, e.g. −0.8 points. (B) Atorvastatin + SOC. Innermost polygon (yellow): reported HRQOL at 24 months in atorvastatin + SOC; intermediate polygon (grey): reported HRQOL at baseline in atorvastatin; indicating worsening in RP domain that exceeds MCID for deterioration, e.g. −0.8 points, but no change in other domains. (C) Placebo + SOC vs atorvastatin + SOC. Innermost polygon (yellow): reported HRQOL at baseline in atorvastatin + SOC group; intermediate polygon (orange) reported HRQOL at baseline in placebo + SOC group, indicating higher values in placebo at baseline in RP, RE and MH domains. (D) Spydergram comparing SLE patients with and without FM. Innermost polygon (red): reported HRQOL at baseline in SLE patients with FM; intermediate polygon (aqua): reported HRQOL at baseline in SLE patients without FM, which closely resembles that reported in the entire protocol population at baseline. Large decrements in HRQOL are evident in those with concomitant FM, particularly in PF, RP and BP of the physical domains and VT, SF and RE of the mental domains. The pattern in SLE patients with FM is similar to other FM patient populations from RCTs and longitudinal observational studies [8].
No differences in SF-36 domains between female and male patients were evident, although women did have lower scores in PF and RE (Table 2). These results were not statistically significant. African Americans reported lower scores in PF (50.5 vs 64.1, P = 0.0031) and higher scores in VT (46.0 vs 38.6, P = 0.041) compared with Caucasians (Table 2). High school education was associated with higher scores for PF (65.0 vs 53.6, P = 0.010) and RE (68.7 vs 54.7, P = 0.021) (Table 2). The presence of FM was associated with significantly lower scores in PF (61.9 vs 33.1, P = 0.0016), RP (52.0 vs 19.4, P = 0.015), BP (55.9 vs 27.9, P = 0.0006), GH (45.6 vs 32.5, P = 0.0052), VT (43.1 vs 24.3, P = 0.0086) and SF (66.9 vs 50.0, P = 0.042) and lower overall mean PCS scores (39.6 vs 26.0, P = 0.0002) (Table 2). A lower PGA of disease activity was associated with better scores in PF, BP, GH, SF, RE and MH (P = 0.024, 0.0018, 0.053, 0.0091, 0.043 and 0.016). A higher PGA was also associated with a lower mean PCS (P = 0.033) as well as lower MCS (P = 0.031) scores. The SELENA-SLEDAI, in contrast, was not associated with any SF-36 domains (Table 2).
Table 2.
Gender, ethnicity, education status, FM, PGA and SF-36 domains at baseline
| PF | RP | BP | GH | VT | SF | RE | MH | MCS | PCS | |
|---|---|---|---|---|---|---|---|---|---|---|
| Gender | ||||||||||
| Female | 59.2 | 50.0 | 54.1 | 44.9 | 42.0 | 65.8 | 62.4 | 69.0 | 44.2 | 38.7 |
| Male | 72.3 | 50.0 | 55.3 | 44.0 | 42.0 | 66.7 | 68.9 | 72.3 | 45.2 | 40.1 |
| P-value | 0.11 | 0.99 | 0.87 | 0.89 | 0.99 | 0.91 | 0.57 | 0.52 | 0.77 | 0.68 |
| Ethnicity | ||||||||||
| Caucasian | 64.1 | 47.8 | 54.9 | 46.0 | 38.6 | 65.8 | 66.7 | 70.1 | 44.6 | 38.9 |
| African American | 50.5 | 50.0 | 50.6 | 41.4 | 46.0 | 65.0 | 54.0 | 67.0 | 43.2 | 37.2 |
| P-value | 0.0031 | 0.75 | 0.31 | 0.20 | 0.041 | 0.86 | 0.049 | 0.27 | 0.51 | 0.36 |
| Education | ||||||||||
| ≤12 years | 53.6 | 49.1 | 53.1 | 43.6 | 43.7 | 64.6 | 54.7 | 67.3 | 42.8 | 38.1 |
| >12 years | 65.0 | 50.7 | 54.9 | 45.7 | 40.7 | 66.8 | 68.7 | 70.6 | 45.3 | 39.3 |
| P-value | 0.010 | 0.80 | 0.65 | 0.53 | 0.40 | 0.57 | 0.021 | 0.22 | 0.19 | 0.55 |
| Fibromyalgia | ||||||||||
| Present | 33.1 | 19.4 | 27.9 | 32.5 | 24.3 | 50.0 | 47.2 | 69.4 | 42.6 | 26.0 |
| Absent | 61.9 | 52.0 | 55.9 | 45.6 | 43.1 | 66.9 | 63.9 | 69.2 | 44.3 | 39.6 |
| P-value | 0.0016 | 0.015 | 0.0006 | 0.0052 | 0.0086 | 0.042 | 0.18 | 0.97 | 0.65 | 0.0002 |
| PGA | ||||||||||
| ≤1 | 62.0 | 52.0 | 56.4 | 46.0 | 43.0 | 67.8 | 65.1 | 70.4 | 45.0 | 39.5 |
| >1 | 46.5 | 34.8 | 37.5 | 36.0 | 34.4 | 51.6 | 46.4 | 60.4 | 38.8 | 33.5 |
| P-value | 0.024 | 0.083 | 0.0018 | 0.053 | 0.11 | 0.0091 | 0.043 | 0.016 | 0.033 | 0.031 |
| SLEDAI | ||||||||||
| ≤4 | 61.7 | 49.9 | 54.6 | 45.5 | 41.8 | 66.4 | 62.7 | 68.8 | 44.0 | 39.3 |
| >4 | 49.6 | 52.0 | 50.6 | 39.9 | 43.4 | 62.0 | 64.0 | 71.0 | 45.8 | 35.8 |
| P-value | 0.069 | 0.83 | 0.50 | 0.27 | 0.76 | 0.47 | 0.89 | 0.59 | 0.52 | 0.21 |
SLEDAI: SELENA-SLEDAI.
In multiple variable regression analysis (not shown), African American ethnicity was an independent associate of PF (P = 0.0078). FM was significantly associated with lower PF (P = 0.0003), RP (P = 0.017), BP (P = 0.0004), GH (P = 0.0430), VT (P = 0.018) and SF (P = 0.031), as well as overall mean PCS scores (P = 0.0002).
Discussion
The LAPS, a 2-year intervention trial of atorvastatin vs placebo, allowed us to measure HRQOL in SLE and to determine changes over time. As shown in previous studies, SLE patients report lower SF-36 scores than the general population or healthy subjects [21–24], comparable with other severe chronic diseases [1, 9]. We found that HRQOL reported by SLE patients in this study generally did not change over 2 years of follow-up. This contrasts with disease activity, which may change rapidly, especially in SLE patients who flare or have a relapsing-remitting pattern of SLE [25–27], and organ damage [3], which gradually accumulates over time.
HRQOL measured by the SF-36 has often been the only patient-reported outcome measured in RCTs in SLE and offers important information unobtainable from measures of disease activity or damage [28, 29]. In a randomized trial using LJP 394 in SLE, improved HRQOL was reported with active treatment compared with placebo [30]. In this trial, patients receiving LJP showed improvement or stabilization in all domains except one, in contrast to the placebo group, which reported deterioration in all domains. Women treated with DHEA had improved HRQOL in the SF-36 RE domain vs placebo [28, 31]. We have shown improvement in HRQOL with the use of hormone therapy [32]. In the SELENA trial of oral contraceptives and oestrogen replacement, the SF-36 demonstrated improvement in those patients receiving oral contraceptive pills. There was improvement in the SF-36 RP and RE domains as well as SF and VT. There was either no change or worsening reported by the placebo group. In the early phase 2/3 randomized clinical trials of epratuzumab, large improvements across all SF-36 domains were evident with active therapy compared with placebo [8, 33]. In the phase 3 trials of belimumab (BLISS-52 and -76) in a combined analysis of both protocols, responders by the SLE Responder Index (SRI) reported statistically better HRQOL at 1 year than non-responders, with twice as many stating they were better or much better than 1 year before by the transition question of the SF-36 [34].
In this study, atorvastatin had no effect, either good or bad, on HRQOL assessed by SF-36. In a trial of atorvastatin in RA patients, atorvastatin 40 mg showed a modest reduction in the 28-joint disease activity score (DAS28) [35]. In non-SLE studies, atorvastatin improved physical activity in a claudication trial [36], but had no effect on HRQOL in a post-coronary artery bypass graft trial [37].
We examined both demographic variables and disease activity as possible associates of HRQOL in SLE. Gender had no influence on any SF-36 domain. Ethnicity had different effects, depending on the SF-36 domain. African Americans had lower scores than Caucasians in PF, but higher scores in VT; however, VT dropped out in our multivariate analysis and a trend was seen towards greater impairment of PF in blacks than whites [38].
High school education was associated with significantly higher scores in PF and RE, but only in univariate analysis. In the general population, perceived health status declines with decreasing educational level [39]. In past studies in SLE, level of education was not consistently associated with HRQOL [17, 21] compared with other diseases, such as seizure [40]. However, better education has been associated with lower mortality rates [41], better coping strategies and better social support.
In our study, FM was significantly associated with poor PF, RP, BP, GH, VT and SF and lower overall mean PCS scores. FM in SLE has been extensively studied by our group [42] and others [17, 43, 44]. Patients with FM with or without SLE have been found to report impaired scores in the SF-36 PF, RP, BP and VT domains, and commensurately PCS scores [43–45]. Our results are in accordance with other studies.
In SLE, poor functional outcome may be attributed to fatigue and pain. Fatigue is common and one of the most important associates of poor HRQOL in SLE [43]. In the SF-36, fatigue is included in the VT domain, and in our study African Americans had higher scores compared with Caucasians. However, fatigue was not correlated with SLE disease severity or activity [15, 46] contrary to other studies [47, 48]. Pain is a predictor of activity limitation [49]. Pain, fatigue and FM are closely related constructs in SLE and should be addressed in the management of SLE.
In our study, higher disease activity measured by the PGA in univariate analysis was associated with poorer PF, BP, SF, RE, MH and GH domain scores. Higher disease activity was also associated with lower overall mean PCS and MCS scores, again in univariate analysis only. Similar results using other measures of disease activity have been reported in the past [5–8, 18, 50]. However, we did not find any correlation of disease activity with SF-36 mental domain scores in our study, in agreement with the study by Benitha et al. [5]. Impairment of all SF-36 domains with higher disease activity, as measured by the Systemic Lupus Activity Measure (SLAM), was reported in the study by Saba et al. [50]. However, the SLAM contains several measures that may not directly measure disease activity. When we analysed the SELENA-SLEDAI, there was no association with any of the SF-36 domains. A 12-month double-blind RCT of abatacept vs placebo showed greater improvements in PCS, MCS, fatigue and sleep problems with abatacept vs placebo [51]. Another randomized, placebo-controlled trial of belimumab showed significant improvement in PCS in the belimumab group [52].
FM is an important factor in the poor quality of life in SLE [53, 54] and appears to have a greater frequency in SLE than in the general female population [55, 56]. The reason for the increased frequency of fibromyalgia in SLE, or even a connection with the pathophysiology of SLE, remains unknown. Much of the progress in FM has centred on the appreciation that it is a central pain sensitization syndrome [57]. Decreased levels of gamma amino butyric acid have been documented in the right anterior insula [58] and an imbalance in µ-opioid receptor availability [59] was recently reported. In our study, multiple regression analyses showed that FM was an independent associate of poorer PF, RP, BP, GH, VT and SF domains. Ethnicity was also an important factor in PF scores. FM is likely to have the greatest impact on important SF-36 domains in randomized clinical trials in SLE.
In summary, our study confirms that SLE adversely affects all domains of HRQOL and that SF-36 scores did not change over 2 years of follow-up. Ethnicity, education, FM and disease activity are important associates in some domains of HRQOL in SLE. Because the SF-36 will continue to be an outcome measure in randomized clinical trials, it is suggested that these explanatory domains be adjusted for on a domain-by-domain basis. These results again underscore the need for effective therapies for FM in SLE.
Rheumatology key messages.
SLE adversely affects all domains of HRQOL.
Fibromyalgia is likely to have the greatest impact on important SF-36 domains in randomized clinical trials in SLE.
Funding: This study was supported by a grant from the Alliance for Lupus Research, the Arthritis Foundation, the Hopkins Lupus Cohort (NIH AR 43727) and by grant number UL1 RR 025005 from the National Center for Research Resources (NCRR).
Disclosure statement: V.S. has served as a consultant to Amgen, Anthera, BMS, Genentech/Roche, HGS/GSK, Lilly, Medimmune, Merck Serono, Neovacs, NovoNordisk, Pfizer, Takeda and UCB. All other authors have declared no conflicts of interest.
References
- 1.Gilboe I, Kvien T, Husby G. Health status in systemic lupus erythematosus compared to rheumatoid arthritis and healthy controls. J Rheumatol. 1999;26:1694–700. [PubMed] [Google Scholar]
- 2.Hanly JG. Disease activity, cumulative damage and quality of life in systemic lupus erythematosus: results of a cross-sectional study. Lupus. 1997;6:243–7. doi: 10.1177/096120339700600305. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Urowitz MB, Ong A, et al. Lack of correlation among the 3 outcomes describing SLE: disease activity, damage and quality of life. Clin Exp Rheumatol. 1996;14:305–8. [PubMed] [Google Scholar]
- 4.Sutcliffe N, Clarke AE, Gordon C, et al. The association of socio-economic status, race, psychosocial factors and outcome in patients with systemic lupus erythematosus. Rheumatology. 1999;38:1130–7. doi: 10.1093/rheumatology/38.11.1130. [DOI] [PubMed] [Google Scholar]
- 5.Benitha R, Mohammed T. Functional disability and health-related quality of life in South Africans with rheumatoid arthritis and systemic lupus erythematosus. Clin Rheumatol. 2007;26:24–9. doi: 10.1007/s10067-006-0215-4. [DOI] [PubMed] [Google Scholar]
- 6.Strand V, Gladman D, Isenberg D, et al. Outcome measures to be used in clinical trials in systemic lupus erythematosus. J Rheumatol. 1999;26:490–7. [PubMed] [Google Scholar]
- 7.Ware J. SF-36 health survey update. Spine. 2000;25:3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Strand V, Crawford B, Singh J, et al. Use of ‘spydergrams’ to present and interpret health-related quality of life data across rheumatic diseases. Ann Rheum Dis. 2009;68:1800–4. doi: 10.1136/ard.2009.115550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juenger J, Schellberg D, Kraemer S. Health-related quality of life in patients with congestive heart failure: comparison with other chronic diseases and relation to functional variables. Heart. 2002;87:235–41. doi: 10.1136/heart.87.3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ragsdale D, Morrow J. Quality of life as a function of HIV classification. Nurs Res. 1990;39:355–9. [PubMed] [Google Scholar]
- 11.Da Costa D, Clarke A, Dobkin P. The relationship between health status, social support and satisfaction with medical care among patients with systemic lupus erythematosus. Int J Qual Health Care. 1999;11:201–7. doi: 10.1093/intqhc/11.3.201. [DOI] [PubMed] [Google Scholar]
- 12.Sutcliffe N, Clarke A, Levinton C, et al. Associates of health status in patients with systemic lupus erythematosus. J Rheumatol. 1999;26:2352–6. [PubMed] [Google Scholar]
- 13.Bae S, Hashimoto H, Karlson E, et al. Variable effects of social support by race, economic status, and disease activity in systemic lupus erythematosus. J Rheumatol. 2001;28:1245–50. [PubMed] [Google Scholar]
- 14.Devins GM, Edworthy SM. Illness intrusiveness explains race-related quality-of-life differences among women with systemic lupus erythematosus. Lupus. 2000;9:534–41. doi: 10.1177/096120330000900710. [DOI] [PubMed] [Google Scholar]
- 15.Wang B, Gladman D, Urowitz M. Fatigue in lupus is not correlated with disease activity. J Rheumatol. 1998;25:892–5. [PubMed] [Google Scholar]
- 16.Zonana-Nacach A, Roseman J, McGwin G., Jr Systemic lupus erythematosus in three ethnic groups. VI: Factors associated with fatigue within 5 years of criteria diagnosis. Lupus. 2000;9:101–9. doi: 10.1191/096120300678828046. [DOI] [PubMed] [Google Scholar]
- 17.Alarcon G, McGwin G, Jr, Uribe A. Systemic lupus erythematosus in a multiethnic lupus cohort (LUMINA). XVII. Predictors of self-reported health-related quality of life early in the disease course. Arthritis Rheum. 2004;51:465–74. doi: 10.1002/art.20409. [DOI] [PubMed] [Google Scholar]
- 18.Stoll T, Kauer Y, Buchi S, et al. Prediction of depression in systemic lupus erythematosus patients using SF-36 mental health scores. Rheumatology. 2001;40:695–8. doi: 10.1093/rheumatology/40.6.695. [DOI] [PubMed] [Google Scholar]
- 19.Petri M, Kim M, Kalunian K. Combined oral contraceptives in women with systemic lupus erythematosus. N Eng J Med. 2005;353:2550–8. doi: 10.1056/NEJMoa051135. [DOI] [PubMed] [Google Scholar]
- 20.Petri M, Hellmann D, Hochberg M. Validity and reliability of lupus activity measures in the routine clinic setting. J Rheumatol. 2002;19:53–9. [PubMed] [Google Scholar]
- 21.Friedman A, Alarcon G, Mcgwin G. Systemic lupus erythematosus in three ethnic groups. IV. Factors associated with self-reported functional outcome in a large cohort study. Arthritis Care Res. 1999;12:256–66. doi: 10.1002/1529-0131(199908)12:4<256::aid-art4>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 22.Stoll T, Gordon C, Seifert B, et al. Consistency and validity of patient administered assessment of quality of life by the MOS SF-36; its association with disease activity and damage in patients with systemic lupus erythematosus. J Rheumatol. 1997;24:1608–14. [PubMed] [Google Scholar]
- 23.Dorsey R, Andresen E, Moore T. Health-related quality of life and support group attendance for patients with systemic lupus erythematosus. J Clin Rheumatol. 2004;10:6–9. doi: 10.1097/01.rhu.0000111311.38407.15. [DOI] [PubMed] [Google Scholar]
- 24.Rinaldi S, Doria A, Salaffi F. Health-related quality of life in Italian patients with systemic lupus erythematosus. I. Relationship between physical and mental dimension and impact of age. Rheumatology. 2004;43:1574–9. doi: 10.1093/rheumatology/keh397. [DOI] [PubMed] [Google Scholar]
- 25.Petri M. Lupus in Baltimore: evidence-based ‘clinical pearls’ from the Hopkins Lupus Cohort. Lupus. 2005;14:970–3. doi: 10.1191/0961203305lu2230xx. [DOI] [PubMed] [Google Scholar]
- 26.Barr S, Zonana-Nacach A, Magder L, et al. Patterns of disease activity in systemic lupus erythematosus. Arthritis Rheum. 1999;42:2682–8. doi: 10.1002/1529-0131(199912)42:12<2682::AID-ANR26>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 27.Thumboo J, Strand V. Health-related quality of life in patients with systemic lupus erythematosus: an update. Ann Acad Med Singapore. 2007;36:115–22. [PubMed] [Google Scholar]
- 28.Crawford B, Strand V. Improvement in health-related quality of life in patients with SLE following sustained reductions in anti-dsDNA antibodies. Expert Rev Pharmacoecon Outcomes Res. 2005;5:317–26. doi: 10.1586/14737167.5.3.317. [DOI] [PubMed] [Google Scholar]
- 29.Strand V, Chu AD. Generic versus disease-specific measures of health-related quality of life in systemic lupus erythematosus. J Rheumatol. 2011;38:1821–3. doi: 10.3899/jrheum.110766. [DOI] [PubMed] [Google Scholar]
- 30.Strand V, Aranow C, Cardiel MH. Improvement in health-related quality of life in systemic lupus erythematosus patients enrolled in a randomized clinical trial comparing LJP 394 treatment with placebo. Lupus. 2003;12:677–86. doi: 10.1191/0961203303lu440oa. [DOI] [PubMed] [Google Scholar]
- 31.Nordmark G, Bengtsson C, Larsson A, et al. Effects of dehydroepiandrosterone supplement on health-related quality of life in glucocorticoid treated female patients with systemic lupus erythematosus. Autoimmunity. 2005;38:531–40. doi: 10.1080/08916930500285550. [DOI] [PubMed] [Google Scholar]
- 32.Petri M, Buyon J, Sigler L, et al. Beneficial effects of hormone therapy on health-related quality of life (HRQOL) in systemic lupus erythematosus (SLE): results of SELENA OCP and HRT randomized controlled trials (RCTs) Arthritis Rheum. 2006;54:S447. [Google Scholar]
- 33.Strand V, Gordon C, Kalunian K, et al. Meaningful improvements in health-related quality of life (HRQOL) with epratuzumab (anti-CD22 mAb targeting B-cells) in patients (Pts) with SLE with high disease activity: results from 2 randomized controlled trials (RCTs) Arthritis Rheum. 2008;1086:S570. [Google Scholar]
- 34.Strand V, Cooper S, Zhong ZJ, et al. Responders in the phase 3 belimumab clinical trials in patients with systemic lupus erythematosus reported improvements in fatigue and health-related quality of life at week 52. Arthritis Rheum. 2011;1369:S535. [Google Scholar]
- 35.McCarey DW, McInnes IB, Madhok R, et al. Trial of atorvastatin in rheumatoid arthritis (TARA): double blind, randomized placebo-controlled trial. Lancet. 2004;363:2015–21. doi: 10.1016/S0140-6736(04)16449-0. [DOI] [PubMed] [Google Scholar]
- 36.Mohler E, Hiatt W, Creager M. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108:1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 37.Fox N, Hoogwerf B, Czajkowski S. Quality of life after coronary artery bypass graft. Chest. 2004;126:487–95. doi: 10.1378/chest.126.2.487. [DOI] [PubMed] [Google Scholar]
- 38.Doninger N, Fink J, Utset T. Neuropsychologic functioning and health status in systemic lupus erythematosus.: does ethnicity matter? J Clin Rheumatol. 2005;11:250–6. doi: 10.1097/01.rhu.0000182149.67967.cc. [DOI] [PubMed] [Google Scholar]
- 39.Regidor E, Barrio G, de la Fuente L, et al. Association between educational level and health related quality of life in Spanish adults. J Epidemiol Community Health. 1999;53:75–82. doi: 10.1136/jech.53.2.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Djibuti M, Shakarishvili R. Influence of clinical, demographic, and socioeconomic variables on quality of life in patients with epilepsy: findings from Georgian study. J Neurol Neurosurg Psychiatry. 2003;74:570–3. doi: 10.1136/jnnp.74.5.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward M. Education level and mortality in systemic lupus erythematosus (SLE): evidence of underascertainment of deaths due to SLE in ethnic minorities with low education levels. Arthritis Rheum. 2004;51:616–24. doi: 10.1002/art.20526. [DOI] [PubMed] [Google Scholar]
- 42.Akkasilpa S, Goldman D, Magder L, et al. Number of fibromyalgia tender points is associated with health status in patients with systemic lupus erythematosus. J Rheumatol. 2005;32:48–50. [PubMed] [Google Scholar]
- 43.Friedman AW, Tew MB, Ahn C. Systemic lupus erythematosus in three ethnic groups: XV prevalence and correlates of fibromyalgia. Lupus. 2003;12:274–9. doi: 10.1191/0961203303lu330oa. [DOI] [PubMed] [Google Scholar]
- 44.Gladman DD, Urowitz MB, Gough J, et al. Fibromyalgia is a major contributor to quality of life in lupus. J Rheumatol. 1997;24:2145–8. [PubMed] [Google Scholar]
- 45.Bennett RM, Schein J, Kosinski MR, et al. Impact of fibromyalgia pain on health-related quality of life before and after treatment with tramadol/acetaminophen. Arthritis Rheum. 2005;53:519–27. doi: 10.1002/art.21319. [DOI] [PubMed] [Google Scholar]
- 46.Bruce I, Mak V, Hallet D, et al. Factors associated with fatigue in patients with systemic lupus erythematosus. Ann Rheum Dis. 1999;58:379–81. doi: 10.1136/ard.58.6.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wysenbeek A, Leibovici L, Weinberger A, et al. Fatigue in systemic lupus erythematosus. Prevalence and relation to disease expression. Br J Rheumatol. 1993;32:633–5. doi: 10.1093/rheumatology/32.7.633. [DOI] [PubMed] [Google Scholar]
- 48.Krupp L, LaRocca N, Muir J, et al. A study of fatigue in systemic lupus erythematosus. J Rheumatol. 1990;17:1450–2. [PubMed] [Google Scholar]
- 49.Greco C, Thomas R, Manzi S. Effects of disease activity, pain and distress on activity limitations in patients with systemic lupus erythematosus. J Rheumatol. 2004;31:260–7. [PubMed] [Google Scholar]
- 50.Saba J, Quinet R, Davis W. Inverse correlation of each functional status scale of the SF-36 with degree of disease activity in systemic lupus erythematosus (m-SLAM) Joint Bone Spine. 2003;70:348–51. doi: 10.1016/s1297-319x(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 51.Merrill JT, Burgos-Vargas R, Westhovens R, et al. The efficacy and safety of abatacept in patients with non-life threatening manifestations of systemic lupus erythematosus. Arthritis Rheum. 2010;62:3077–87. doi: 10.1002/art.27601. [DOI] [PubMed] [Google Scholar]
- 52.Navarra SV, Guzman RM, Gallacher AE, et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomized, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–31. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 53.Buskila D, Press J, Abu-Shakra M. Fibromyalgia in systemic lupus erythematosus: prevalence and clinical implications. Clin Rev Allergy Immunol. 2003;25:25–8. doi: 10.1385/CRIAI:25:1:25. [DOI] [PubMed] [Google Scholar]
- 54.Da Costa D, Dobkin PL, Fitzcharles MA, et al. Determinants of health status in fibromyalgia: a comparative study with systemic lupus erythematosus. J Rheumatol. 2000;27:365–72. [PubMed] [Google Scholar]
- 55.Wolfe F, Ross K, Anderson J, et al. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38:19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 56.Middleton GD, McFarlin JE, Lipsky PE. The prevalence and clinical impact of fibromyalgia in systemic lupus erythematosus. Arthritis Rheum. 1994;37:1181–8. doi: 10.1002/art.1780370812. [DOI] [PubMed] [Google Scholar]
- 57.Arnold LM, Clauw DJ. Fibromyalgia syndrome: practical strategies for improving diagnosis and patient outcomes. Am J Med. 2010;123:S2. doi: 10.1016/j.amjmed.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 58.Foerster BR, Petrou M, Edden RAE, et al. Reduced insular γ-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64:579–83. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harris RE, Clauw DJ, Scott DJ, et al. Decreased central mu-opioid receptor availability in fibromyalgia. J Neurosci. 2007;27:10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]