Abstract
The spore of Tricholoma matsutake is considered to be the starting point of the mushroom growth cycle, but the mechanism of mycelial development from the spore stage is not yet clarified. In this study, we tried to measure how far the spores of T. matsutake disperse from a fruiting body located at a Pinus densiflora stand in Korea. We established 16 slide glasses coated with glycerin near a fruiting body in four directions separated by four different distance intervals within a mushroom productive stand after removing all other fruiting bodies from three plots. The number of dispersed spores increased with time from the first day (475 spores/cm2) to the fourth day (836 spores/cm2) after the pileus opened. The number of spores dispersed downward was about 1.5 times greater than that dispersed toward the ridge. The number of dispersed spores decreased exponentially as the distance from each fruiting body increased. More than 95% of the spores dropped within a meter from the fruiting body, with 75% dropping within 0.5 m. Even so, the number of spores dispersed over 5 m from the fruiting body was more than 50 million when considering the total number of spores produced by a fruiting body is about 5 billion.
Keywords: Fruiting body, Pine mushroom, Pinus densiflora, Spore dispersion, Tricholoma matsutake
Pine mushroom is a famous ectomycorrhizal mushroom common in east Asia, specifically Korea and Japan. As the mushroom is the symbiotic product of a fungus (Tricholoma matsutake) and certain trees such as Pinus densiflora, it is difficult to produce the mushroom artificially. Due to difficulties in cultivation, production of the mushroom is restricted to autumn, which results in high prices. The mushroom has intensely been studied from an ecological viewpoint, in particular its growth pattern [1-3] and productivity by focusing on environmental control [1, 4]. Although some reports have demonstrated successful inoculation of the fungus into a host plant [5-7], there is still too much unknown about the life cycle of the fungus.
Understanding the process of spore dispersion and germination of T. matsutake is a prerequisite step to revealing the whole life cycle of the mushroom. Since spore germination is the starting point for initializing a new mycelial cluster, or so-called 'fairy ring', spore dynamics would be a key factor. Spore dispersion may depend on the size and shape of the spores as well as site factors and climatic conditions such as wind strength. The flight path of spores is largely influenced by gravity as well as the air flow surrounding the fruiting body [8]. However, finding a report related to the spores of T. matsutake is not easy, especially regarding spore dispersion in situ. Although there are some papers on the dispersion of various spores in a forest environment from a theoretical or modeling perspective [9, 10], there seems to be an absence of reports on the practical dispersion of fungal spores, which makes sense since it is not easy to track dispersed spores in situ. Therefore, as a fundamental study on the life cycle of T. matsutake, we tried to determine how far the spores disperse from a fruiting body of the fungus.
Materials and Methods
Assay of amount of spores produced by a fruiting body
The amount of spores produced by each fruiting body was enumerated. Three fruiting bodies of T. matsutake were used to measure the number of spores produced from each fruiting body. The samples used for the enumeration of spores were similar in size used for the investigation of spore dispersion, and their veils under each pileus had just started to open. We cut the stem of each mushroom and set the pileus above a piece of aluminum foil for 4 days to obtain spore prints. All of the spores on the foil were collected and measured for weight, and the numbers of spores in a serial-diluted solution were enumerated using a Haemacytometer. The data were statistically analyzed using SAS software ver. 9 (SAS Institute Inc., Cary, NC, USA).
Spore collection in situ
The experimental site was mainly composed of 65-year-old Japanese red pines (Pinus densiflora S. et Z.), although a few oaks (Quercus variabilis BL.) occupied less than 20%. The aspect of the stand was southwesterly, and the slope was around 30%. The site had been surveyed for mushroom growth for more than 4 yr, and the locations of pine mushroom had been thoroughly documented [11].
Collection of spores was conducted during late September. Only one fruiting body of T. matsutake remained within each site; all others, including other mycorrhizal mushrooms, were thoroughly removed. We established three sites for study, and the distances from each site were more than 50 m. We established 48 slide glasses coated with glycerin nearby each fruiting body in four directions separated by four different distance intervals at three heights. The direction was decided by the slope direction: upward, downward and perpendicular. The distances were set as 50, 100, 200 and 500 cm from the fruiting body. At each point, we installed a stick containing three slide glasses that were 0, 50 and 100 cm above the ground. The dispersed spores were collected from the time immediately after the veils of pileus were torn out. We installed the collecting glasses when the fruiting body was hemispherical and stopped when it became planar, a total of 4 days as described by Ka et al. [2]. The slide glasses were installed at 10 AM every day and were replaced by a new one after 24 hr.
Enumeration of collected spore
The number of spores on each slide was enumerated by the line-transect method under a microscope (×400) using an image analyzer (Leica, Sasem onAirTV). The plate was overlapped by grids of 116 µm×118 µm for counting, and triplicates were applied for the enumeration.
Results and Discussion
Number of spores produced by a fruiting body
The fruiting bodies used for spore counting were 230 ± 22 g in fresh weight, 12 ± 0.3 cm in pileus diameter, 15 ± 0.3 cm in stipe length and 3.5 ± 0.1 cm in stipe diameter. Although the fruiting bodies were relatively larger and heavier than generally harvested mushrooms as goods, they could be considered as representative fruiting bodies of overmatured mushrooms. The spore of T. matsutake showed an oval shape with a small prominence. The spores collected from each fruiting body reached about 150 mg in total mass and were counted as 4.5~5 billion, which varied depending on the size of the mushroom.
Difficulties in assay
Although we set the slide glasses at three heights (0, 50 and 100 cm above the ground), the data collected from the slides located at 50 and 100 cm above the ground were unusable due to sparseness with large variation. As Deering et al. [8] showed for the airflow surrounding a simulated mushroom, the spores of T. matsutake did not fly at high elevations but instead streamed down along the forest floor. Thus, we only used the data from the slides set on the ground for the enumeration.
Dispersion pattern of spores
The number of spores increased with time but rapidly dropped as the pileus turned over (on the fifth day); 475 spores/cm2 on the first day, 497 spores/cm2 on the second day, 599 spores/cm2 on the third day and 836 spores/cm2 on the fourth day after the veils of pileus opened. Ogawa [1] noted that the most abundant time for spore production is 3~4 days after the veil of pileus is torn down. He noted that spores are immature if the veil is not torn down or the pileus turned over, and the amount of spores also becomes smaller. Thus, we used the cumulative numbers of spores over 4 days to compare the differences in the amount of spores among directions and/or distances.
The spores dispersed more along the slope compared to those within the slope (Table 1). Although we did not measure the intensity of the wind within the forest, the intensity of the wind was stronger along the slope than that within the slope. Thus, we estimated that the difference in intensity of the wind resulted in the differences in spore dispersion. The number of spores dispersed downward was about 1.5 times greater than that dispersed upward, which indicates that gravity was another key factor in the dispersion of spores, as noted by Deering et al. [8]. Although spores may usually spread more toward the lower part of mountains, pine mushrooms are mainly produced on the upper parts. This implies that the decline in the productivity of pine mushroom was not due to an insufficient number of spores but actually other factors, such as unsuitable site conditions for growth.
Table 1.
Number of spores accumulated in each direction
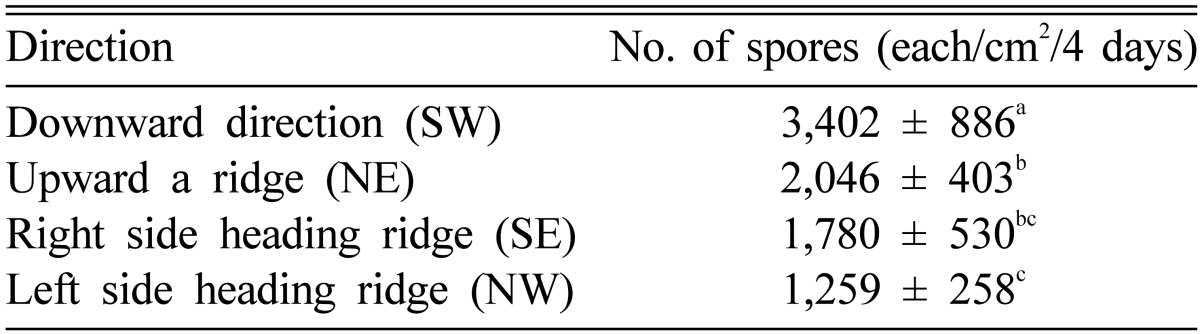
SW, southwest; NE, northwest; SE, southeast; NW, northwest.
a,b,cThe same letters above each value indicate that the values were not significantly different at the 5% level.
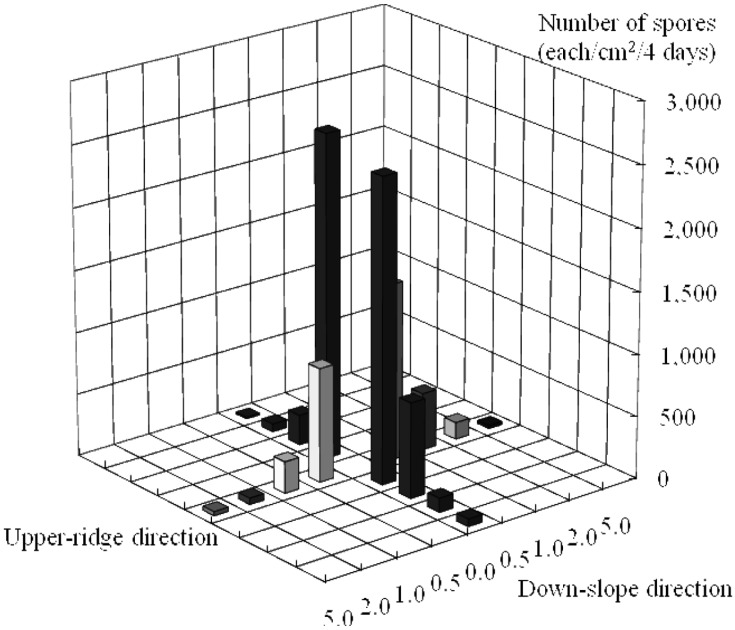
Further, the number of spores decreased exponentially as the distance increased from each fruiting body (Fig. 1). More than 95% of the spores dropped within a meter of the fruiting body, with about 75% of them within 0.5 m (Table 2). However, the spores dispersed over 5 m from the fruiting body could not be discounted as they numbered more than 50 million, considering that the number of spores produced by a fruiting body is about 5 billion.
Fig. 1.
Number of spores surrounding the fruiting body of Tricholoma matsutake.
Table 2.
Number of spores dropped at several distances from a fruiting body
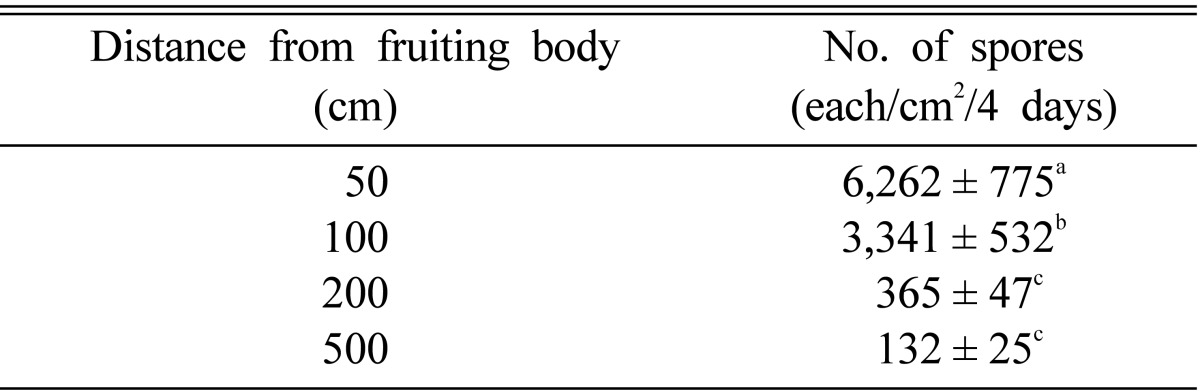
a,b,cThe same letters above each value indicate that the values were not significantly different at the 5% level.
The pattern of spore dispersion was also dependent upon the stand density and other micro-environmental factors (Table 3). As stand density increased, the number of dropped spores on the sampled slides decreased (2,605 spores/cm2 for dense, 2,089 spores/cm2 for middle and 1,670 spores/cm2 for sparse), which indicates that stand density might have affected air flow and therefore spore dispersion.
Table 3.
Number of spores at three different sites under various site conditions
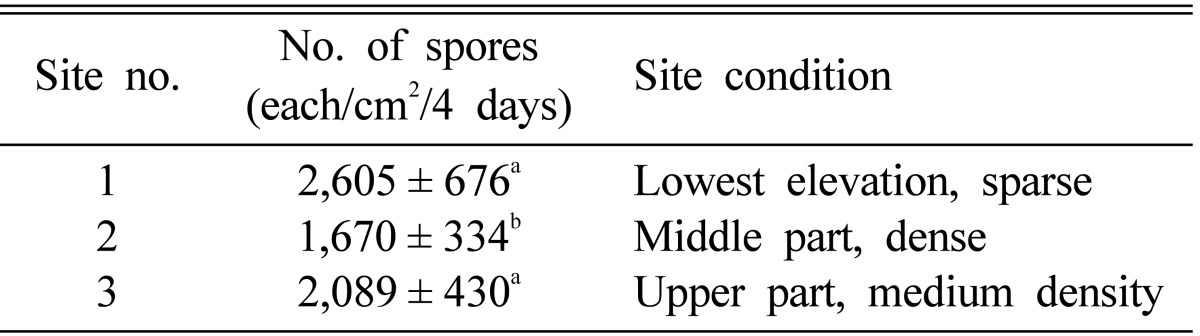
a,bThe same letters above each value indicate that the values were not significantly different at the 5% level.
Ogawa [1] reported that spore dispersion is largely dependent upon the climatic conditions or fructification time. He mentioned that the amount of mature spores would be quite few if the air temperature is very high or low. In addition, he stated that relative humidity is a key factor in spore dispersion; for example, colonized spores are high in number after rain or fog. In some cases, a spore print was formed on the surface of litter, which indicates that the spores were not widely spread out. Thus, stand density and other site factors could be major factors in the dispersal of spores, but microclimatic factors such as wind are more important in spore dispersion.
References
- 1.Ogawa M. Biology of matsutake mushroom. Tokyo. Tsukiji Shokan Co., Ltd.: 1978. pp. 168–175. [Google Scholar]
- 2.Ka KH, Park H, Huh TC, Kim HS, Kim KS, Kim HJ, Lee WK, Kim JW, Lee MW. Study on the growth mechanism of fruiting bodies of Tricholoma matsutake. FRI J For Sci. 1998;58:35–39. [Google Scholar]
- 3.Ka KH, Park H, Hur TC, Yoon KH, Bak WC, Yeo WH, Lee MW. Fairy ring growth of Tricholoma matsutake in 65-year-old pine (Pinus densiflora) forest stand. Korean J Mycol. 2002;30:95–98. [Google Scholar]
- 4.Park H, Kim SH, Kim KS. Effects on the pine mushroom yield of controlling environmental conditions at the pine stands in Namwon, Korea. J Korean For Soc. 1997;86:399–404. [Google Scholar]
- 5.Yamada A, Maeda K, Ohmasa M. Ectomycorrhiza formation of Tricholoma matsutake isolates on seedlings of Pinus densiflora in vitro. Mycoscience. 1999;40:455–463. [Google Scholar]
- 6.Vaario LM, Guerin-Laguette A, Gill WM, Lapeyrie F, Suzuki K. Only two weeks are required for Tricholoma matsutake to differentiate ectomycorrhizal Hartig net structures in roots of Pinus densiflora seedlings cultivated on artificial substrate. J For Res. 2000;5:293–297. [Google Scholar]
- 7.Park H, Lee BH, Ka KH, Ryu SR, Bak WC. Acclimation of ectomycorrhizal pine (Pinus densiflora) seedlings inoculated with Tricholoma matsutake by the treatment of PDMP and Tween solutions. J Korean For Soc. 2009;98:357–362. [Google Scholar]
- 8.Deering R, Dong F, Rambo D, Money NP. Airflow patterns around mushrooms and their relationship to spore dispersal. Mycologia. 2001;93:732–736. [Google Scholar]
- 9.Lacey J. Spore dispersal: its role in ecology and disease: the British contribution to fungal aerobiology. Mycol Res. 1996;100:641–660. [Google Scholar]
- 10.Kuparinen A, Markkanen T, Riikonen H, Vesala T. Modeling air-mediated dispersal of spores, pollen and seeds in forested areas. Ecol Model. 2007;208:177–188. [Google Scholar]
- 11.Park H, Ka KH, Ryoo CI, Kim KS, Kim HJ. Ectomycorrhizal mushroom occurrence around the fairy ring of Tricholoma matsutake at a pine-mushroom forest. Korean J Mycol. 1998;26:306–313. [Google Scholar]