Abstract
Single ascospore isolates of Cordyceps bassiana were observed for their colony pigmentation on Sabouraud Dextrose agar plus Yeast Extract (SDAY) plates and were inoculated in a brown rice medium for production of fruiting bodies. Colony pigmentation did not show any relationship with perithecial stromata formation. The isolates were also grown on opposite sides of SDAY agar plates and were observed for vegetative compatibility. Neither vegetative compatibility nor perithecial stromata could be found to be related to each other. It was concluded that fertile fruiting body production was independent of colony pigmentation and vegetative compatibility. Synnemata formation was found to be more common than perithecial stromata formation. This might be due to its highly conidiogenous anamorphic stage, i.e., Beauveria bassiana.
Keywords: Cordyceps bassiana, Perithecial stromata, Synnemata
Until now, only two Cordyceps species out of more than 500 reported have been shown as having Beauveria as their anamorphs. The first of these, C. brongniartii, was grown in vitro from Coleopteran larvae infected with B. brongniartii in Japan [1]. Nearly two decades later, it was recently reported as wild, growing on Coleopteran larva in Japan [2]. The second species, C. bassiana, was described as growing from a Lepidopteran cadaver in the wild, from China [3, 4]. Recently, C. bassiana has been successfully grown in vitro, with the aim of developing it for commercial use [5-7].
Cordyceps have been a subject of curiosity for hundreds of years. They have been prized for their hidden medicinal values, especially in the Eastern Asian society. A more recent, worldwide interest in these fungi as a medicinal mushrooms has instigated the development of much research. The challenge to develop it commercially is in understanding their growth habit in culture conditions. The growth of C. militaris, the cosmopolitan species, was the first to be attempted in vitro [8, 9]. Ever since then, many researchers have tried to grow its stromata under different nutritional and environmental conditions. Besides the biotic factors, the bipolar heterothallic type of mating system has been shown as the principal genetic factor for fertile stromata production of C. militaris [10]. The mysteries of Cordyceps are slowly being deciphered.
A wide range of variation in colony texture, pigmentation and growth rate has been observed in C. militaris [11]. Also, it has been shown that colony pigmentation has no correlation with mating type in C. militaris [12]. C. bassiana isolates produce synnemata very profusely from both single isolates and their combinations, although perithecial stromata are also produced, usually from crossings of mating-compatible isolates [5]. C. bassiana isolates either produce yellow pigmentation or do not produce any pigmentation and remain white. It was also observed that two isolates, when inoculated on opposite sides of agar plates, either avoided each other at the meeting line or overlapped each other and induced thick mycelial growth. Hence, the aim of this study is to understand relationship between fruiting body formation and colony pigmentation and vegetative compatibility in C. bassiana.
Materials and Methods
Fungal isolates
Ten single ascospore isolates were derived from in vitro stromata produced from crossing two isolates of C. bassiana EFCC 12511a and b [5]. The colony pigmentations of the isolates were observed on Sabouraud Dextrose agar plus Yeast Extract (SDAY) plates, following the methods of Shrestha et al. [11]. The isolates were numbered from EFCC 12511a × b 1 to EFCC 12511a × b 10. Among the isolates, isolate numbers. EFCC 12511a × b 1, 3, 6, 9 and 10 did not produce any pigmentation and remained white. The remaining isolates, EFCC 12511a × b 2, 4, 5, 7 and 8, produced yellow pigmentation.
Relationship between colony pigmentation and fruiting body formation
In order to acertain the relationship between colony pigmentation and fruiting body formation, all ten isolates of EFCC 12511a × b were inoculated in brown rice medium following the methods of Shrestha et al. [10] and observed for the fertile stromata formation.
Relationship between vegetative compatibility and fruiting body formation
For observation of vegetative compatibility, all isolates were inoculated on opposite sides of SDAY agar plates in all possible combinations of two and incubated for 3 wk at 25℃. The meeting lines between the isolates on SDAY agar plates were observed. The isolates were also inoculated in brown rice medium for fruiting body formation, as previously mentioned.
Results and Discussion
Relationship between colony pigmentation and fruiting body formation
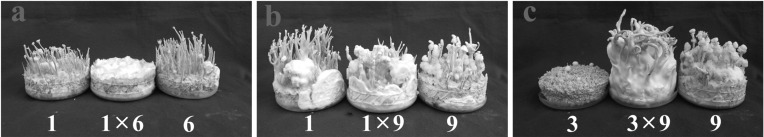
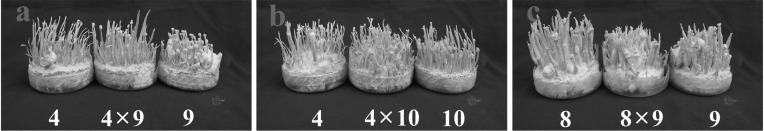
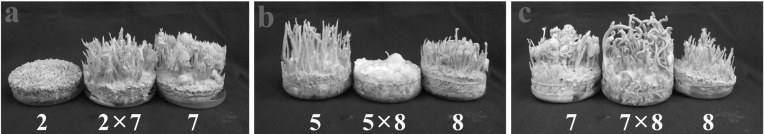
Single ascospore isolates of C. bassiana principally showed two types of pigmentations: white and yellow. In wild, the color of C. bassiana is yellow. White colonies have also been reported, though much less in numbers, in C. militaris [11, 12], but it is quite common in C. bassiana. Crossings between white isolates EFCC 12511a × b 1 × 6 and 1 × 9 produced only mycelial growth or synnemata, but not perithecial stromata, while the crossing 3 × 9 produced perithecial fruiting bodies (Fig. 1). None of the crossings between yellow and white isolates 4 × 9, 4 × 10 and 8 × 9 produced perithecial fruiting bodies (Fig. 2). From the crossings between yellow isolates, only 7 × 8 produced perithecial fruiting bodies, while 2 × 7 and 5 × 8 produced only synnemata or none at all (Fig. 3). Here, it can be noted that colony pigmentation does not correlate with the fertile fruiting body production. In this experiment, none of the crossings involving white and yellow isolates produced perithecial fruiting bodies, while crossings involving only white isolates or yellow isolates produced fertile fruiting bodies. However, synnemata production was very frequent. For synnemata production, crossing was not necessary; rather it was even detrimental. Isolates 1 and 6, and 5 and 8 produced synnemata while inoculated in single, but their crossings did not produce any synnemata at all (Figs. 1a and 3b). Similarly, isolate 7 in single produced synnemata, but crossing with isolate 2 did not enhance synnemata production (Fig. 3a).
Fig. 1.
Fruiting body formation from white isolates of Cordyceps bassiana EFCC 12511a × b. Only crossing of isolates 3 × 9 produced perithecial fruiting bodies.
Fig. 2.
Fruiting body formation from white and yellow isolates of Cordyceps bassiana EFCC 12511a × b. None of the crossings produced perithecial fruiting bodies.
Fig. 3.
Fruiting body formation from yellow isolates of Cordyceps bassiana EFCC 12511a × b. Only crossing of isolates 7 × 8 produced perithecial fruiting bodies.
Relationship between vegetative compatibility and fruiting body formation
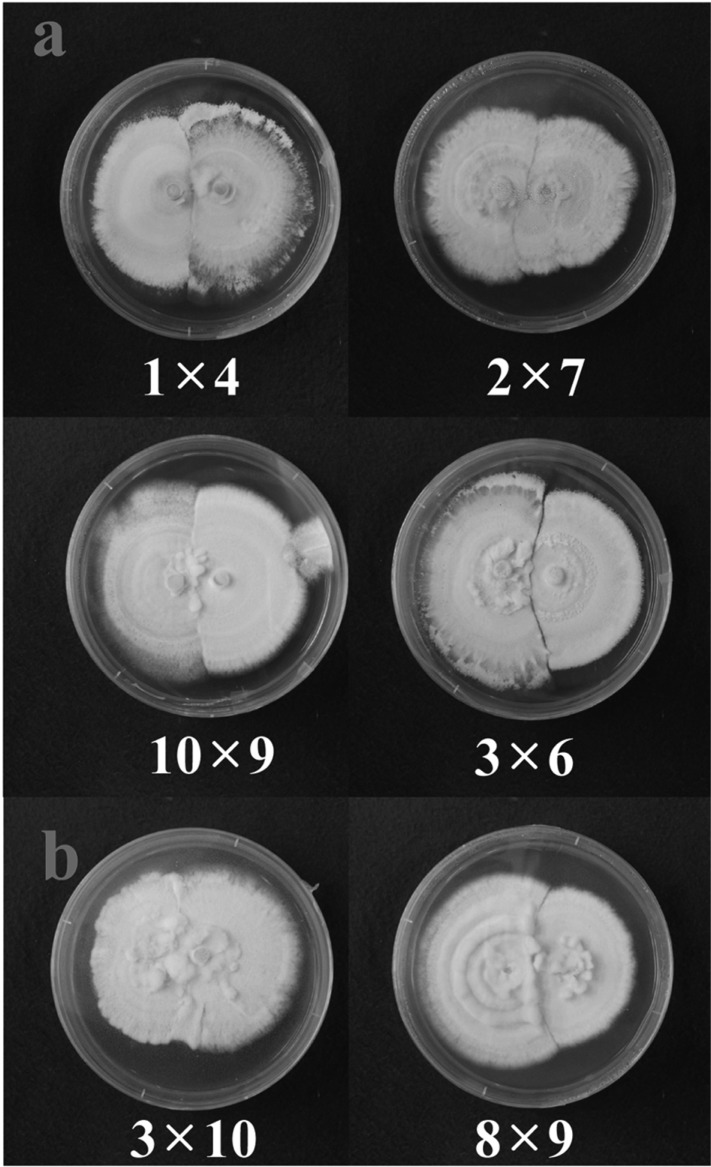
In this study, vegetative compatibility was assessed by observing the growth patterns at the meeting line between two isolates inoculated on opposite sides of agar plates. Combinations 1 × 4, 2 × 7, 10 × 9 and 3 × 6 showed somewhat distinct meeting lines as growth was avoided, showing that they are vegetatively incompatible with one another (Fig. 4A). Combinations 3 × 10 and 8 × 9 did not produce a distinct meeting line between the isolates (Fig. 4B). The isolates overlapped each other and induced thicker mycelial growth at the meeting line, showing vegetative compatibility between these isolates (Fig. 4B). Colony pigmentation did not show any correlation with vegetative compatibility. White isolates showed both vegetative compatibility and incompatibility between them, similar to yellow isolates (Fig. 4).
Fig. 4.
Vegetative compatibility of Cordyceps bassiana EFCC 12511a × b isolates on SDAY agar plates. A, A distinct line could be observed between two isolates; B, No distinct line was observed between the isolates. Further, mycelial growth was enhanced at the meeting line.
Crossings 1 × 4 and 2 × 7 did not produce fruiting bodies, although the isolates produced synnemata when inoculated in single (Fig. 5A). On the other hand, crossings 10 × 9 and 3 × 6 produced perithecial fruiting bodies (Fig. 5B). Crossings 3 × 10 and 8 × 9 did not produce any fruiting bodies or produced only synnemata, but not perithecial fruiting bodies (Fig. 5B). Thus, it could be said that vegetative incompatibility can induce fertile fruiting body production, while vegetative compatibility does not guarantee it. Further studies are necessary to understand the nature of vegetative compatibility in C. bassiana by using chemicals or mutant isolates.
Fig. 5.
Fruiting body formation from Cordyceps bassiana EFCC 12511a × b isolates. A, Fruiting body formation from vegetative incompatible isolates; B, Fruiting body formation from vegetative compatible isolates.
Although C. bassiana shows the pattern of bipolar heterothallic type of mating system, the fertile fruiting body production is very unstable, which is in contrast with C. militaris [5, 10]. Also, synnemata production is much more common than perithecial stromata production in C. bassiana. The colonies tend to show easy dispersal of conidia on agar plates. These characteristics might be due to its highly conidiogenous anamorphic state. In nature, C. bassiana is quite rare, probably because its anamorphic state is highly dominant in nature.
The environmental factors are very important for the production of fruiting bodies in mushrooms. The experiments so far have shown that both perithecial stromata and synnemata are produced under the same nutritional and environmental conditions. Perhaps the range of environmental factors is more narrow for perithecial stromata formation, compared to synnemata formation. Thus, further studies are necessary to understand the specific biotic factors that can enhance fertile fruiting body production. For genetic improvement of the fungal isolates, perithecial stromata formation in culture is very important so that genetically stable strains can be derived from ascospores.
Acknowledgements
The authors wish to acknowledge the financial support from Ministry for Food, Agriculture, Forest and Fishery. Also, acknowledgement goes to Cordyceps Research Institute for providing facilities to carry out this study.
References
- 1.Shimazu M, Mitsuhashi W, Hashimoto H. Cordyceps brongniartii sp. nov., the teleomorph of Beauveria brongniartii. Trans Mycol Soc Jpn. 1988;29:323–330. [Google Scholar]
- 2.Sasaki F, Miyamoto T, Tamai Y, Yajima T. Note on Cordyceps brongniartii Shimazu collected from the wild in Japan. Mycoscience. 2007;48:312–315. [Google Scholar]
- 3.Huang B, Li CR, Li ZG, Fan MZ, Li ZZ. Molecular identification of the teleomorph of Beauveria bassiana. Mycotaxon. 2002;81:229–236. [Google Scholar]
- 4.Li ZZ, Li CR, Huang B, Fan MZ. Discovery and demonstration of the teleomorph of Beauveria bassiana (Bals.) Vuill., an important entomogenous fungus. Chin Sci Bull. 2001;46:751–753. [Google Scholar]
- 5.Lee JO, Shrestha B, Kim TW, Sung GH, Sung JM. Stable formation of fruiting body in Cordyceps bassiana. Mycobiology. 2007;35:230–234. doi: 10.4489/MYCO.2007.35.4.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sung JM, Lee JO, Humber RA, Sung GH, Shrestha B. Cordyceps bassiana and production of stromata in vitro showing Beauveria anamorph in Korea. Mycobiology. 2006;34:1–6. doi: 10.4489/MYCO.2006.34.1.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JO, Shrestha B, Sung GH, Han SK, Sung JM. Successful development of Cordyceps bassiana stromata from Beauveria bassiana. Mycobiology. 2010;38:13–16. doi: 10.4489/MYCO.2010.38.1.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Bary A. Zur kenntniss insectentodtender Pilze. Bot Zeit. 1867;25:1–7. 9–13, 17–21. [Google Scholar]
- 9.de Bary A. Comparative morphology and biology of the fungi, mycetozoa and bacteria, English translation. Oxford: Clarendon Press; 1887. [Google Scholar]
- 10.Shrestha B, Kim HK, Sung GH, Spatafora JW, Sung JM. Bipolar heterothallism, a principal mating system of Cordyceps militaris in vitro. Biotechnol Bioprocess Eng. 2004;9:440–446. [Google Scholar]
- 11.Shrestha B, Lee WH, Han SK, Sung JM. Observations on some of the mycelial growth and pigmentation characteristics of Cordyceps militaris isolates. Mycobiology. 2006;34:83–91. doi: 10.4489/MYCO.2006.34.2.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shrestha B, Choi SK, Kim HK, Kim TW, Sung JM. Genetic analysis of pigmentation in Cordyceps militaris. Mycobiology. 2005;33:125–130. doi: 10.4489/MYCO.2005.33.3.125. [DOI] [PMC free article] [PubMed] [Google Scholar]