Abstract
We identified a gene for β-1,3-glucan synthesis (GBG1), a nonessential gene whose disruption alters cell wall synthesis enzyme activities and cell wall composition. This gene was cloned by functional complementation of defects in β-1,3-glucan synthase activity of the the previously isolated Saccharomyces cerevisiae mutant LP0353, which displays a number of cell wall defects at restrictive temperature. Disruption of the GBG1 gene did not affect cell viability or growth rate, but did cause alterations in cell wall synthesis enzyme activities: reduction of β-1,3-glucan synthase and chitin synthase III activities as well as increased chitin synthase I and II activities. GBG1 disruption also showed altered cell wall composition as well as susceptibility toward cell wall inhibitors such as Zymolyase, Calcofluor white, and Nikkomycin Z. These results indicate that GBG1 plays a role in cell wall biogenesis in S. cerevisiae
Keywords: β-1,3-glucan synthase; Cell wall composition; Chitin synthase; GBG1/AYR1; Saccharomyces cerevisiae
The cell wall of Saccharomyces cerevisiae supposedly plays a role in determining cell morphology and maintaining osmotic stability [1]. The major components of S. cerevisiae are mannoproteins, β-glucans, and chitin [2]. Yeast cell wall biogenesis is an example of cellular morphogenesis, since the wall expands during cell growth and a septum is synthesized at a specific time and location during the cell division cycle [3, 4]. Recently, there has been evidence to support connections between cell wall synthesis and other areas such as signal transduction, control of bud placement and emergence, and the roles of intermediate filaments [1, 5, 6]. From a practical standpoint, S. cerevisiae also provides a model for cell wall synthesis in pathogenic fungi. Therefore, investigating cell wall biogenesis, which is critical to maintaining cell wall integrity, can help elucidate which steps of the process are susceptible to antifungal agents [7]. To date, a variety of strategies have been used to identify cell wall synthesis mutants as well as genes involved in cell wall assembly [1]. Thus, thorough understanding of how a yeast cell synthesizes and assembles its wall components has been developed. However, identification of new cell wall biogenesis genes as well as the genetic interactions between them is still required to disclose the full mechanism of cell wall biogenesis.
We previously identified the two genes responsible for β-1,3-glucan biosynthesis in S. cerevisiae using a conditional osmo-sensitive mutant with lower β-1,3-glucan content and lower β-1,3-glucan synthase activity at nonpermissive temperature (37℃). We also cloned SOO1/RET1, which is essential and responsible for the defective β-1,3-glucan synthase activity as well as the temperature-dependent osmo-sensitive (Tos-) phenotype of mutant strain LP0353 by functional complementation [8]. We identified a gene for β-1,3-glucan synthesis (GBG1), a non-essential gene that partially complements defective β-1,3-glucan synthase activity but not the Tos- phenotype. Disruption of GBG1 altered not only the activities of cell wall synthesis enzymes but also cell wall susceptibility toward various agents in S. cerevisiae. These results suggest that GBG1 plays a role in cell wall biosynthesis in S. cerevisiae.
Materials and Methods
Strains and growth media
S. cerevisiae LP0353 (MATa ura3-52 lys2-801 soo1-1 Bgs-) and F808 (MATa ura3-52 leu2-3,112 his4-519 ade1-100) were used for cloning and disruption experiments. S. cerevisiae X2180-1A (MATa SUC2 mal mel gal2 cup1) cells were used to prepare synchronous culture. Rich medium containing glucose (yeast-extract peptone, dextrose [YPD]) and synthetic minimal medium (synthetic dextrose) for yeast culture were prepared as described elsewhere [9]. Escherichia coli DH5α was used for the propagation of all plasmids.
DNA manipulation and yeast genetic techniques
Transformation of E. coli, plasmid preparation, restriction mapping, DNA ligation, and other DNA manipulations were performed by standard techniques [10]. Transformation of yeast cells was carried out by the lithium acetate procedure [11]. The yeast plasmids YEplac195, YCplac33, and YIplac128 were used for cloning and gene disruption experiments.
Construction of GBG1 null mutant
For gene disruption, ca 600 bp internal BalI-SpeI fragment of GBG1 cloned in YEplac195 was replaced by the PCR-amplified LEU2 gene in YIpalc128 using primers (LEU2-SpeI-R: 5'-AAC TAG TTA GGC GTA TCA CGA GGC C-3', underlined is SpeI site, LEU2-BalI-L: 5'-TTG GCC AAG ACG AAA GGG CCT ACC C-3', underlined is BalI site). The LEU2 gene in the resulting plasmid flanked on either side by the GBG1 fragment was amplified by PCR using primers (B2-LEU2-L: 5'-GTG ATG ACG GTG AAA ACC TCC AAC TCT GAC CGG CAT TG-3', B2LEU2-R: 5'-CCT CGT GAT ACG CCT ATT TTC CCT TGC CTG AAA CCT CA-3') followed by introduction into the haploid F808 strain.
Induction of synchrony and Northern blot analysis
To synchronize yeast culture with α-factor, the X2180-1A strain was grown to early log phase at 30℃ in YPD medium. α-factor purchased [12] from Sigma Chemical Co. (St Louis, MO, USA) was added to a final concentration of 5 µg/mL. The cells were further incubated at 30℃ for 1 hr, collected by centrifugation, washed with distilled water and then transferred to fresh medium. X2180-1A cells synchronized with α-factor were then washed and resuspended in fresh YPD medium. Aliquots of the cell suspension were injected into five separate flasks containing fresh media. After shaking at 30℃ for 100 min, cultures were harvested at 10 min intervals for 40 min. Total RNA was extracted by hot acid phenol method [12]. For Northern blotting, 30 to 40 µg of RNA was separated on a 1% agarose gel containing 6% formamide, followed by transfer to a Hybond-N+ membrane (Amersham, Buckinghamshire, UK) through capillary blotting and UV cross-linking. Northern hybridization was performed with modified church buffer using [α-32P] dCTP-labeled probe and a random priming kit (Stratagene, La Jolla, CA, USA). The probe used was a 600 bp portion of the EcoRI-BalI fragment from GBG1. Following hybridization, the blot was exposed to X-ray film.
Preparation of holoenzyme from yeast and measurement of cell wall biosynthesis enzyme activities
Membrane fractions of cells were prepared using glass beads [8, 13]. Glucan synthase activity was measured following a previously described method [8, 14]. However, chitin synthase activity was measured by the method of Choi and Cabib [13]. All reactions were performed in duplicate and terminated by the addition of 10% trichloroacetic acid. The radioactivity of insoluble materials was counted by a liquid scintillator after filtration through glass fiber filters (Whatman Inc., Clifton, NJ, USA). Specific activities of β-1,3-glucan synthase and chitin synthase were expressed as nanomoles of Glu and GlcNAc incorporated per hour per milligram of protein, respectively. The concentration of used protein was measured by the method of Lowry et al. [15] using bovine serum albumin as a standard.
Fractionation and analysis of cell wall preparations
Total cell wall fractionation was prepared by following a previously described method [8, 16]. Glucan content of the fractions was determined by the orcinol-sulphuric acid method [17]. For determination of chitin content, the amount of GlcNAc liberated after treatment of the acid-insoluble fraction with chitinase was measured by a previous method [18].
Susceptibility to cell wall damaging agents
Zymolyase resistance test was performed following a modified method [19]. Briefly, yeast cells were grown in YPD medium containing 1.2M sorbitol to mid-log phase and then harvested, after which the cell pellet was washed and resuspended in distilled water to OD600 = 0.4. Zymolyase 100T in 10 mM Tris-HCl (pH 8.0) was added to a final concentration of 2 mg/mL followed by incubation at 30℃. Every 90 min, aliquots were removed and read optically. Determination of minimum inhibitory concentration (MIC) was performed according to the method of Kurtz et al. [20].
Results and Discussion
Characterization of the GBG1 gene
In a previous study, we isolated a clone that complements the defective glucan synthase activity of LP0353 [21]. Sequence analysis of the selected 8.5 kb DNA fragment revealed two open reading frames (ORFs), Yil124w and Yil125w. ORF Yil125w was identified as KGD1, which encodes α-ketoglutarate dehydrogenase [22]. Although both ORFs can complement defective glucan synthase activity (data not shown), the function of ORF Yil124w was not clear at the beginning of this study. We therefore performed functional analysis of the unknown gene, Yil124w, which is an ORF consisting of 891 bp that encode a polypeptide of 297 amino acids and with a calculated mass of 32.8 kDa. Based on previous results, this gene was named as GBG1.
Even during our own study, the function of ORF Yil124w has been reported by other groups [23, 24]. The ORF Yil124w, named as AYR1, encodes a NADPH dependent 1-acyl dihydroxyacetone phosphate reductase found in lipid particles, the endoplasmic reticulum, and mitochondrial outer membrane [23, 24]. Thus AYR1 is involved in phosphatidic acid and phospholipid biosynthesis, and is also required for spore germination but not vegetative growth [24]. However, there are no reports on the function of Gbg1/Ayr1 in cell wall biogenesis.
In order to explore the effect of copy number of the GBG1 gene on β-1,3-glucan synthase activity, 1.6 kb EcoRI fragment containing the whole ORF of GBG1 was cloned into a high-copy number plasmid (YEplac195) and low-copy number plasmid (Ycplac33). Each of the resulting recombinant plasmids were introduced into mutant LP0353. The levels of β-1,3-glucan synthase activity were almost identical, but both were lower than that of wild type (Table 1). Additional evidence that GBG1 plays a role in β-1,3-glucan synthase activity was that the LP0353 strain expressing GBG1 produces an increased amount of β-1,3-glucan, based on quantitative analysis of cell wall composition. Although further experiments including an allele test should be performed, these results indicate that GBG1 may account for the defect in β-1,3-glucan synthase activity [21].
Table 1.
Assay of β-1,3-glucan synthase activity and quantitative analysis of cell wall composition in LP0353 stain expressing GBG1
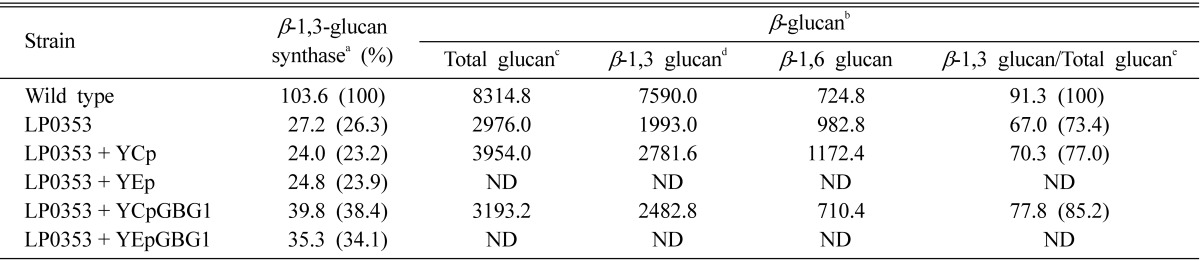
GBG, gene for β-1,3-glucan synthesis; ND, not detected.
aEnzyme activity was measured in membrane fractions and was expressed as mg (UDPG)/h·mg (protein).
bThe units of β-glucan were expressed as mg/mg of cell wall dry-weight.
cThe amount of total β-glucan was determined based on the carbohydrate content of the Zymolyase-insoluble pellet and solubilized supernatant before dialysis.
dThe amount of β-1,3-glucan was the difference in between total carbohydrate content and β-1,6-glucan.
eThe amount of β-1,3-glucan in GS-1-36 was taken as 100.
GBG1 disruption reduces cell wall polysaccharide synthesis
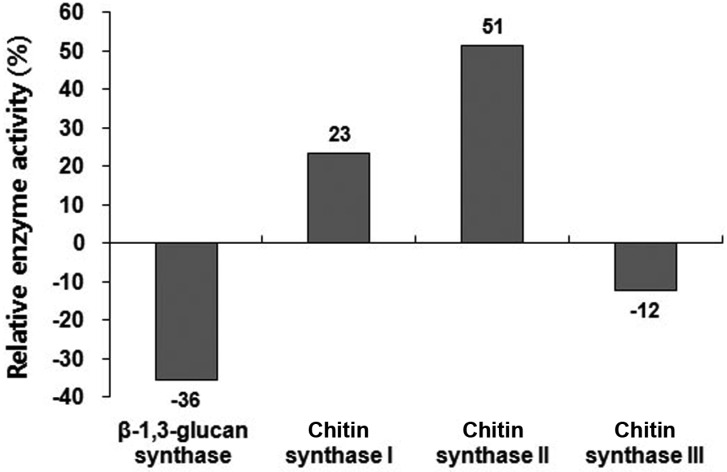
To predict the in vivo function of the GBG1 gene with regards to cell wall biogenesis, a GBG1 disruption mutant was constructed and confirmed by Southern blot (Fig. 1). No difference in growth rate between the GBG1 null mutant and isogenic wild type was observed (data not shown). However, as expected, the GBG1 null mutant showed 36% reduction in β-1,3-glucan synthase activity (Table 2). On the other hand, GBG1 disruption increased chitin synthase I and II activities by 23% and 51%, respectively, and decreased chitin synthase III activity by 12% (Fig. 2). The reduction in β-1,3-glucan and chitin III synthase activity by the disruption of a single is not well understood. However, the increase in chitin synthase I and II activities can be explained by the fact that gene(s) for cell wall biogenesis are activated by defect(s) in the cell wall caused by mutation. This compensation mechanism is known to increase chitin synthesis in a FKS1 deletion mutant [25]. In this study, GBG1 disruption caused defects in β-1,3-glucan synthase, which then may have altered chitin synthase activity.
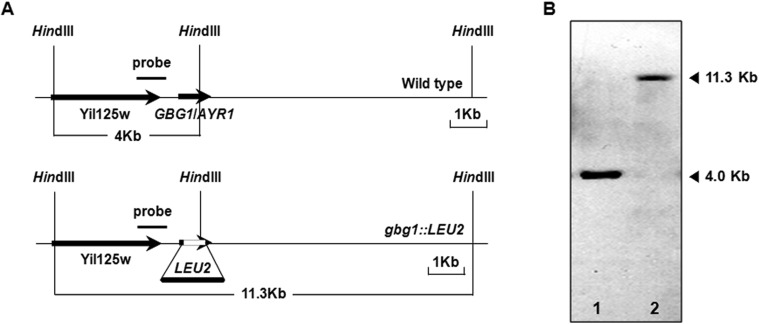
Fig. 1.
Gene disruption of gene for β-1,3-glucan synthesis (GBG1) in Saccharomyces cerevisiae. A, A schematic representation of the gene disruption of GBG1. Black arrows stand for open reading frames. The predicted sizes of fragments generated by HindIII-digestion are depicted. A 4 kb fragment indicates GBG1 wild type allele while the 11 kb fragment indicates the gbg1::LEU2 allele disrupted by inserted LEU2; B, The disruption of GBG1 was detected by Southern blot using the indicated probe. Analysis of wild type (lane 1) and Δgbg1 (lane 2) is shown.
Table 2.
Assay of β-1,3-glucan synthase activity and quantitative analysis of cell wall composition in Δgbg1 mutant

gbg, gene for β-1,3-glucan synthesis.
aEnzyme activity was measured in membrane fractions and was expressed as mg (UDPG)/h·mg (protein).
bThe units of β-glucan were expressed as µg/mg cell wall dry-weight.
cThe amount of total β-glucan was determined based on the carbohydrate content of the Zymolyase-insoluble pellet and solubilized supernatant before dialysis.
dThe amount of β-1,3-glucan was the difference between total carbohydrate content total and β-1,6-glucan.
eThe amount of β-1,3-glucan in F808 was taken as 100.
Fig. 2.
Assays of enzymes related to cell wall biogenesis in Δgbg1. The values are presented as relative enzyme activities normalized to that of wild type. The experiment was performed three times. gbg, gene for β-1,3-glucan synthesis.
Although the null mutant showed no significant change in the amount of cell wall carbohydrates in the alkali-soluble fraction, there was dramatic reduction in the acid-insoluble fraction containing β-1,3-glucan and chitin. Consistent with the result of the chemical extraction, the amount of GlcNAc liberated by chitinase treatment of the acid-insoluble fraction was also dramatically reduced (Table 2). According to a recent model of yeast cell wall architecture, chitin is linked to the cell wall through both β-1,3-glucan and β-1,6-glucan. Thus, a decrease in β-1,3-glucan and/or β-1,6-glucan synthesis in combination with defective chitin synthesis could dramatically reduce chitin content in the cell wall.
GBG1 disruption alters susceptibility toward various agents affecting cell wall biogenesis
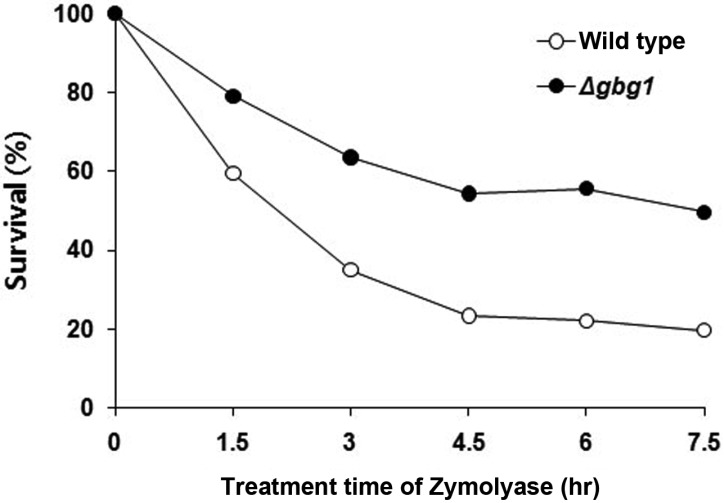
In order to investigate other cell wall properties that are altered by GBG1 disruption, the susceptibility of the strains toward various agents affecting cell wall biogenesis was tested. Usually, a reduction in cell wall components will increase resistance toward cell wall lytic enzyme. Upon Zymolyase treatment, the GBG1 null mutant revealed more than two-fold increase in resistance, implying that β-1,3-glucan content in cell wall of the null mutant was reduced (Fig. 3). This is consistent with the result from the cell wall analysis that revealed a dramatic decrease in β-1,3-glucan. Many cell wall defects affect sensitivity to Calcofluor white, a chitin specific dye [26]. When measuring the MIC (minimum inhibitory concentration) of the strains, GBG1 null mutant revealed an increased MIC value toward Calcofluor white and Nikkomycin Z (Table 3). Calcofluor white is known to interfere with the normal synthesis and assembly of cell wall polysaccharides by binding to the growing chitin polymer [26, 27]. Thus, decreased chitin content increases cell resistance toward this dye. On the other hand, Nikkomycin Z, an inhibitor of chitin synthesis, is a specific inhibitor of chitin synthase III in S. cerevisiae [28]. Therefore, the results from the MIC determination are consistent with those from the cell wall analysis and chitin synthase activity assays that showed reduction in chitin content and chitin synthase III activity. However, sensitiviteis to Polyoxin D, which inhibits chitin synthesis, as well as L-688,786 and L-733,560, which inhibit glucan synthesis, were not different between null mutant and wild type [28].
Fig. 3.
The viability of Δgbg1 cells after treatment with Zymolyase. Cells were grown to mid-exponential phase followed by treatment with Zymolyase 100T (final concentration is 2 µg/mL). Cell survival was measured as OD600 every 90 min for 7.5 hr. Data are the mean of three independent experiments. gbg, gene for β-1,3-glucan synthesis.
Table 3.
Sensitivity of Δgbg1 mutant to cell wall damaging agents
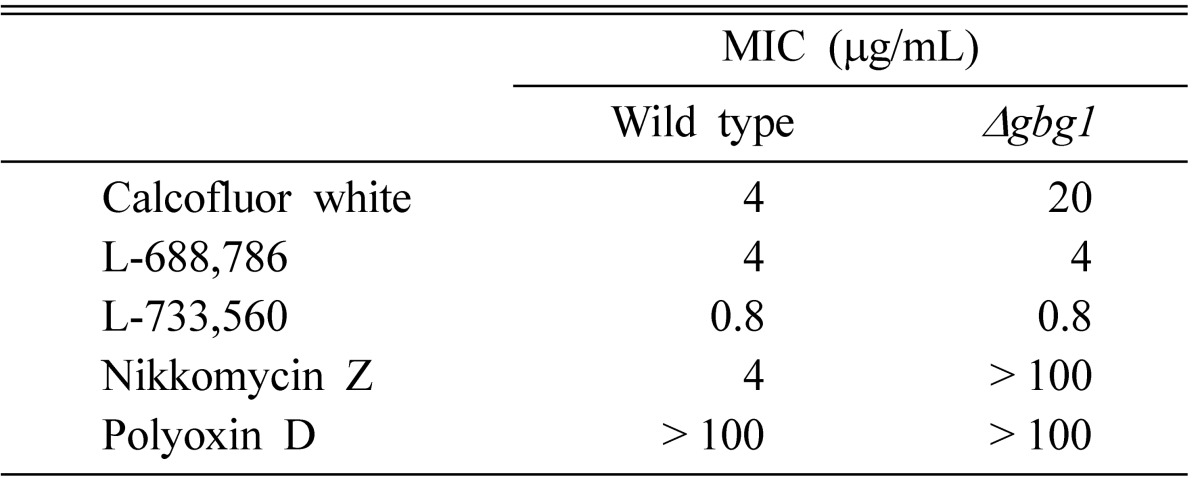
gbg, gene for β-1,3-glucan synthesis; MIC, minimum inhibitory concentration.
GBG1 is expressed in a cell cycle-dependent manner
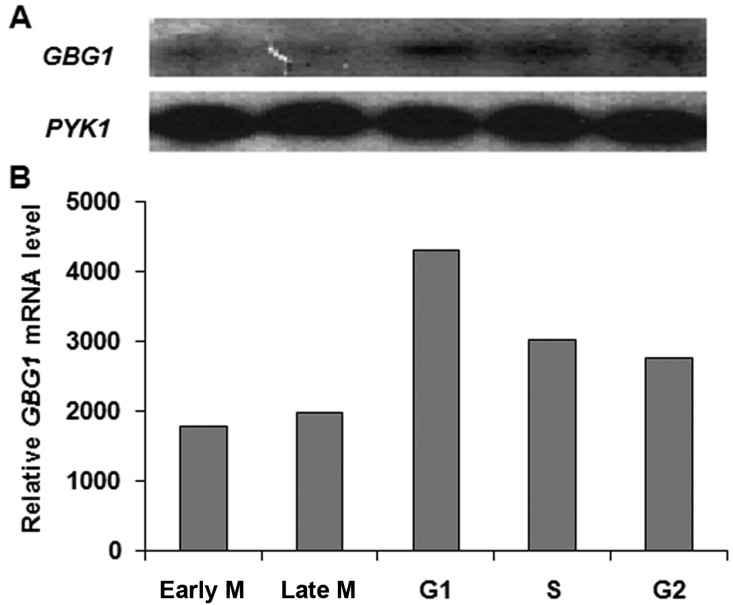
In fungi various cell types cause change in cell wall structure and composition [3]. Morphological differentiation in S. cerevisiae was observed during the cell cycle and mating process as well as in response to nutrient limitation [4]. It was also reported that FKS1, a β-1,3-glucan synthase gene, is mainly expressed for vegetative growth, whereas FKS2 is induced for sporulation under starvation and in response to mating pheromones [6, 29]. To test the expression of GBG1 during vegetative growth, RNA preparation from vegetative cells was subjected to Northern blot analysis. As shown in Fig. 4, GBG1 was expressed during vegetative growth with a maximum level of transcription at G1 phase, after which it was decreased during the remainder of the cell cycle. A bud was observed in G1 phase, and maturation was observed in S phase. Increased GBG1 transcription during G1 phase may be able to explain abnormally elongated bud formation in Δayr1 mutant [30]. These results also suggest that GBG1 may play a role during G1 phase when the bud starts to emerge.
Fig. 4.
Analysis of gene for β-1,3-glucan synthesis (GBG)1 transcription during vegetative cell cycle. Total RNA was isolated from the X2180-1A strain synchronized with α-factor. A, Northern blotting of GBG1 and pyruvate kinase (PYK)1 was performed during the synchronized cell cycle. PYK1 was used as a loading control; B, Level of GBG1 mRNA was determined by densitometry of the Northern blot in A. The values were normalized to PYK1 mRNA.
In this study, we showed that disruption of GBG1 reduced the activities of β-1,3-glucan synthase and chitin synthase III while increasing chitin synthase I and II activities. GBG1 disruption also altered cell wall composition and thus increased cell wall resistance to inhibitors such as Zymolyase, Calcoluor white, and Nikkomycin Z. Since GBG1/AYR1 codes for NADPH-dependent 1-acyl dihydroxyacetone phosphate reductase, a key enzyme of the phosphatidic acid biosynthesis pathway [23], and not for a cell wall synthesis enzyme, the effect of GBG1 deletion on cell wall biogenesis might be indirect and therefore explainable by the following. Phosphatidic acid plays a central role in the metabolism of phospholipids [31], which in turn determine the total amounts of membrane lipids [32]. The nature of lipids in the membrane affects membrane fluidity, protein-mediated transport, and membrane-associated enzyme activities [32, 33]. Although further experiments are required for the confirmation, alternations of yeast cell wall biogenesis caused by the GBG1 deletion reported here can be supported by the low level of phospholipids and phosphatidic acid in the gbg1/ayr1 deletion mutant [23, 31]. G1-specific expression of GBG1 is also explainable by a previous report that found GBG1 is required for spore germination, which is a G1-specific event in the yeast cell cycle [23, 30, 31]. The results presented here indicate that GBG1 is not essential for the viability and growth of cells but instead is involved in cell wall biogenesis by affecting the activities of cell wall synthesis enzymes.
Acknowledgments
Part of this work was supported by a grant from the KOSEF (961-0501-003-2).
References
- 1.Vink E, Vossen JH, Ram AF, van den Ende H, Brekelmans S, de Nobel H, et al. The protein kinase Kic1 affects 1,6-beta-glucan levels in the cell wall of Saccharomyces cerevisiae. Microbiology. 2002;148:4035–4048. doi: 10.1099/00221287-148-12-4035. [DOI] [PubMed] [Google Scholar]
- 2.Shepherd MG. Cell envelope of Candida albicans. Crit Rev Microbiol. 1987;15:7–25. doi: 10.3109/10408418709104445. [DOI] [PubMed] [Google Scholar]
- 3.Spreghini E, Davis DA, Subaran R, Kim M, Mitchell AP. Roles of Candida albicans Dfg5p and Dcw1p cell surface proteins in growth and hypha formation. Eukaryot Cell. 2003;2:746–755. doi: 10.1128/EC.2.4.746-755.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohmann S, Mager WH. Yeast stress responses. New York: Springer; 2003. [Google Scholar]
- 5.Roh DH, Bowers B, Riezman H, Cabib E. Rho1p mutations specific for regulation of β(1→3)glucan synthesis and the order of assembly of the yeast cell wall. Mol Microbiol. 2002;44:1167–1183. doi: 10.1046/j.1365-2958.2002.02955.x. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara S, Hirata A, Nogami S, Beauvais A, Latge JP, Ohya Y. Homologous subunits of 1,3-beta-glucan synthase are important for spore wall assembly in Saccharomyces cerevisiae. Eukaryot Cell. 2007;6:143–156. doi: 10.1128/EC.00200-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cabib E, Bowers B, Sburlati A, Silverman SJ. Fungal cell wall synthesis: the construction of a biological structure. Microbiol Sci. 1988;5:370–375. [PubMed] [Google Scholar]
- 8.Lee DW, Ahn GW, Kang HG, Park HM. Identification of a gene, SOO1, which complements osmo-sensitivity and defect in in vitro beta1,3-glucan synthase activity in Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1450:145–154. doi: 10.1016/s0167-4889(99)00041-5. [DOI] [PubMed] [Google Scholar]
- 9.Burke D, Dawson D, Stearns T. Methods in yeast genetics: a Cold Spring Harbor Laboratory course manual. Plainview: Cold Spring Harbor Laboratory Press; 2000. [Google Scholar]
- 10.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview: Cold Spring Horbor Laboratory Press; 1989. [Google Scholar]
- 11.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ausuble FM, Brent R, Kingston RE, Moore DD, Seidmen JG, Smith JA, et al. Current protocols in molecular biology. New York: Greene Publishing & Wiley Interscience; 1998. [Google Scholar]
- 13.Choi WJ, Cabib E. The use of divalent cations and pH for the determination of specific yeast chitin synthetases. Anal Biochem. 1994;219:368–372. doi: 10.1006/abio.1994.1278. [DOI] [PubMed] [Google Scholar]
- 14.Kang MS, Cabib E. Regulation of fungal cell wall growth: a guanine nucleotide-binding, proteinaceous component required for activity of (1-3)-β-D-glucan synthase. Proc Natl Acad Sci U S A. 1986;83:5808–5812. doi: 10.1073/pnas.83.16.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 16.Manners DJ, Masson AJ, Patterson JC. The structure of a β-(1→3)-D-glucan from yeast cell walls. Biochem J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.White CA, Kennedy JF. Oligosaccharides. In: Chaplin MF, Kennedy JF, editors. Carbohydrate analysis: a practical approach. Oxford: IRL Press; 1986. pp. 38–39. [Google Scholar]
- 18.Tracey MV. Chitin. In: Peach K, Tracey MV, editors. Modern methods of plant analysis. Berlin: Springer-Verlag; 1956. pp. 264–274. [Google Scholar]
- 19.Ishiguro J, Saitou A, Durán A, Ribas JC. cps1+, a Schizosaccharomyces pombe gene homolog of Saccharomyces cerevisiae FKS genes whose mutation confers hypersensitivity to cyclosporin A and papulacandin B. J Bacteriol. 1997;179:7653–7662. doi: 10.1128/jb.179.24.7653-7662.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurtz MB, Douglas C, Marrinan J, Nollstadt K, Onishi J, Dreikorn S, et al. Increased antifungal activity of L-733,560, a water-soluble, semisynthetic pneumocandin, is due to enhanced inhibition of cell wall synthesis. Antimicrob Agents Chemother. 1994;38:2750–2757. doi: 10.1128/aac.38.12.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin EH, Lee DW, Kim JM, Park HM. Cloning of a gene involved in biosynthesis of β-1,3-glucan in Saccharomyces cerevisiae. Korean J Mycol. 1995;23:129–138. [Google Scholar]
- 22.Repetto B, Tzagoloff A. Structure and regulation of KGD1, the structural gene for yeast alpha-ketoglutarate dehydrogenase. Mol Cell Biol. 1989;9:2695–2705. doi: 10.1128/mcb.9.6.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zahedi RP, Sickmann A, Boehm AM, Winkler C, Zufall N, Schönfisch B, et al. Proteomic analysis of the yeast mitochondrial outer membrane reveals accumulation of a subclass of preproteins. Mol Biol Cell. 2006;17:1436–1450. doi: 10.1091/mbc.E05-08-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Athenstaedt K, Daum G. 1-Acyldihydroxyacetone-phosphate reductase (Ayr1p) of the yeast Saccharomyces cerevisiae encoded by the open reading frame YIL124w is a major component of lipid particles. J Biol Chem. 2000;275:235–240. doi: 10.1074/jbc.275.1.235. [DOI] [PubMed] [Google Scholar]
- 25.García-Rodriguez LJ, Trilla JA, Castro C, Valdivieso MH, Durán A, Roncero C. Characterization of the chitin biosynthesis process as a compensatory mechanism in the fks1 mutant of Saccharomyces cerevisiae. FEBS Lett. 2000;478:84–88. doi: 10.1016/s0014-5793(00)01835-4. [DOI] [PubMed] [Google Scholar]
- 26.Pringle JR, Preston RA, Adams AE, Stearns T, Drubin DG, Haarer BK, et al. Fluorescence microscopy methods for yeast. Methods Cell Biol. 1989;31:357–435. doi: 10.1016/s0091-679x(08)61620-9. [DOI] [PubMed] [Google Scholar]
- 27.Dodou E, Treisman R. The Saccharomyces cerevisiae MADS-box transcription factor Rlm1 is a target for the Mpk1 mitogen-activated protein kinase pathway. Mol Cell Biol. 1997;17:1848–1859. doi: 10.1128/mcb.17.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.el-Sherbeini M, Clemas JA. Nikkomycin Z supersensitivity of an echinocandin-resistant mutant of Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1995;39:200–207. doi: 10.1128/aac.39.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazur P, Morin N, Baginsky W, el-Sherbeini M, Clemas JA, Nielsen JB, et al. Differential expression and function of two homologous subunits of yeast 1,3-β-D-glucan synthase. Mol Cell Biol. 1995;15:5671–5681. doi: 10.1128/mcb.15.10.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M, Watanabe D, Nogami S, Morishita S, Ohya Y. Comprehensive and quantitative analysis of yeast deletion mutants defective in apical and isotropic bud growth. Curr Genet. 2009;55:365–380. doi: 10.1007/s00294-009-0251-0. [DOI] [PubMed] [Google Scholar]
- 31.Loewen CJ, Gaspar ML, Jesch SA, Delon C, Ktistakis NT, Henry SA, et al. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science. 2004;304:1644–1647. doi: 10.1126/science.1096083. [DOI] [PubMed] [Google Scholar]
- 32.Kamboj A, Kiran R, Sandhir R. N-acetylcysteine ameliorates carbofuran-induced alterations in lipid composition and activity of membrane bound enzymes. Mol Cell Biochem. 2006;286:107–114. doi: 10.1007/s11010-005-9100-8. [DOI] [PubMed] [Google Scholar]
- 33.McElhaney RN. Bronner F, Kleinzeller A. Current topics in membranes and transport. New York: Academic Press; 1982. Effects of membrane lipids on transport and enzymic activities; pp. 317–380. [Google Scholar]