Abstract
Rodents exposed to a 15-min pretest swim in the forced swimming test (FST) exhibit prolonged immobility in a subsequent 5-min test swim, and antidepressant treatment before the test swim reduces immobility. At present, neuronal circuits recruited by antidepressant before the test swim remain unclear, and also less is known about whether antidepressants with different mechanisms of action could influence neural circuits differentially. To reveal the neural circuits associated with antidepressant effect in the FST, we injected desipramine or citalopram 0.5 h, 19 h, and 23 h after the pretest swim and observed changes in c-Fos expression in rats before the test swim, namely 24 h after the pretest swim. Desipramine treatment alone in the absence of pretest swim was without effect, whereas citalopram treatment alone significantly increased the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala and bed nucleus of the stria terminalis, where this pattern of increase appears to be maintained after the pretest swim. Both desipramine and citalopram treatment after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the ventral lateral septum and ventrolateral periaqueductal gray before the test swim. These results suggest that citalopram may affect c-Fos expression in the central nucleus of the amygdala and bed nucleus of the stria terminalis distinctively and raise the possibility that upregulation of c-Fos in the ventral lateral septum and ventrolateral periaqueductal gray before the test swim may be one of the probable common mechanisms underlying antidepressant effect in the FST.
Keywords: Antidepressant, Forced swimming test, Fos, Lateral septum
INTRODUCTION
Forced swimming test (FST) is composed of a 15-min pretest swim and a 5-min test swim 24 h later [1], and rodents subjected to the pretest swim exhibit increased immobility in the subsequent test swim. Treatment with antidepressant, known to exert their effects by blocking the reuptake of serotonin and/or norepinephrine at nerve terminals [2], after the pretest swim reduces immobility during the test swim. Because of these reasons, FST has been commonly used for screening antidepressant efficacy [3] and studying mechanisms of antidepressant actions [4]. However, at present, much is not known about the neuronal circuits selectively recruited by antidepressant.
One of the possible markers to investigate the neuronal circuits involved with antidepressant effect in the FST may be c-Fos. Since c-Fos is an immediate early gene and rapidly induced by external stimuli in relevant brain areas, c-Fos has been widely used as a marker for neuronal activation and to explore the neuronal circuits affected by external stimuli. Growing evidence has been shown that forced swimming or antidepressant treatment elicits c-Fos expression in brain regions implicated with depression, including the medial prefrontal cortex, nucleus accumbens, hippocampus, lateral septum, bed nucleus of the stria terminalis, hypothalamic paraventricular nucleus, central nucleus of the amygdala, raphe nucleus, locus ceruleus, and periaqueductal gray [5-7]. In addition, antidepressant treatment blunts the forced swimming induced expression of c-Fos in some brain areas [5,8].
Interestingly, these studies usually assessed the antidepressant effect on the changes in c-Fos expression after the test swim of FST. Accordingly, there is paucity of studies delineating the alterations in c-Fos expression by antidepressant before test swim. Considering that c-Fos acts as a transcription factor and could be involved in neuroplastic changes, it is plausible that changes in c-Fos expression before the test swim of FST are more likely related to antidepressant effect observed in the FST. Moreover, it is unclear whether antidepressants with different mechanisms of action elicit c-Fos expression in a common or distinct neural circuits. To answer this question, we injected desipramine or citalopram 0.5 h, 19 h, and 23 h after the pretest swim and observed changes in c-Fos expression in rat brain before the test swim, namely 24 h after the pretest swim. Desipramine and citalopram are reported as highly selective reuptake inhibitors of norepinephrine and serotonin, respectively [9].
METHODS
Animals
Male Sprague-Dawley rats (weight 280~300 g, Orient, Seoul, Korea) were adapted to experimental condition for a week. Rats were housed two per cage under standard conditions at 21~22℃, with a 12-h light/dark cycle (lights on at 6:00 am), and food and water were given ad libitum. Rats were daily handled for 2~3 min before experiment to reduce nonspecific stress. All the procedures used in this study were consistent with the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council 2003).
Forced swim test (FST)
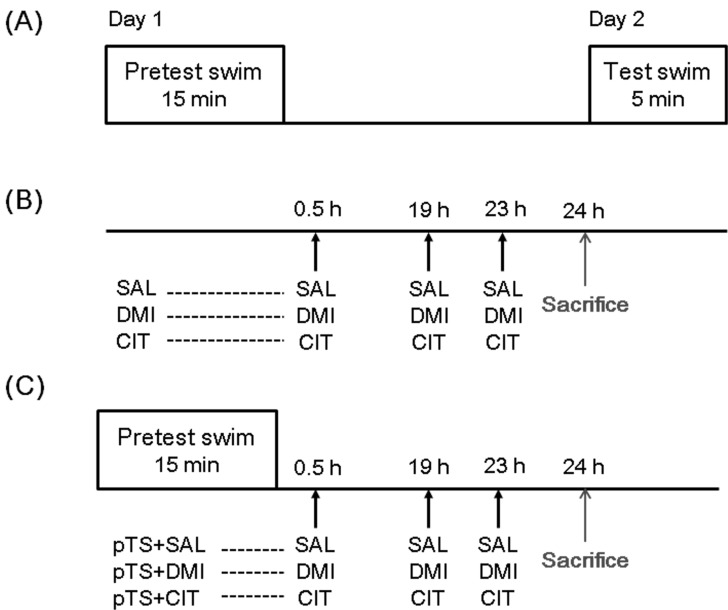
The FST procedure is composed of two forced swimming sessions as previously described [10,11]. In this experiment, rats were separately placed in a clear Plexiglas cylinder (25 cm diameter by 65 cm height containing 30 cm of water at 25℃) for 15 min (pretest). They were then removed and allowed to dry in a separate cage before returning to their home cages (Fig. 1).
Fig. 1.
Schematic representation of the experimental procedures and groups. (A) the forced swimming test (FST) process consists of two sessions: a pretest swim (pTS, 15 min) and a test swim (5 min) 24 h later. (B) one set of rats was injected with 0.9% saline (SAL, 1 ml/kg, i.p.), desipramine (DMI, 15 mg/kg/ml, i.p.), or citalopram (CIT, 10 mg/kg/ml, i.p.) according to the antidepressant treatments schedule (0.5 h, 19 h, and 23 h after the pretest swim), but not exposed to the pretest swim. (C) another set of rats was treated with saline, desipramine or citalopram after the pretest swim according to the antidepressant treatments schedule. Behaviorally naïve rats not exposed to the forced swimming and antidepressant served as baseline controls (CON) for each set. All rats were sacrificed 1 h after the last antidepressant or saline injection. Abbreviations used: SAL, saline-treated rats not exposed to the pretest swim; DMI, desipramine-treated rats not exposed to the pretest swim; CIT, citalopram-treated rats not exposed to the pretest swim; pTS+SAL, pretest swim-exposed, saline-treated rats; pTS+DMI, pretest swim-exposed, desipramine-treated rats; pTS+CIT, pretest swim-exposed, citalopram-treated rats.
Drug administration paradigm
Desipramine (Tocris; 15 mg/kg/ml, i.p.) or citalopram (kindly donated by H. Lundbeck A/S, Copenhagen, Denmark; 10 mg/kg/ml, i.p.) was administered at 0.5 h, 19 h, and 23 h after the pretest swim [4,11,12]. The doses of antidepressant in the present experiment have been shown to be effective in reducing the immobility during the test swim of FST [11,13]. Vehicle control rats were administered saline (1 ml/kg, i.p.).
Tissue preparation and Fos immunohistochemistry
At the end of the forced-swim test, rats were given an overdose of sodium pentobarbital (100 mg/kg, i.p.) and perfused intracardially with 0.9% saline, followed by 4% paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.2 (PPB) and stored in 20% sucrose. Serial coronal sections (30 µm) were prepared by using a freezing microtome (Microm International GmbH, Walldorf, Germany), and stored in a cryoprotectant [30% RNase free sucrose, 30% ethylene glycol, and 1% polyvinylpyrrolidone (PVP-40) in 100 mM PPB, pH 7.4] at -20℃ until required. Sections were first incubated in rabbit anti-c-Fos (Ab-5, 1:10,000, Calbiochem, La Jolla, CA) for 48 h at 4℃. After a thorough rinse in PBS, sections were rinsed again and were incubated with biotinylated goat anti-rabbit IgG (1:500, Vector Laboratories, Burlingame, CA, USA) for 2 h at room temperature. After several rinses, sections were incubated in avidin-biotin-peroxidase complex (1:250, Vector Laboratories, USA) for 1 h at room temperature. Moreover, sections were reacted in diaminobenzidine solution containing 0.3% H2O2. Sections were then mounted, dried, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA, USA). Images of brain sections were captured by a DP72 camera (Olympus Optical, Tokyo, Japan) mounted on an Olympus BX-51 microscope (Olympus Optical, Tokyo, Japan).
Data analysis
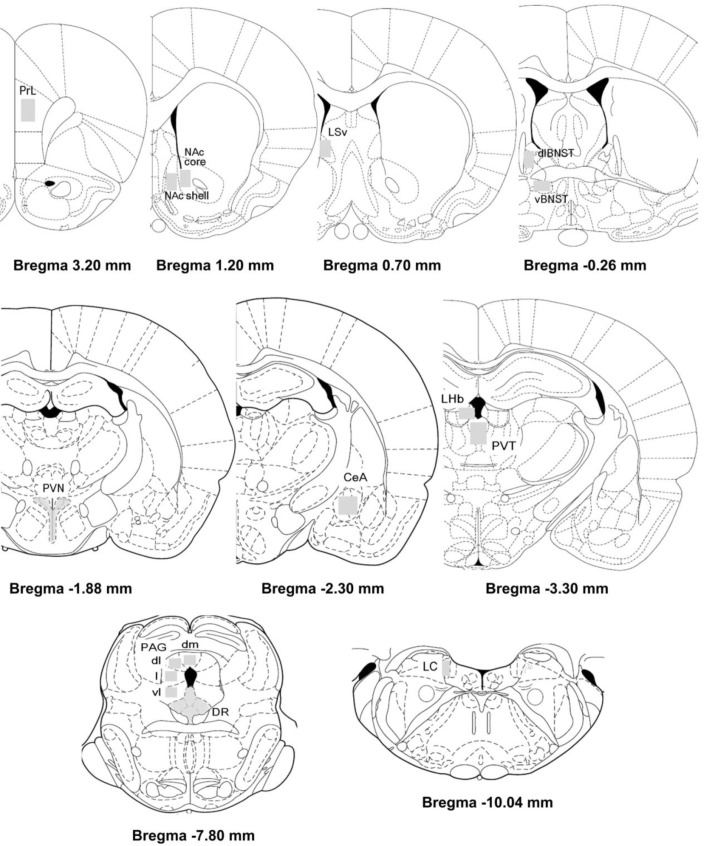
Fos-immunolabeled profiles from each brain region were quantified bilaterally from at least two sections per rat and averaged [14]. Regions of interest were selected according to the stereotaxic atlas of Paxinos and Watson [15] (Paxinos & Watson 1998) (Fig. 2). The mean densities in each brain area of interest were measured with known standard value using the Image J analysis program (version 1.43q, NIH, USA) by constructing a third degree polynomial calibration curve. By using the Image J analysis program, the number of c-Fos-like immunoreactive was counted according to two criteria, threshold grey values and a limited cellular diameter [16].
Fig. 2.
Schematic diagrams adapted from the Paxinos and Watson atlas showing selected brain areas in which c-Fos-like immunoreactive cells were counted. PrL, prelimbic cortex (3.20~2.70 mm from bregma, 25×104 µm2); NAc core, nucleus accumbens core (1.70~1.00 mm from bregma, 10×104 µm2); NAc shell, nucleus accumbens shell (1.70~1.00 mm from bregma, 10×104 µm2); LSv, ventral lateral septal nucleus (1.00~0.70 mm from bregma, 10×104 µm2); dlBNST, dorsolateral bed nucleus of the stria terminalis (-0.26~-0.30 mm from bregma, 4×104 µm2); vBNST, ventral bed nucleus of the stria terminalis (-0.26~-0.30 mm from bregma, 12.5×104 µm2); PVN, hypothalamic paraventricular nucleus (-1.80~-1.88 mm from bregma); CeA, central nucleus of the amygdala (-1.88~-2.80 mm from bregma, 6×104 µm2); PVT, paraventricular thalamic nucleus (-3.30~-4.16 mm from bregma); LHb, lateral habenula (-3.30~-4.16 mm from bregma); PAG [periaqueductal gray (dm, dorsomedial; dl, dorsolateral; l, lateral; vl, ventrolateral)] (-6.30~-8.00 mm from bregma, 1×104 µm2); DR, dorsal raphe (-7.30~-8.00 mm from bregma); LC, locus ceruleus (-9.68~-10.04 mm from bregma).
Statistical analyses
Statistical significance of data with equal variances was assessed by one-way analyses of variance (ANOVAs) followed by post hoc Fisher's least significant difference (LSD) test and significance was accepted for p-values less than 0.05. Statistical significance of data with unequal variances was assessed by nonparametric Kruskal-Wallis test and post hoc Mann-Whitney U test; significant p-values denote comparisons surviving Bonferroni correction (*p<0.05/6=0.00833; ***p<0.001/6=0.00016). The quantitative data are reported as means±the standard errors of the means (S.E.M.).
RESULTS
Effects of antidepressants on the pretest swim-induced changes in c-Fos expression in the ventral lateral septum
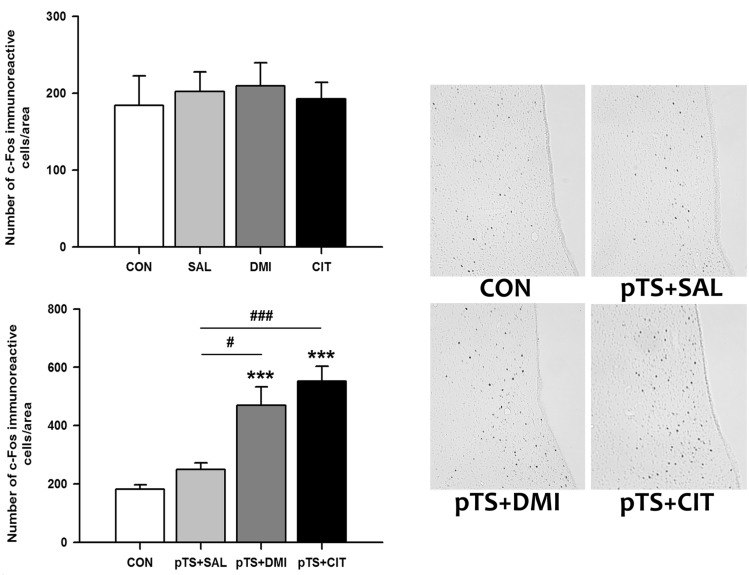
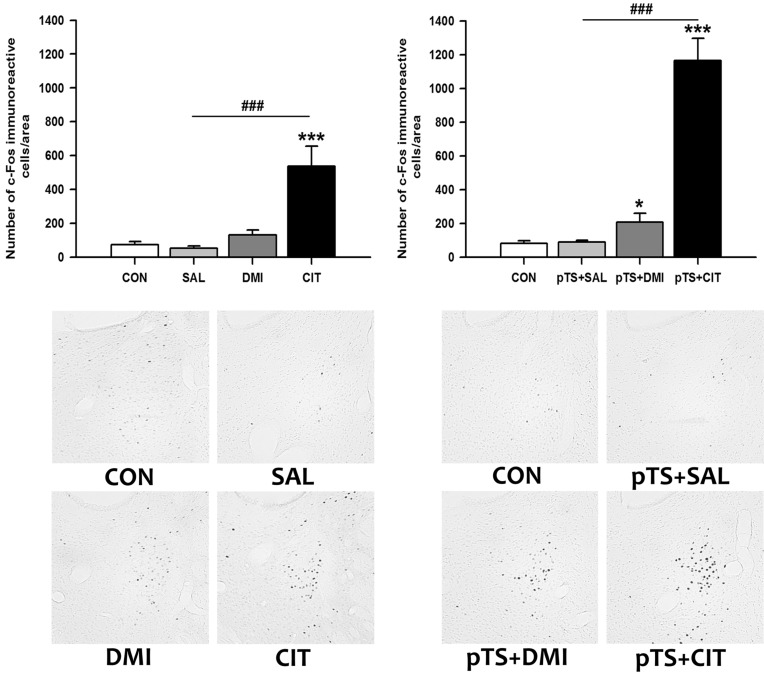
Treatment with either desipramine or citalopram alone in the absence of pretest swim did not alter the number of c-Fos-like immunoreactive cells in the ventral lateral septum compared with naïve and vehicle control not previously exposed to pretest swim (Fig. 3, upper left). Both desipramine (+158% vs. CON) and citalopram treatment (+204.3% vs. CON) after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells before the test swim, namely 24 h after the pretest swim, compared with naïve control and vehicle control (Fig. 3, lower left). There was a tendency towards an increase in the number of c-Fos-like immunoreactive cells in vehicle control (+37.8% vs. CON), but it did not reach a significance (Fig. 3, lower left).
Fig. 3.
Effects of antidepressant treatments on the number of c-Fos-like immunoreactive cells in the ventral lateral septum in the FST. Saline, desipramine or citalopram was administered at 0.5 h, 19 h, and 23 h after the pretest swim as depicted in Fig. 1. Rats were killed 1 h after the last injection of antidepressant or saline. Behaviorally naïve rats not exposed to forced swimming or antidepressants served as baseline controls (CON). The results with antidepressant treatments obtained under basal conditions without the pretest swim (upper left) and after the pretest swim (lower left) are presented in the bar graphs. The results are presented as the means±standard error of the means (S.E.M). Significant differences among groups were determined by the nonparametric Kruskal-Wallis ANOVA followed by post hoc Mann-Whitney U test with Bonferroni correction (***p<0.00016 vs. CON; #p<0.00833 and ###p<0.00016 vs. pTS+SAL). Representative images at ×100 magnification show c-Fos-like immunoreactivity in the ventral lateral septum from rats with antidepressant or saline treatments after the pretest swim.
Effects of antidepressants on the pretest swim-induced changes in c-Fos expression in the central nucleus of the amygdala
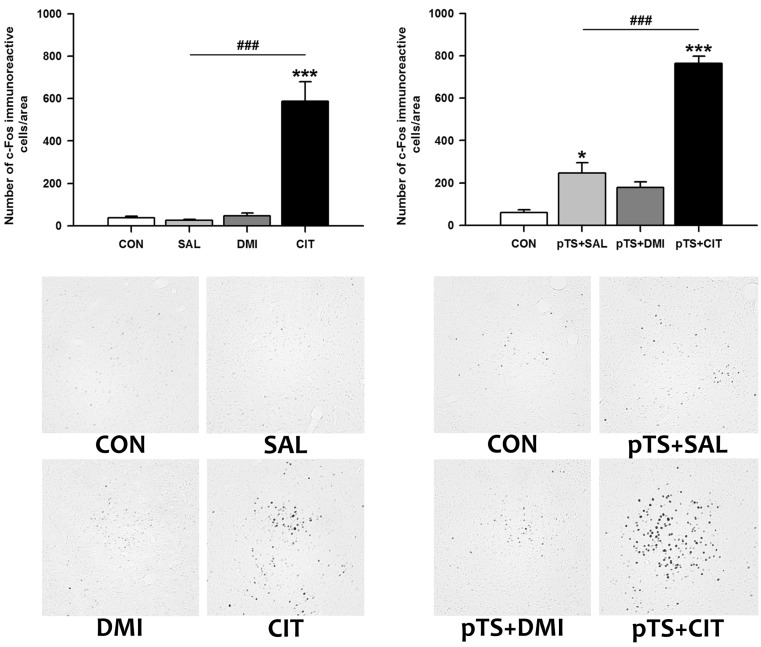
Citalopram treatment alone in the absence of pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala (+1,469.4% vs. CON), while treatment with either saline or desipramine alone had no effect on the number of c-Fos-like immunoreactive cells (Fig. 4, upper left). Citalopram treatment after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells (+1,170.7% vs. CON) before the test swim. This increase was also observed after the treatment with either saline (+310.8% vs. CON) or desipramine (+195.8% vs. CON), although the magnitude of increase is profoundly smaller than that induced by citalopram (Fig. 4, lower left).
Fig. 4.
Effects of antidepressant treatments on the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala during the FST. Experimental groups, animal numbers per group, and all the procedures are the same as described in Fig. 1. The results obtained with antidepressant or saline treatments alone (upper left) or in combination with prior exposure to pretest swim (upper right) are presented as bar graphs. Significant differences among groups were determined by the nonparametric Kruskal-Wallis ANOVA followed by post hoc Mann-Whitney U test with Bonferroni correction (*p<0.00833 and ***p<0.00016 vs. CON; ###p< 0.00016 vs. pTS+SAL). Representative images at ×100 magnification show c-Fos-like immunoreactivity in the central nucleus of the amygdala from rats with antidepressant or saline treatments alone (lower left) or in combination with prior exposure to pretest swim (lower right). Abbreviations used are the same as in Fig. 1.
Effects of antidepressants on the pretest swim-induced changes in c-Fos expression in the dorsal bed nucleus of the stria terminalis
Citalopram treatment alone in the absence of pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the dorsal bed nucleus of the stria terminalis (+640.8% vs. CON), while treatment with either saline or desipramine alone had no significant effect on the number of c-Fos-like immunoreactive cells (Fig. 5, upper left). Citalopram after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells before the test swim (+1,350.0% vs. CON) compared with naïve and vehicle control (Fig. 5, upper right). The increase was also observed after desipramine treatment (+157.4% vs. CON), but the magnitude of increase is profoundly smaller than that induced by citalopram (Fig. 5, upper right).
Fig. 5.
Effects of antidepressant treatments on the number of c-Fos-like immunoreactive cells in the dorsolateral bed nucleus of stria terminalis during the FST. Experimental groups, animal numbers per group, and all the procedures are the same as described in Fig. 1. The results obtained with antidepressant or saline treatments alone (upper left) or in combination with prior exposure to pretest swim (upper right) are presented as bar graphs. Significant differences among groups were determined by the nonparametric Kruskal-Wallis ANOVA followed by post hoc Mann-Whitney U test with Bonferroni correction (*p<0.00833 and ***p<0.00016 vs. CON; ###p<0.00016 vs. pTS+SAL). Representative images at ×100 magnification show c-Fos-like immunoreactivity in the dorsolateral bed nucleus of stria terminalis from rats with antidepressant or saline treatments alone (lower left) or in combination with prior exposure to pretest swim (lower right). Abbreviations used are the same as in Fig. 1.
Effects of antidepressants on the pretest swim-induced changes in c-Fos expression in the ventrolateral periaqueductal gray
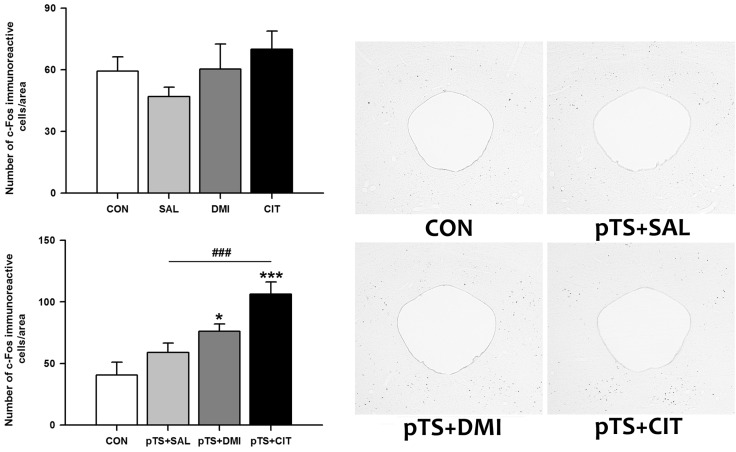
Antidepressant or saline treatment alone in the absence of pretest swim had no significant effect on the number of c-Fos-like immunoreactive cells in the ventrolateral periaqueductal gray (Fig. 6, upper left). Both desipramine (+36.1% vs. CON) and citalopram treatment (+85.7% vs. CON) after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells before the test swim compared with naïve and vehicle control (Fig. 6, lower left).
Fig. 6.
Effects of antidepressant treatments on the number of c-Fos-like immunoreactive cells in the ventrolateral periaqueductal gray during the FST. Experimental groups, animal numbers per group, and all the procedures are the same as described in Fig. 1. The results obtained with antidepressant or saline treatments alone (left upper) or in combination with prior exposure to pretest swim (right upper) are presented as bar graphs. Statistical significance of data with equal variances was assessed by one-way analyses of variance (ANOVAs) followed by post hoc Fisher's least significant difference (LSD) test and significance was accepted for p-values less than 0.05 (*p<0.05 and ***p<0.001 vs. CON; ###p< 0.001 vs. pTS+SAL). Representative images at ×40 magnification show c-Fos-like immunoreactivity in the ventrolateral periaqueductal gray from rats with antidepressant or saline treatments after the pretest swim (right). Abbreviations used are the same as in Fig. 1.
Effects of antidepressants on the pretest swim-induced changes in c-Fos expression in the prelimbic area, nucleus accumbens, and ventral bed nucleus of the stria terminalis
Without an exposure to the pretest swim, there was only a nonsignificant tendency towards an increase in the number of c-Fos-like immunoreactive cells after the treatment with either saline, desipramine, or citalopram in the prelimbic area, nucleus accumbens core and shell, as well as ventral bed nucleus of the stria terminalis, except for the group with citalopram treatment in the ventral bed nucleus of the stria terminalis in which a significant increase was noted (Table 1). In contrast, treatment with either saline, desipramine or citalopram after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the prelimbic area, nucleus accumbens core and shell, as well as ventral bed nucleus of the stria terminalis before the test swim (Table 1).
Table 1.
Effects of antidepressant treatments on the pretest swim-induced changes in c-Fos expression in the prelimbic cortex, nucleus accumbens core, nucleus accumbens shell, and ventral bed nucleus of the stria terminalis
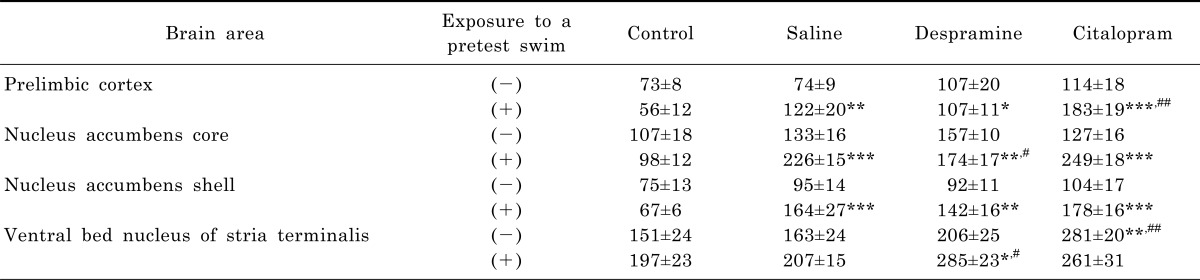
Statistical significance of data with equal variances was assessed by one-way analyses of variance (ANOVAs) followed by post hoc Fisher's least significant difference (LSD) test and significance was accepted for p-values less than 0.05 (*p<0.05, **p<0.01, and ***p<0.001 vs. CON; #p<0.05 and ##p<0.01 vs. pTS+SAL).
DISCUSSION
The results of the present study demonstrate that citalopram treatment alone in the absence of the pretest swim significantly increases the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala and bed nucleus of the stria terminalis, where this pattern of increase appears to be maintained after the pretest swim. Both desipramine and citalopram treatment after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the ventral lateral septum and ventrolateral periaqueductal gray before the test swim. These results suggest that citalopram may affect c-Fos expression in the central nucleus of the amygdala and bed nucleus of the stria terminalis distinctively and raise the possibility that upregulation of c-Fos expression in the ventral lateral septum and ventrolateral periaqueductal gray before the test swim may be one of the probable common mechanisms underlying antidepressant effect in the FST.
Lateral septum
Although there was only a tendency towards an increase in the number of c-Fos-like immunoreactive cells in the ventral lateral septum before the test swim, namely 24 h after the pretest swim, in the present study, another shows that c-Fos expression is increased in the lateral septum 2 h after the test swim of FST [5]. Moreover, desipramine or citalopram treatment alone had no effect on the number of c-Fos immunoreactive cells in the ventral lateral septum, but this result is in contrast to previous finding that acute treatment with antidepressants (fluoxetine, imipramine, and mirtazapine) significantly decreases c-fos mRNA expression within the septum in non-stressed rats [17]. The discrepancy from previous studies is likely to reflect the difference in the time elapsed after the swimming (24 h after the pretest swim in our study vs. 2 h after the test swim), a number of antidepressant treatment before observation (three times injection in our study vs. a single injection), or analytical detection method (protein vs. mRNA). In addition, the tendency towards an increase in the number of c-Fos-like immunoreactive cells 24 h after the pretest swim was significantly potentiated by desipramine and citalopram treatment.
Previous studies point to a key role for the lateral septum in modulating stress coping behaviour [18-20]. For example, bilateral lesions of lateral septum by ibotenic acid in rats show an increase in floating and decrease in struggling/swimming behavior in the FST indicative of a more passive coping behavior [19]. Moreover, c-Fos expression in the lateral septum is significantly reduced in rats showing a behavioral despair in learned helplessness paradigm, an animal model of depression [20]. In this context, and based on the ability of desipramine and citalopram to induce c-Fos expression in the lateral septum, it is conceivable that elevated c-Fos expression in the ventral lateral septum could be involved in the active coping behavior such as swimming and climbing in the FST, an effect commonly observed by antidepressant. The potential beneficial role for increased c-Fos expression in the lateral septum in stress coping behavior is consistent with the previous finding that treatment with cannabinoid receptor 1 (CB1) antagonist (SR141716) prior to acute restraint stress produces a significant increase in c-Fos expression in the lateral septum which appears to be in parallel with an enhancement of escape behavior during the restraint stress [18]. Interestingly, SR141716 is also known to increase active behavior in the mouse FST [21]. Considering together, these results suggest that the ventral lateral septum may be a neural substrate for antidepressant effect in the FST and elevated c-Fos expression in the ventral lateral septum could facilitate active coping behavior in the FST.
Central nucleus of the amygdala
In the present study, citalopram treatment alone in the absence of pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala, while treatment with either saline or desipramine alone had no effect. The increase in the number of c-Fos-like immunoreactive cells after treatment with citalopram is in agreement with the previous study showing that fluoxetine and citalopram significantly increase c-Fos expression in the central nucleus of the amygdala [22,23]. In contrast to the lack of effect of desipramine treatment in the present study, single administration of desipramine significantly increases c-Fos expression in the central nucleus of the amygdala [22]. This discrepancy presumably relates to the difference in the number of desipramine administration. Saline treatment after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala, and similar increase in c-Fos expression 2 h after the test swim is reported in diverse brain regions including amygdala [7]. This result suggests that a 15-min pretest swim exerts a rather long-term influence on c-Fos expression in the central nucleus of the amygdala. Interestingly, although both desipramine and citalopram treatment increased the number of c-Fos-like immunoreactive cells in the central nucleus of the amygdala, it appears that each degree of increase by the treatment with desipramine, citalopram, or saline did not differ either in the presence of or in the absence of the pretest swim. These results suggest that antidepressant appears not to alter the degree of increase in c-Fos expression in response to the pretest swim.
It is well documented that the central nucleus of the amygdala is implicated in many aspects of fear and anxiety [24,25]. Therefore, it is possible that elevated c-Fos expression in the central nucleus of the amygdala may be involved, in part, with the anxiogenic effect of antidepressant, an adverse experience often occurs shortly after acute treatment with selective serotonin reuptake inhibitors [26-28]. This possibility is substantiated by the finding that c-Fos expression in the central nucleus of the amygdala and other brain areas is induced by several known anxiogenic drugs [29]. However, it should be pointed out that anxiogenic effect of selective serotonin reuptake inhibitors appears to be more related to c-Fos expression in the locus ceruleus [30] and also that c-Fos expression in the central nucleus of the amygdala could be induced by other psychotropic drugs such as diazepam, haloperidol, and clozapine [23]. These results suggest that c-Fos induction in the central nucleus of the amygdala may not be specific for the selective serotonin reuptake inhibitors, and anxiogenic effect by selective serotonin reuptake inhibitors could be attributed to the elevated c-Fos induction in the locus ceruleus. In addition, given that desipramine and citalopram did not alter the degree of increase in the pretest swim-induced c-Fos expression in the central nucleus of the amygdala, these results suggest that increased c-Fos expression in the central nucleus of the amygdala before the test swim may not play an important role in the antidepressant effect during the test swim.
Bed nucleus of the stria terminalis
In the present study, citalopram treatment alone in the absence of pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the dorsal bed nucleus of the stria terminalis, but desipramine treatment was without effect. Both desipramine and citalopram treatment after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells, but the magnitude of increase by citalopram is far greater than that by desipramine. The increase in c-Fos expression after citalopram treatments is in agreement with previous studies showing that different types of selective serotonin reuptake inhibitors such as fluvoxamine [31], citalopram [32,33], fluoxetine [34] significantly increase c-Fos expression in the dorsal bed nucleus of the stria terminalis. Similar absence of changes in c-Fos expression in the dorsal bed nucleus of the stria terminalis for desipramine was reported previously [23]. However, no significant changes in the number of c-Fos-like immunoreactive cells after the pretest swim are inconsistent with the results of other studies in which forced swimming significantly increases c-Fos expression in the dorsal bed nucleus of the stria terminalis [7,35]. This discrepancy could be explained by the time elapsed after the forced swimming, since the forced swimming-induced increase in c-Fos expression is usually observed ~2 h after the forced swimming [7,35], but not 24 h after the pretest swim as in the present study.
It is well known that the bed nucleus of the stria terminalis is involved with the expression of fear, stress, and anxiety [36]. Moreover, bed nucleus of the stria terminalis is supposed to play an important role in the FST, because transient inactivation of bed nucleus of the stria terminalis by microinjection of cobalt chloride shows antidepressant-like effect in the FST [37]. Considering that both desipramine and citalopram significantly increased c-Fos expression in the dorsal bed nucleus of the stria terminalis, although the degree of increase is different, elevated number of c-Fos-like immunoreactive cells in the dorsal bed nucleus of the stria terminalis before the test swim could exert a beneficial effect during the test swim of FST. However, recent study showed that fluoxetine and venlafaxine treatments, similar to our antidepressant injection schedule, significantly blunt the increase in c-Fos expression in the bed nucleus of the stria terminalis 2 h after the test swim [7]. Although differences in the time of sacrifice after forced swimming (before or after the test swim) and antidepressants used could explain the discrepant results, it is not clear at present what the role of c-Fos in the bed nucleus of the stria terminalis in the FST. Additional work will be necessary to identify the role of c-Fos in the bed nucleus of the stria terminalis.
Periaqueductal gray
The PAG is known to be a brain area that initiates active or passive emotional coping behavior depending on the type of stressor (escapable or inescapable) [38]. Distinct longitudinal portions of neurons (dorsomedial, dorsolateral, lateral, ventrolateral) in the PAG are engaged in differential emotional coping strategy, and growing evidence indicates that dorsolateral/lateral and ventrolateral PAG are involved in active and passive coping behavior, respectively [38,39]. Since FST is a type of stress elicited by an exposure to inescapable environment and immobility observed in the FST is regarded either as the development of passive coping behavior or a failure of escape-directed behavior [3], PAG is supposedly involved in immobility behavior in the FST [40,41]. In the current study, saline treatment after the pretest swim showed a nonsignificant increase in the number of c-Fos immunoreactive cells in the ventrolateral PAG, but not in other portions of PAG, while previous studies have shown that forced swimming induces c-Fos expression in the most portions of PAG [41,42]. The discrepancy from previous studies may reflect the difference in the time point of observation in that we observed changes in c-Fos expression 24 h after the pretest swim, not 2 h after forced swimming reported in previous studies [41,42]. Notably, the tendency towards an increase in c-Fos expression in the ventrolateral PAG after the pretest swim was significantly enhanced by desipramine and citalopram treatment, although treatment with desipramine or citalopram alone was without effect. In contrast to our results, antidepressant treatments over 24 h after the pretest swim significantly ameliorate the swimming-induced increase in c-Fos expression in the PAG [41]. This disparity may be also attributed to the difference in the time point of observation, as noted above [41].
Given that ventrolateral PAG is closely linked to passive emotional coping behavior in response to inescapable stressor such as FST, it is likely that elevated c-Fos expression in the ventraolateral PAG by desipramine and citalopram would facilitate passive emotional coping behavior such as immobility. In parallel with this proposition, previous study showed that neuronal activation in the dorsal PAG by local administration of glutamate increases the latency to immobility, an effect similar to antidepressant [40]. However, same author [41] demonstrated that c-Fos expression particularly in the dorsolateral PAG is positively correlated with the immobility during the test swim, suggesting that neuronal activation in the dorsolateral PAG may promote passive coping behavior. This result raises the possibility that anatomical classification of PAG into the dorsolateral and ventrolateral portion does not necessarily imply increased propensity to adopt active and passive emotional coping behavior, respectively. In this regard, it is noteworthy that repeated inescapable stress in mice significantly increased ΔFosB in the ventrolateral PAG and this increase is inversely correlated with the degree of escape deficit after inescapable stress [43]. Similar increase in ΔFosB in the ventrolateral PAG is observed after repeated social defeat paradigm, an animal model sensitive to antidepressant treatment and there is also a negative relationship between the level of ΔFosB and the individual degree of escape deficit [43]. Taken together, although the role of increased c-Fos expression in the ventrolateral PAG by desipramine and citalopram is not clear at present, these results suggest a preferential involvement of ventrolateral PAG in the neuroplastic changes and antidepressant effect before the test swim, and also raise the possibility that the increase in c-Fos expression in the ventrolateral PAG could contribute, in part, to antidepressant-mediated reduction in immobility in the FST, as demonstrated by the role of induction of ΔFosB in the ventrolateral PAG for the development of resilience to inescapable stress. Further research will be necessary to elucidate the role of c-Fos induction in the ventrolateral PAG.
Other brain areas (prelimbic area, nucleus accumbens core and shell, and ventral bed nucleus of the stria terminalis)
Treatment with either saline, desipramine or citalopram after the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the prelimbic area, nucleus accumbens core and shell, as well as ventral bed nucleus of the stria terminalis before the test swim, but only citalopram treatment alone in the absence of the pretest swim significantly increased the number of c-Fos-like immunoreactive cells in the ventral bed nucleus of the stria terminalis. These results suggest that these brain areas could be activated in response to forced swimming, and a trend towards an increase is rather long-lasting until before the test swim. However, antidepressant treatment significantly alters the pretest swim-induced changes in c-Fos expression, although there are some significant differences among groups. These results raise the possibility that prelimbic area, nucleus accumbens and ventral bed nucleus of the stria terminalis may not play an important role in antidepressant action in the FST.
The results of the present study demonstrate that citalopram treatment alone in the absence of the pretest swim distinctively increased c-Fos expression in the central nucleus of the amygdala and bed nucleus of the stria terminalis. Although both desipramine and citalopram treatment per se had no significant effect on c-Fos expression in the ventral lateral septum and ventrolateral periaqueductal gray, both antidepressant treatment after the pretest swim significantly increased c-Fos expression in these brain areas before the test swim. Considering together, the present results suggest that citalopram may affect c-Fos expression in the central nucleus of the amygdala and bed nucleus of the stria terminalis distinctively and raise the possibility that upregulation of c-Fos expression in the ventral lateral septum and ventrolateral periaqueductal gray before the test swim may be one of the probable common mechanisms underlying antidepressant effect in the FST.
ACKNOWLEDGEMENTS
This study was supported by grants from the National Research Foundation of Korea (NRF-2010-0024048) and Korea University (K0823171).
ABBREVIATIONS
- FST
forced swimming test
- DMI
desipramine
- CIT
citalopram
- PrL
prelimbic cortex
- NAc core
nucleus accumbens core
- NAc shell
nucleus accumbens shell
- LSv
ventral lateral septal nucleus
- dlBNST
dorsolateral bed nucleus of the stria terminalis
- vBNST
ventral bed nucleus of the stria terminalis
- PVN
hypothalamic paraventricular nucleus
- CeA
central nucleus of the amygdala
- PVT
paraventricular thalamic nucleus
- LHb
lateral habenula
- PAG
periaqueductal gray
- DR
dorsal raphe
- LC
locus ceruleus
References
- 1.Detke MJ, Lucki I. Detection of serotonergic and noradrenergic antidepressants in the rat forced swimming test: the effects of water depth. Behav Brain Res. 1996;73:43–46. doi: 10.1016/0166-4328(96)00067-8. [DOI] [PubMed] [Google Scholar]
- 2.Richelson E. Synaptic effects of antidepressants. J Clin Psychopharmacol. 1996;16(3 Suppl 2):1S–7S. doi: 10.1097/00004714-199606002-00001. [DOI] [PubMed] [Google Scholar]
- 3.Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23:238–245. doi: 10.1016/s0165-6147(02)02017-5. [DOI] [PubMed] [Google Scholar]
- 4.Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Rev. 2005;29:547–569. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Duncan GE, Knapp DJ, Johnson KB, Breese GR. Functional classification of antidepressants based on antagonism of swim stress-induced fos-like immunoreactivity. J Pharmacol Exp Ther. 1996;277:1076–1089. [PubMed] [Google Scholar]
- 6.Kawahara R, Soeda F, Kawaura K, Honda S, Miki R, Noguchi T, Shirasaki T, Takahama K. Effect of tipepidine with novel antidepressant-like action on c-fos-like protein expression in rat brain. Brain Res. 2013;1513:135–142. doi: 10.1016/j.brainres.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 7.Silva M, Aguiar DC, Diniz CR, Guimarães FS, Joca SR. Neuronal NOS inhibitor and conventional antidepressant drugs attenuate stress-induced fos expression in overlapping brain regions. Cell Mol Neurobiol. 2012;32:443–453. doi: 10.1007/s10571-011-9775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jama A, Cecchi M, Calvo N, Watson SJ, Akil H. Interindividual differences in novelty-seeking behavior in rats predict differential responses to desipramine in the forced swim test. Psychopharmacology (Berl) 2008;198:333–340. doi: 10.1007/s00213-008-1126-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Owens MJ, Morgan WN, Plott SJ, Nemeroff CB. Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther. 1997;283:1305–1322. [PubMed] [Google Scholar]
- 10.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 11.Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- 12.Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi: 10.1016/0014-2999(78)90118-8. [DOI] [PubMed] [Google Scholar]
- 13.Kang S, Kim HJ, Kim HJ, Shin SK, Choi SH, Lee MS, Shin KH. Effects of reboxetine and citalopram pretreatment on changes in cocaine and amphetamine regulated transcript (CART) expression in rat brain induced by the forced swimming test. Eur J Pharmacol. 2010;647:110–116. doi: 10.1016/j.ejphar.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 14.Briand LA, Vassoler FM, Pierce RC, Valentino RJ, Blendy JA. Ventral tegmental afferents in stress-induced reinstatement: the role of cAMP response element-binding protein. J Neurosci. 2010;30:16149–16159. doi: 10.1523/JNEUROSCI.2827-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paxinos G, Watson C. The rat brain in stereotaxic coordinates (Deluxe Edition). Fourth Edition. 4th ed. San Diego: Academic Press; 1998. [Google Scholar]
- 16.Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JM. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- 17.Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B. Comparison of alterations in c-fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology. 2005;30:1278–1287. doi: 10.1038/sj.npp.1300717. [DOI] [PubMed] [Google Scholar]
- 18.Patel S, Roelke CT, Rademacher DJ, Hillard CJ. Inhibition of restraint stress-induced neural and behavioural activation by endogenous cannabinoid signalling. Eur J Neurosci. 2005;21:1057–1069. doi: 10.1111/j.1460-9568.2005.03916.x. [DOI] [PubMed] [Google Scholar]
- 19.Singewald GM, Rjabokon A, Singewald N, Ebner K. The modulatory role of the lateral septum on neuroendocrine and behavioral stress responses. Neuropsychopharmacology. 2011;36:793–804. doi: 10.1038/npp.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steciuk M, Kram M, Kramer GL, Petty F. Decrease in stress-induced c-Fos-like immunoreactivity in the lateral septal nucleus of learned helpless rats. Brain Res. 1999;822:256–259. doi: 10.1016/s0006-8993(99)01134-8. [DOI] [PubMed] [Google Scholar]
- 21.Tzavara ET, Davis RJ, Perry KW, Li X, Salhoff C, Bymaster FP, Witkin JM, Nomikos GG. The CB1 receptor antagonist SR141716A selectively increases monoaminergic neurotransmission in the medial prefrontal cortex: implications for therapeutic actions. Br J Pharmacol. 2003;138:544–553. doi: 10.1038/sj.bjp.0705100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beck CH. Acute treatment with antidepressant drugs selectively increases the expression of c-fos in the rat brain. J Psychiatry Neurosci. 1995;20:25–32. [PMC free article] [PubMed] [Google Scholar]
- 23.Sumner BE, Cruise LA, Slattery DA, Hill DR, Shahid M, Henry B. Testing the validity of c-fos expression profiling to aid the therapeutic classification of psychoactive drugs. Psychopharmacology (Berl) 2004;171:306–321. doi: 10.1007/s00213-003-1579-7. [DOI] [PubMed] [Google Scholar]
- 24.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.LeDoux J. The amygdala. Curr Biol. 2007;17:R868–R874. doi: 10.1016/j.cub.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 26.Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- 27.Belzung C, Le Guisquet AM, Barreau S, Calatayud F. An investigation of the mechanisms responsible for acute fluoxetine-induced anxiogenic-like effects in mice. Behav Pharmacol. 2001;12:151–162. doi: 10.1097/00008877-200105000-00001. [DOI] [PubMed] [Google Scholar]
- 28.Kurt M, Arik AC, Celik S. The effects of sertraline and fluoxetine on anxiety in the elevated plus-maze test in mice. J Basic Clin Physiol Pharmacol. 2000;11:173–180. doi: 10.1515/jbcpp.2000.11.2.173. [DOI] [PubMed] [Google Scholar]
- 29.Singewald N, Salchner P, Sharp T. Induction of c-Fos expression in specific areas of the fear circuitry in rat forebrain by anxiogenic drugs. Biol Psychiatry. 2003;53:275–283. doi: 10.1016/s0006-3223(02)01574-3. [DOI] [PubMed] [Google Scholar]
- 30.Salchner P, Singewald N. Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology. 2002;43:1238–1248. doi: 10.1016/s0028-3908(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 31.Veening JG, Coolen LM, Spooren WJ, Joosten H, van Oorschot R, Mos J, Ronken E, Olivier B. Patterns of c-fos expression induced by fluvoxamine are different after acute vs chronic oral administration. Eur Neuropsychopharmacol. 1998;8:213–226. doi: 10.1016/s0924-977x(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 32.Thomsen C, Helboe L. Regional pattern of binding and c-Fos induction by (R)- and (S)-citalopram in rat brain. Neuroreport. 2003;14:2411–2414. doi: 10.1097/00001756-200312190-00024. [DOI] [PubMed] [Google Scholar]
- 33.Morelli M, Pinna A, Ruiu S, Del Zompo M. Induction of Fos-like-immunoreactivity in the central extended amygdala by antidepressant drugs. Synapse. 1999;31:1–4. doi: 10.1002/(SICI)1098-2396(199901)31:1<1::AID-SYN1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 34.Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimarães FS. Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res Bull. 2001;55:747–754. doi: 10.1016/s0361-9230(01)00566-4. [DOI] [PubMed] [Google Scholar]
- 35.Muigg P, Hoelzl U, Palfrader K, Neumann I, Wigger A, Landgraf R, Singewald N. Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol Psychiatry. 2007;61:782–796. doi: 10.1016/j.biopsych.2006.08.035. [DOI] [PubMed] [Google Scholar]
- 36.Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 37.Crestani CC, Alves FH, Correa FM, Guimarães FS, Joca SR. Acute reversible inactivation of the bed nucleus of stria terminalis induces antidepressant-like effect in the rat forced swimming test. Behav Brain Funct. 2010;6:30. doi: 10.1186/1744-9081-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bandler R, Keay KA, Floyd N, Price J. Central circuits mediating patterned autonomic activity during active vs. passive emotional coping. Brain Res Bull. 2000;53:95–104. doi: 10.1016/s0361-9230(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 39.Keay KA, Bandler R. Parallel circuits mediating distinct emotional coping reactions to different types of stress. Neurosci Biobehav Rev. 2001;25:669–678. doi: 10.1016/s0149-7634(01)00049-5. [DOI] [PubMed] [Google Scholar]
- 40.Lino-de-Oliveira C, De Lima TC, Carobrez AP. Dorsal periaqueductal gray matter inhibits passive coping strategy elicited by forced swimming stress in rats. Neurosci Lett. 2002;335:87–90. doi: 10.1016/s0304-3940(02)01119-9. [DOI] [PubMed] [Google Scholar]
- 41.Lino-de-Oliveira C, de Oliveira RM, Pádua Carobrez A, de Lima TC, del Bel EA, Guimarães FS. Antidepressant treatment reduces Fos-like immunoreactivity induced by swim stress in different columns of the periaqueductal gray matter. Brain Res Bull. 2006;70:414–421. doi: 10.1016/j.brainresbull.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Bellchambers CE, Chieng B, Keay KA, Christie MJ. Swim-stress but not opioid withdrawal increases expression of c-fos immunoreactivity in rat periaqueductal gray neurons which project to the rostral ventromedial medulla. Neuroscience. 1998;83:517–524. doi: 10.1016/s0306-4522(97)00399-0. [DOI] [PubMed] [Google Scholar]
- 43.Berton O, Covington HE, 3rd, Ebner K, Tsankova NM, Carle TL, Ulery P, Bhonsle A, Barrot M, Krishnan V, Singewald GM, Singewald N, Birnbaum S, Neve RL, Nestler EJ. Induction of deltaFosB in the periaqueductal gray by stress promotes active coping responses. Neuron. 2007;55:289–300. doi: 10.1016/j.neuron.2007.06.033. [DOI] [PubMed] [Google Scholar]