Abstract
Agrocybe aegerita is an important mushroom cultivated in Korea, with good feel and a peculiar fragrance. A. aegerita can be cultivated throughout the year using culture bottles but is more susceptible to contamination than other mushrooms. Twenty-two pathogens were isolated from the fruiting bodies and compost of A. aegerita, and seven isolates were isolated from Pleurotus ostreatus to compare with the A. aegerita isolates, collected from Gimje, Iksan, Gunsan of Chonbuk, and Chilgok of Gyeongbuk Province in 2009. These isolates were identified based on morphological and molecular characteristics. Of the 29 isolates, 26 were identified as Trichoderma spp. and the remaining three were Aspergillus spp., Mucor spp., and Penicillium spp. A phylogenetic analysis revealed that the 26 isolates of Trichoderma were divided into four taxa, namely T. harzianum, T. pleuroticola, T. longibrachiatum, and T. atroviride. Among the Trichoderma spp., 16 isolates (55.2%) were identified as T. harzianum, six as T. pleuroticola (20.7%), two as T. longibrachiatum, and the remaining two were T. atroviride.
Keywords: Agrocybe aegerita, Phylogenetic analysis, rDNA internal transcribed spacer sequence, Trichoderma spp.
Higher fungi have been used by humans for millennia. First, they are used as part of a regular diet for their nutritional value, as they contain minerals, vitamins, and nutritive compounds such as proteins and polysaccharides and have a low fat content [1, 2]. Secondly, mushroom fruiting bodies are also appreciated as a delicacy. Indeed, their palatability is exploited as taste and flavor enhancers in food preparation and cooking [3]. Thirdly, higher fungi are used for medicinal purposes, and some species clearly demonstrate higher antioxidant properties than others [4, 5]. Agrocybe aegerita has a high antioxidant effect and free-radical scavenging ability, which is correlated with total phenolic content [6]. A. aegerita is a kind of saprophyte fungi and a basidiomycetes. It belongs to the family Bolitiaece, most of which are distributed in Korea, Japan, Europe, and Africa, A. aegerita is very fibrous, has a good feel and peculiar fragrance compared to others, and can be cultivated year round in bottle cultivation facilities. However, it requires long duration culturing in culture bottles and is more susceptible to contamination than others.
During this rapid expansion period, the mushroom industry suffered several disease epidemics. During severe outbreaks, no mushrooms are produced from a contaminated bed. Various pathogens such as Aspergillus spp., Mucor spp., Penicillium spp., and Trichoderma spp. damage mushroom by inhibiting growth. Penicillium competes for preoccupancy with green spores and inhibits the formation of fruiting bodies, resulting in the spores spreading out in the middle and top portion of the mushrooms bottles. Aspergillus spp. and Mucor spp. form black colored mycelia on the top of mushroom culture bottles and inhibit the formation of fruiting bodies.
Green mold caused by Trichoderma species was once recognized as an indicator of poor compost quality but was of minor significance in the cultivation of the commercial mushroom, Agricus bisporus. Typical green mold symptoms are the appearance of green fungal sporulation on oyster mushroom substrates. Sinden and Houser [7] were the first to recognize Trichoderma spp. as a potentially important pathogen and/or competitor that affects white button mushroom production.
Trichoderma spp. grow rapidly under various conditions and utilize various kinds of substrates. T. harzianum, T. viride, T. virens, T. longibrachiatum, T. koningii, and T. polysporum are found frequently associated with mushrooms. Among these, T. harzianum has been recognized as the most important species and cause of potential losses [8]. The devastating nature of green mold was undocumented in the mushroom industry until 1985 when it was first observed in Ireland [9]. Since then, growers in England [10], Canada [11], and the United States [12] have experienced outbreaks of Trichoderma green mold resulting in millions of dollars in crop losses. However, in the past decade, green mold has become a destructive disease of cultivated mushrooms. Crop losses have been estimated at ≤ 3~4 million in the UK and Ireland [13] and at more than $20 million in Pennsylvania, USA [14].
With mushroom production located primarily in rural areas, the industry makes a significant contribution to the rural economy and provides a major alternative to traditional farming enterprises. However, the occurrence and diversity of Trichoderma spp. associated A. aegerita have not been well studied. The objectives of this study were to identify and characterize Trichoderma spp. and other pathogens present in fruiting bodies of commercial A. aegerita and substrates based on morphological and molecular characteristics.
Materials and Methods
Collection of fungal isolates
Twenty-two pathogenic fungi were isolated from the fruiting bodies and compost of A. aegerita. Seven isolates from Pleurotus ostreatus were used to compare with the A. aegerita isolates. The isolates were collected from Gimje, Iksan, Gunsan of Chonbuk, and Chilgok of Gyeongbuk Province from January to April 2009.
Morphological characterization
Pure cultures of the collected isolates were grown in pre-sterilized plates on potato dextrose agar (PDA) using standard laboratory techniques. The cultures were incubated at 25℃ in a biological oxygen demand incubator for 1 wk. Morphological characters such as colony color, size of hypha, and color and shape of conidia were recorded. Conidia and conidiophores were observed under a microscope and by scanning electron microscopy (JSM-5410LV; JEOL, Tokyo, Japan). These morphological data were compared with previous descriptions of related pathogens.
DNA extraction, PCR amplification of the rDNA internal transcribed spacer (ITS) region, and sequence analysis
For DNA extraction, mycelia cultures were raised individually on PDA at 25℃ for 7 days. These pure mycelia were used for sequence analysis of the rDNA ITS region (ITS-1 region, 5.8S gene, and ITS-2 region). The genomic DNA was extracted using a DNeasy plant mini kit (Qiagen, Valencia, CA, USA) following the manufacturer's instructions. Amplification and sequencing of the isolates were performed using a pair of universal primers: ITS1 (5'-TCCGTAGGTGAACCTGCGG-3') and ITS4 (5'-TCCTCCGCTTATTGATATGC-3') [15] for the region containing ITS1, ITS2, and the 5.8S rDNA. These primers were also used as a positive control in the subsequent diagnostic PCR. The amplification was conducted in a 20 µL reaction mixture containing 50 nM genomic DNA, 10 mM Tris-HCl, 1.5 mM MgCl2, 50 mM KCl, 0.01% gelatin, 0.2 mM dNTP, 200 ng of each primer, and 1 unit Taq DNA polymerase (Promega, Madison, WI, USA). The reaction mixtures were denatured at 94℃ for 10 min and subjected to 35 cycles of 1 min at 94℃, annealing at 55℃ for 1 min, extension at 72℃ for 1 min, and a final extension step of 7 min at 72℃. The amplified PCR product was separated on a 1.5% agarose gel, followed by purification with a DNA purification kit (Core-one™; Core-Bio, Seoul, Korea), according to the manufacturer's instructions. Both amplicon strands were sequenced using the same primers, reactions were monitored with BigDye Terminator Cycle Sequencing kits (Applied Biosystems, Foster City, CA, USA), and run on an ABIPRISM 3130 automated DNA sequencer (Applied Biosystems), as described previously [16]. The rDNA ITS sequence data were analyzed using the DNASTAR program (DNASTAR Inc, Madison, WI, USA) and aligned by the CLUSTAL W method [17]. MEGA ver. 4.0 was used for the phylogenetic analysis [18, 19].
Results and Discussion
Of 29 isolates, 26 were Trichoderma spp. and one isolate each was Mucor spp., Penicillium spp., and Aspergillus spp. (Table 1). Contamination in mushrooms that spreads mainly due to contaminated air or by a worker can be easily identified from the outside, as the contamination is green to dark green, black, yellowish green, or red in color due to the spores (Fig. 1).
Table 1.
Pathogens isolated from Agrocybe aegerita and Pleurotus ostreatus

Fig. 1.

Fruiting bodies and symptoms of some pathogens occurring on Agrocybe aegerita. A, A. aegerita fruiting body; B~D, Symptoms occurring in A. aegerita as a result of pathogens.
Symptoms of mushroom pathogens
Mucor spp., Penicillium spp., and Aspergillus spp. caused damage in a similar manner by competing for space resulting in growth inhibition and hampered fruiting body development. The pathogens grew quicker than the mushrooms and occupied all of the surface area, which prevented growth of the mushrooms. The pathogens could be identified with the naked eye due to their colored mycelia. A contaminated bottle can spread infection to others so it must be discarded quickly. The Mucor spp. and Aspergillus spp. formed a black color on the top of mushroom bottles after sporulation (Fig. 1). Initially, Penicillium spp. appeared as a green-gray colored powder and turned black as timed passed. The spores of Trichoderma spp. and Penicillium spp. were round and globular in shape. The Mucor spp. and Aspergillus spp. had shapes similar to an onion flower stake, and their spores had spikes on the outer surface (Fig. 2). Similar morphological characters were reported in Penicillium spp. by Jo et al. [20], and in Mucor spp. [21], and Aspergillus spp. [22].
Fig. 2.
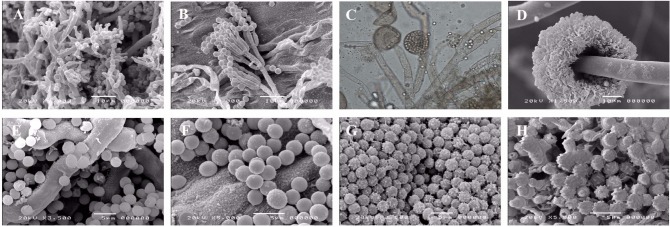
Morphological characteristics of the pathogenic fungi from Agrocybe aegerita. A, E, Trichoderma pleuroticola; B, F, Penicillium crustosum; C, G, Mucor racemosus f. racemosus; D, H, Aspergillus tubingensis.
Trichoderma spp. initially produced a dense pure white mycelium, which was difficult to distinguish from the mushroom mycelium. However, the mycelial mat gradually turned green in color due to heavy sporulation, which is a characteristic symptom of green mold disease. Trichoderma spp. infected the newly developing primordia. This produced brownish lesions and spots on the developing fruiting bodies, which later joined and completely covered the mushroom fruiting bodies. The emerging fruiting bodies in the affected portion of the substrate were badly spotted, brownish in color, and showed reduced growth and yield [8]. The main Trichoderma spp. have a green, green-yellow, or white color on the mushroom bottles and cause parasitic damage, compete with other mushrooms for nutrients, and produce a mycotoxin [23].
Morphological characteristics of Trichoderma spp
Four species of Trichoderma were isolated from A. aegerita and P. ostreatus. Differences in the morphological characteristics of the four species are described in Table 2 and Fig. 3. All Trichoderma spp. were cultured on PDA for 10 days. They developed hyaline mycelia and produced conidiophores on exposed fertile branches after 1 wk. The conidia of Trichoderma are generally oblong, rarely globose, and mostly green or hyaline but rarely yellow. The sizes of the T. longibrachiatum, T. harzianum, T. pleuroticola, and T. atroviride conidia were 3.8~5.4 × 2.7~3.2 µm, 2.7~3.7 × 2.4~3.4 µm, 2.6~3.7 × 2.1~3.0 µm, and 2.7~4.0 × 2.4~3.4 µm, respectively. The conidia size and length/weight ratio of T. longibrachiatum was the largest followed by T. atroviride, whereas the size of the T. pleuroticola hyphae was longest followed by T. longibrachiatum, and the smallest was T. atroviride. The conidia of all four species were ovate and green in color, while the T. pleuroticola conidia were yellowish green. Growth of T. atroviride was slower than that of the other species, and conidia were restricted to concentric rings. T. longibrachiatum tended to form fasciculate conidiation initially, which turned coalescent, often forming greenish yellow conidial crusts with dense conidiation. T. longibrachiatum was distinguished by aggregated conidiophores with weakly developed pustules. As a representative isolate, T. longibrachiatum was usually cultivated until dark green spore bands were 3 cm from the colony center, as the pathogen was mixed together with white mushroom mycelia and light-green pathogen spores. T. harzianum was mass-produced at the end of mycelium with black spores, unlike T. longibrachiatum and T. pleuroticola. Differences in the anamorphs, growth characteristics, and DNA sequences were observed among the species. For the closely related species, significant differences were more likely visible in the Trichoderma anamorph morphology than in the anatomy or morphology of the telemorph. Seaby [24] reported that distinguishing Trichoderma spp. using classic microscopic features alone was difficult, as their characteristics differ widely on different media, and spore sizes vary significantly with incubation temperature. Choi et al. [25] reported that the conidia of Trichoderma spp. are ellipsoidal and ovoid and that the phialides were lageniform and bowling pin shaped. The phialides of T. cf. virens and T. harzianum tended to cluster but were solitary in T. longibrachiatum. T. cf. virens was characterized by predominantly effuse, penicillate type conidiation, which was sparingly branched and fertile to the apex. Samuels et al. [26] also reported similar dimensions for Trichoderma spp. isolated from Agaricus bisporus. The most critical and vulnerable stage of mushroom cultivation is the culture stage, so the utmost care has to taken at this time. If mushrooms become infected at the culture stage, none of them can be harvested.
Table 2.
Morphological characteristics of Trichoderma spp. from Agrocybe aegerita and Pleurotus ostreatus
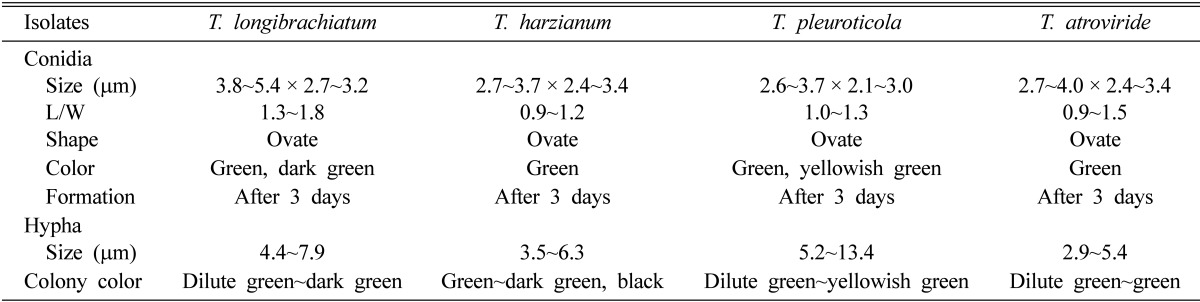
L/W, length/width.
Fig. 3.
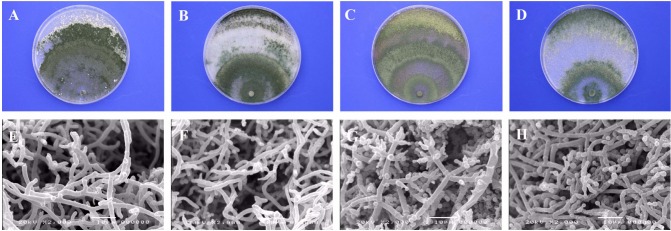
Cultural and morphological characteristics of isolated Trichoderma spp. from Agrocybe aegerita and Pleurotus ostreatus on potato dextrose agar for 10 days. A, E, Trichoderma longibrachiatum; B, F, T. harzianum; C, G, T. pleuroticola; D, H, T. atroviride.
PCR amplification of the rDNA ITS region and a sequence analysis
The rDNA ITS sequence data of 38 isolates were compared and analyzed phylogenetically, including 29 isolates from Jeonbuk and Gyeongbuk Provinces, six isolates of Trichoderma spp., and one isolate each of Mucor, Penicillium, and Aspergillus spp. with the publicly available gene sequences by BLAST against the GenBank database (http://www.ncbi.nlm.nih.gov/).
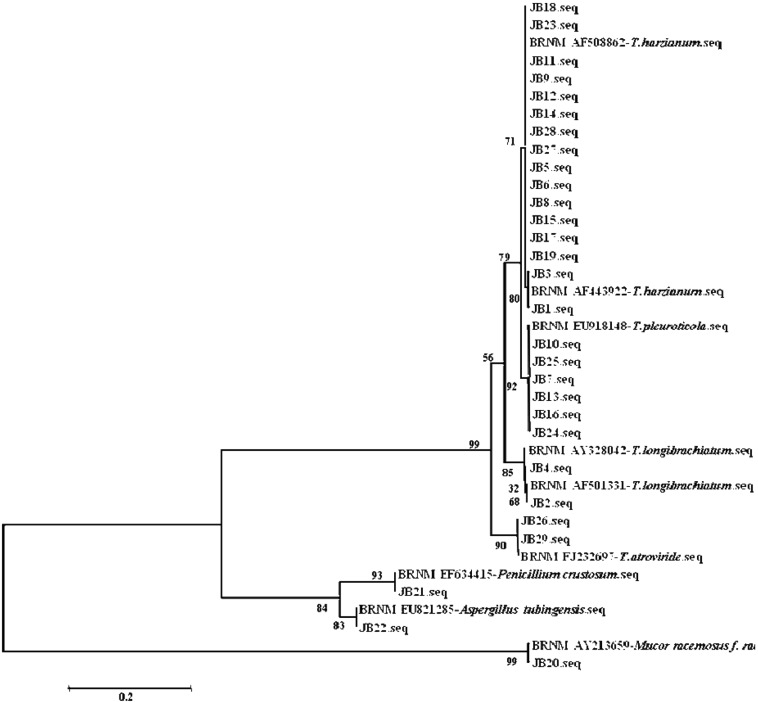
The size of the entire rDNA ITS region was 500~600 bp, yielding 539 bp for Trichoderma spp., 515 bp for Penicillium spp. and Aspergillus spp., and 600 bp for Mucor spp. aligned nucleotide positions. The nucleotide sequences obtained from the GenBank BLAST search in the rDNA ITS region of the isolates was compared with that of other pathogens (Table 1). The phylogenetic analysis of the nucleotide sequence revealed that 26 isolates of Trichoderma were divided into four taxa, namely T. harzianum, T. pleuroticola, T. longibrachiatum, and T. atroviride. However, JB20, JB21, and JB22 were classified as Mucor racemosus f. recemosus, Penicillium crustosum, and Aspergillus tubigenesis, respectively. Among the Trichoderma spp., 16 isolates (55.2%) were identified as T. harzianum, six as T. pleuroticola (20.7%), two as T. longibrachiatum, and the remaining two as T. atroviride. All four groups of Trichoderma spp. isolates were different in their cultural and morphological characteristics, as described earlier (Table 2, Fig. 3). The introduction of DNA sequencing and cladistic analysis in the early 1990s opened a new era for fungal systematics by providing a new set of independently derived data that could be analyzed in tandem with more classically derived data [23]. The molecular and morphological characterization of green mold, Trichoderma spp., isolated from P. ostreatus and P. eryngii beds, were grouped into three species. The occurrence of different species of Trichoderma was T. cf. virens (70.8%), T. longibrachiatum (16.7%), and T. harzianum (12.5%) [25]. In addition to showing phylogenetic relationships among Trichoderma spp., studies in which phenotype and DNA analyses were combined have tended to confirm species monophyly, as distinguished by Bissett [27, 28] (Fig. 4). Many Trichoderma spp. have demonstrated antifungal or plant-growth-stimulating activities, which has led to their exploitation as biological control agents, and some isolates are used in commercially available applications [26].
Fig. 4.
Phylogenetic tree of the rDNA internal transcribed spacer sequences of Agrocybe aegerita isolates and other reference sequences obtained from GenBank (by Laser gene 7.0).
Acknowledgements
This research was supported by a grant from the Korean Rural Development Administration (Agenda Program, PJ006467201003).
References
- 1.Manzi P, Gambelli L, Marconi S, Vivanti V, Pizzoferrato L. Nutrients in edible mushrooms: an inter-species comparative study. Food Chem. 1999;65:477–482. [Google Scholar]
- 2.Mattila P, Suonpaa K, Piironen V. Functional properties of edible mushrooms. Nutrition. 2000;16:694–696. doi: 10.1016/s0899-9007(00)00341-5. [DOI] [PubMed] [Google Scholar]
- 3.Misaki A, Kishida E. Straw mushroom, Fukurotake, Volvariella volvacea. Food Rev Int. 1995;11:219–223. [Google Scholar]
- 4.Zhou H, Chen Q, Wang S. Anti-aging effect of the polysaccharides from Auricularia auricula and Tremella fuciformis. Zhongguo Yaoke Duxue Xuebao. 1989;20:303–306. [Google Scholar]
- 5.Zhu M, Chang Q, Wong LK, Chong FS, Li RC. Triterpene antioxidants from Ganoderma lucidum. Phytother Res. 1999;13:529–531. doi: 10.1002/(sici)1099-1573(199909)13:6<529::aid-ptr481>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 6.Lo KM, Cheung PC. Antioxidant activity of extracts from the fruiting bodies of Agrocybe aegerita var. alba. Food Chem. 2005;89:533–539. [Google Scholar]
- 7.Sinden JW, Houser E. Nature and control of three mildew diseases of mushrooms in America. Mushroom Sci. 1953;2:177–180. [Google Scholar]
- 8.Singh SK, Sharma VP, Sharma SR, Kumar S, Tiwari M. Molecular characterization of Trichoderma taxa causing green mould disease in edible mushrooms. Curr Sci. 2006;90:427–431. [Google Scholar]
- 9.Seaby DA. Infection of mushroom compost by Trichoderma species. Mushroom J. 1987;179:355–361. [Google Scholar]
- 10.Grogan HM, Gaze RH. Growth of Trichoderma harzianum in traditional and experimental compost. In: Elliot TJ, editor. Mushroom science XIV. vol. 2. Balkema: Rotterdam; 1995. pp. 653–660. [Google Scholar]
- 11.Rinker DL. Trichoderma green mold: a seminar by Dr. Donald Betterley, Monterey Labs. Mushroom News. 1994;42:20–23. [Google Scholar]
- 12.Romaine CP, Royse DJ, Wuest PJ, Beyer DM. Mushroom green mold: cause, edaphic factors and control. Mushroom News. 1996;44:20–23. [Google Scholar]
- 13.Fletcher JT. Trichoderma and Penicillium disease of Agaricus bisporus: a literature review for the Horticultural Development Council. London: ADAS; 1990. [Google Scholar]
- 14.Ospina-Giraldo MD, Royse DJ, Thon MR, Chen X, Romaine CP. Phylogenetic relationships of Trichoderma harzianum causing mushroom green mold in Europe and North America to other species of Trichoderma from world-wide sources. Mycologia. 1998;90:76–81. [Google Scholar]
- 15.White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Inis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. London: Academic press; 1990. pp. 315–322. [Google Scholar]
- 16.Yun HY, Kim YH, Hong SG, Lee KJ. Fist description of Coleosporium plectranthi causing perilla rust in Korea. Plant Pathol J. 2007;23:7–12. [Google Scholar]
- 17.Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 20.Jo WS, Rew YH, Kim SH, Yun JT, Choi BS. Occurrence of bluish green mold of Pleurotus eryngii by Penicillium corylophilum. Korean J Mycol. 1999;27:412–414. [Google Scholar]
- 21.Schipper MA. On Mucor circinelloides, Mucor racemosus and related species. Stud Mycol. 1976;12:1–40. [Google Scholar]
- 22.Samson RA, Hong SB, Frisvad JC. Old and new concept of specific differentiation in Aspergillus. Med Mycol. 2006;44(Suppl):S133–S148. doi: 10.1080/13693780600913224. [DOI] [PubMed] [Google Scholar]
- 23.Samuels GJ. Trichoderma: a review of biology and systematics of the genus. Mycol Res. 1996;100:923–935. [Google Scholar]
- 24.Seaby DA. Differentiation of Trichoderma taxa associated with mushroom production. Plant Pathol. 1996;45:905–912. [Google Scholar]
- 25.Choi IY, Hong SB, Yadav MC. Molecular and morphological characterization of green mold, Trichoderma spp. isolated from oyster mushrooms. Mycobiology. 2003;31:74–80. [Google Scholar]
- 26.Samuels GJ, Dodd SL, Gams W, Castlebury LA, Petrini O. Trichoderma species associated with the green mold epidemic of commercially grown Agaricus bisporus. Mycologia. 2002;94:146–170. [PubMed] [Google Scholar]
- 27.Bissett J. Trichoderma atroviride. Can J Bot. 1992;70:639–641. [Google Scholar]
- 28.Bissett J. A revision of the genus Trichoderma: II. infrageneric classification. Can J Bot. 1991;69:2357–2372. [Google Scholar]