Abstract
β-Glucans have been known to exhibit antitumor activities by potentiating host immunity by an unknown mechanism. The C-type lectin dectin-1, a β-glucan receptor, is found on the macrophage and can recognize various β-glucans. Previously, we demonstrated the presence of β-glucan receptor, dectin-1, on the Raw 264.7 cells as well as on murine mucosal organs, such as the thymus, the lung, and the spleen. In order to investigate immunopotentiation of innate immunity by β-glucan, we stimulated a murine macrophage Raw 264.7 cell line with β-glucans from Pleurotus ostreatus, Saccharomyces cerevisiae, and Laminaria digitata. Then, we analyzed cytokines such as tumor necrosis factor (TNF)-α and interleukin (IL)-6 by reverse transcription-polymerase chain reaction (RT-PCR). In addition we analyzed gene expression patterns in β-glucan-treated Raw 264.7 cells by applying total mRNA to cDNA microarray to investigate the expression of 7,000 known genes. When stimulated with β-glucans, the macrophage cells increased TNF-α expression. When co-stimulation of the cells with β-glucan and lipopolysaccharide (LPS), a synergy effect was observed by increased TNF-α expression. In IL-6 expression, any of the β-glucans tested could not induce IL-6 expression by itself. However, when co-stimulation occurred with β-glucan and LPS, the cells showed strong synergistic effects by increased IL-6 expression. Chip analysis showed that β-glucan of P. ostreatus increased gene expressions of immunomodulating gene families such as kinases, lectin associated genes and TNF-related genes in the macrophage cell line. Induction of TNF receptor expression by FACS analysis was synergized only when co-stimulated with β-glucan and LPS, not with β-glucan alone. From these data, β-glucan increased expressions of immunomodulating genes and showed synergistic effect with LPS.
Keywords: β-Glucan, Cytokines, Laminarin, Microarray, Pleurotus ostreatus
β-Glucan is a family of polysaccharides that have linkages of β-(1,3), β-(1,4) and β-(1,6). Among the linkages, only β-(1,3)-glucan has immunomodulating activities. Diverse biological functions of β-glucan have been reported as having anti-tumor [1], anti-fungal [2], radioprotective [3], and cholesterol reducing [4] activities: these effects could be efficacious to anti-aging efforts by preventing infection, tumor, radiation, and oxidation.
Dectin-1 belongs to a C-type lectin type II and functions as a β-glucan receptor on the various immune cells. Type II C-type lectins contain only one carbohydrate recognition domains at their carboxy-terminal extracellular domain [5]. The cytoplasmic domains of the C-type lectins are diverse and contain several conserved motifs that are important for antigen uptake: a tyrosine-containing coated-pit intracellular targeting motif, a triad of acidic amino acids and a dileucine motif [6]. Dectin-1 was found to be widely expressed in mouse tissues, acting as a pattern recognition receptor, recognizing a variety of carbohydrates containing β-1,3- and/or β-1,6-glucan linkages and intact Saccharomyces cerevisiae and Candida albicans [7].
Lipopolysaccharide (LPS) is amphipathic glycolipid, constituting the outer membrane of Gram-negative bacteria. In plasma, the acute phase LPS-bindng protein (LBP) dissociates LPS aggregates by LBP and transfers LPS to CD14. The LPS receptor CD14 is anchored on the plasma membrane by a glycoslyphosphatidylinositol anchor and therefore is unable to transduce signals to the interior of the cell [8, 9]. LPS is the causal agent of gram-negative infection and of septic shock in particular.
cDNA microarray analysis allows us to examine the expression of tens of thousands of genes that can be monitored simultaneously and rapidly and, in turn, provides an opportunity to determine the effects of certain agents. To analyze gene expression modulated by β-glucan (Pleurotus ostreatus) treated murine Raw 264.7 macrophage, the total mRNA was applied to cDNA microarray to investigate the expression of 7,000 known genes. The gene expression profiles of various types of immunomodulation by β-glucan were analyzed using cDNA microarray. This information may contribute to the determination of β-glucan's molecular mechanism of action.
The aim of the present investigation is to study the effect of a β-glucan alone, or in combination with LPS, on cytokine mRNA production activity determined by cDNA microarray and reverse transcription (RT)-PCR in β-glucan treated murine macrophage Raw 264.7 cells.
Materials and Methods
Reagents
β-Glucans of P. ostreatus, and Saccharomyces cerevisiae, Laminarin from Laminaria digitata and LPS were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). PCR primers were synthesized by Takara-Korea Biomedicals Inc. (Seoul, Korea). A two-step RNA PCR kit was purchased from Takara Biomedicals (Shiga, Japan). DMEM, RPMI, penicillin-streptomycin and TRIzol reagent were purchased from Gibco-BRL (Grand Island, NY, USA). AMV reverse transcriptase was purchased from Promega (Sunnyvale, CA, USA). Mouse-7.4k set 1 cDNA chip was purchased from Digital-Genomics (Seoul, Korea).
Cell culture
Mouse monocyte-macrophage Raw 264.7 (ATCC, Rockville, MD, USA) cells were maintained in DMEM medium (Life Technologies, Inc., Eggenstein, Germany) and MEM medium (Sigma-Aldrich Co.) supplemented with 10% heat inactivated fetal bovine serum (JRH Biosciences Co., Lenexa, KS, USA), penicillin (100 U/mL)-streptomycin (100 µg/mL) (Life Technologies, Inc.) and incubated at 37℃ in 5% CO2.
RNA isolation
Total RNA was extracted from the cultured Raw 264.7 cells with TRIzol reagent used according to the manufacturer's instructions. The cells were lysed by adding 1 mL of TRIzol reagent to a 6-well plate, and passing the cell lysate several times through a pipette. The 0.2 mL of chloroform was added and the lysates were shaken vigorously by hand for 15 sec and incubated at room temperature for 2 to 3min. After centrifugation for 15 min, the aqueous phase was transferred to a fresh tube. The RNA was washed with isopropyl alcohol and with 75% ethanol. The RNA pellet was briefly dried, dissolved in RNase-free water, and stored at -70℃ until used.
cDNA microarray
Raw 264.7 macrophage cells were treated with the following: PBS as a control, 100 ng/mL LPS single treatment, and co-treatment of 100 µg/mL of β-glucans from P. ostreatus and 100 ng/mL of LPS. Total mRNA from each sample was isolated by TRIzol according to RNA isolation protocols. RNA yields were measured by UV absorbance and its quality was assessed by agarose gel electrophoresis with ethidium bromide staining for visualization of ribosomal RNA band integrity. In general, the standard RNA processing and hybridization protocols were followed as recommended by Digital-Genomics. cDNA for each sample was synthesized using a Superscript II RTase Synthesis kit (Invitrogen, Carlsbad, CA, USA) and anchored oligo (dT). Then, the Cy-dyelabeled cRNA was transcribed in vitro from cDNA using a mixture (dUTP nucleotide mix, dUTP Cy-dye-labeled nucleotide, Cyscript reverse transcriptase, 5× cyscript buffer, 0.1M DTT) and purified using the Cyscribe GFX kit. The purification cRNA was fragmented by incubation in fragmentation buffer at 95℃ for 2 min and chilled on ice. The fragmented labeled cDNA was applied for the mouse-7.4k set 1 cDNA chip (Digital-Genomics), which contains 7,365 mouse gene (known genes: 6,990) cDNA probes, and hybridized to the probes. After washing and staining, the arrays were scanned using an Array Biochip Reader (Applied Precision, Inc., Greenland, NH, USA). Two independent experiments were performed to verify the reproducibility of results. The gene expression levels of samples were normalized and analyzed using ImaGene 5 and Gene Sight 3.2 (BioDiscovery, Inc., Marina del Rey, CA, USA).
RT-PCR
A two-step RNA PCR kit (Takara Biomedicals) was used for the reverse transcription from RNA to cDNA using AMV reverse transcriptase (Promega) and subsequent amplification in utilizing AMV-optimized Taq DNA polymerase. For the amplification of tumor necrosis factor (TNF)-α and interleukin (IL)-6, denatured at 94℃, 30 sec, denatured at 65℃ and 55℃, 30 sec and polymerized at 72℃, 60 sec and finally 1 minute at 72℃, respectively. The PCR products were identified by 2% agarose gel electrophoresis. The PCR primers were designed on the basis of mouse TNF-α (NM_013693) and IL-6 (X54542) sequences:
TNF-αF (forward primer): 5'-CGGGATCCATGAGCACAGAAAGCAT-3'
TNF-αR (reverse primer): 5'-CCCAAGCTTTCACAGAGCAATGACTCC-3'
IL-6-Fa (forward primer): 5'-ATGAAGTTCCTCTCTGCAAG-3'
IL-6-Ra (reverse primer): 5'-GGTTTGCCGAGTACATCTCA-3'
Flow cytometry
Cells of 1 × 106 were washed twice with PBS, and then resuspended in PBS at a concentration of 1 × 106 cells/mL. One hundred µL of cell suspension was transferred to 1.5 mL centrifuge tube. Cells were then incubated at room temperature in the dark for 15 min with 5 µL of 1 µg/5 µL Phycoerythrin hamster anti-mouse TNF receptor (TNFR) type II (p75) monoclonal antibody (BD Biosciences Pharmingen, San Diego, CA, USA). After adding binding buffer or PBS (400 µL), cells were analyzed by flow cytometry with FACSCalibur™ (Becton Dickinson Co., Bectin Dr. Franklin Lakes, NJ, USA). The percentage of cells was determined by assessing the percentage bound TNF receptor-PE. At least 5,000 cells were analyzed for each sample.
Results
cDNA microarray analysis
cDNA microarray technology is a powerful screening tool for identification genes regulated by β-glucan and/or LPS. In the intestinal environment, macrophage cells resident in Peyer's patch can be activated by the absorbed β-glucan together with penetrated intestinal Gram-negative bacteria. We stimulated Raw 264.7 macrophage cells with PBS, LPS, and co-treatment with β-glucan from P. ostreatus and LPS for 6 hr. Total control was the sample PBS treatment versus 100 ng/mL of LPS single treatment and total tester was the sample 100 ng/mL of LPS single treatment versus co-treatment of 100 µg/mL of β-glucans from P. ostreatus and 100 ng/mL of LPS. Analysis of gene expression induced by β-glucan was detected that compared total tester gene expression ratio with total control gene expression ratio. The gene expression of Raw 264.7 murine macrophage was assessed using cDNA microarray. When genes were classified by molecular function, LPS or LPS plus β-glucan from P. ostreatus treatment up-regulated those genes of immunomodulating genes and signal transducing-related kinases (Table 1).
Table 1.
Classification of genes with altered expression in Raw 264.7 cells treated with LPS alone or LPS plus β-glucan for 6 hr
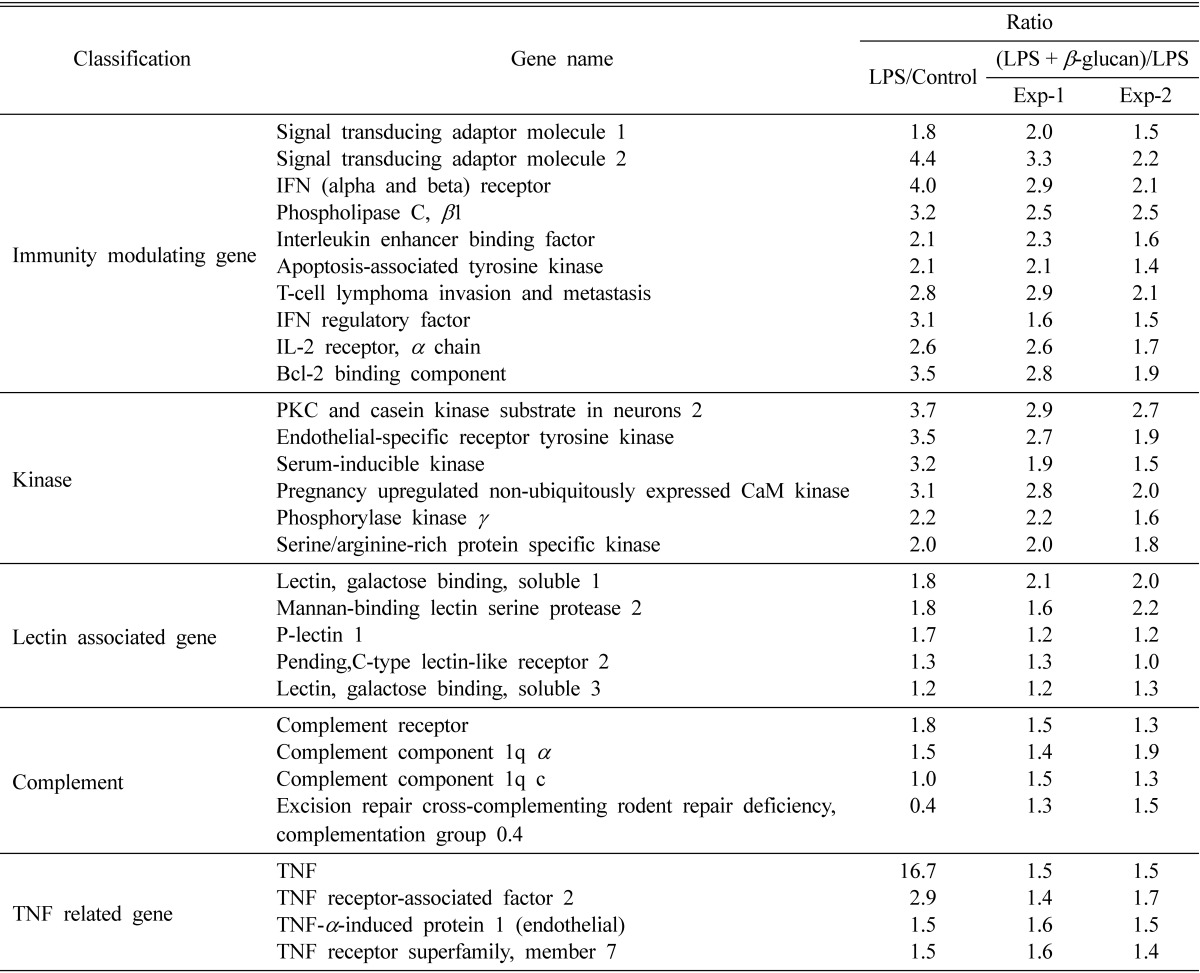
Treatment concentration: LPS (100 ng/mL), β-glucan of Pleurotus ostreatus (100 µg/mL).
LPS, lipopolysaccharide; IFN, interferon; IL, interleukin; PKC, protein kinase C; TNF, tumor necrosis factor.
LPS and β-glucans induced TNF-α and IL-6 transcriptionally
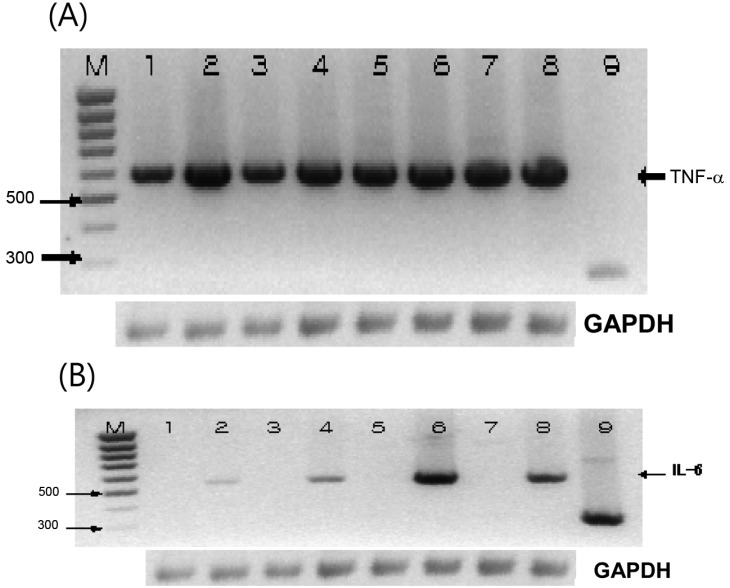
In our previous report, we found high expression of dectin-1 on the Raw 264.7 murine macrophage cell line [10]. Therefore, we stimulated Raw 264.7 cells with various β-glucans for the analysis of immunomodulating genes. In order to investigate whether β-glucans could lead to the state of activation of cells, which are responsible for the cytokine production and activation of immune system, we treated Raw 264.7 cells with β-glucan alone or together with LPS for 12 hr. Those β-glucans used were originated from P. ostreatus, S. cerevisiae and Laminaria digitata. When we treated macrophage cell line with β-glucan, not only LPS induced TNF-α, but also all the β-glucans alone induced TNF-α very strongly. Transcriptional expression of TNF-α seemed to reach a plateau using β-glucan alone, since no synergistic effects were observed using co-stimulation with LPS to the assay system (Fig. 1A). IL-6 induction, however, was not observed in the system treated with β-glucan alone. When we added LPS to this system, the induction of IL-6 was obvious and showed a synergistic effect of co-stimulation with β-glucan and LPS. In the IL-6 induction assay, β-glucan from P. ostreatus showed the strongest effect among the β-glucans tested on the macrophage cell line (Fig. 1B).
Fig. 1.
Co-stimulation with lipopolysaccharide (LPS) and β-glucan induced tumor necrosis factor (TNF)-α and interleukin (IL)-6 mRNA detected by reverse transcription (RT)-PCR. Raw 264.7 cell were treated with LPS (100 ng/mL), Saccharomyces cerevisiae β-glucan (100 µg/mL), Pleurotus ostreatus β-glucan (100 µg/mL), and laminarin 100 µg/mL for 12 hr. M: Marker, 1: Control, 2: LPS (100 ng/mL), 3: S. cerevisiae β-glucan (100 µg/mL), 4: S. cerevisiae β-glucan (100 µg/mL) and LPS (100 ng/mL), 5: P. ostreatus β-glucan (100 µg/mL), 6: P. ostreatus β-glucan (100 µg/mL) and LPS (100 ng/mL), 7: Laminarin (100 µg/mL), 8: Laminarin (100 µg/mL) and LPS (100 ng/mL), (A)-9: TNF-α positive control DNA (275 bp), and (B)-9: IL-6 positive control. (A) and (B) RT-PCR for GAPDH was used as control.
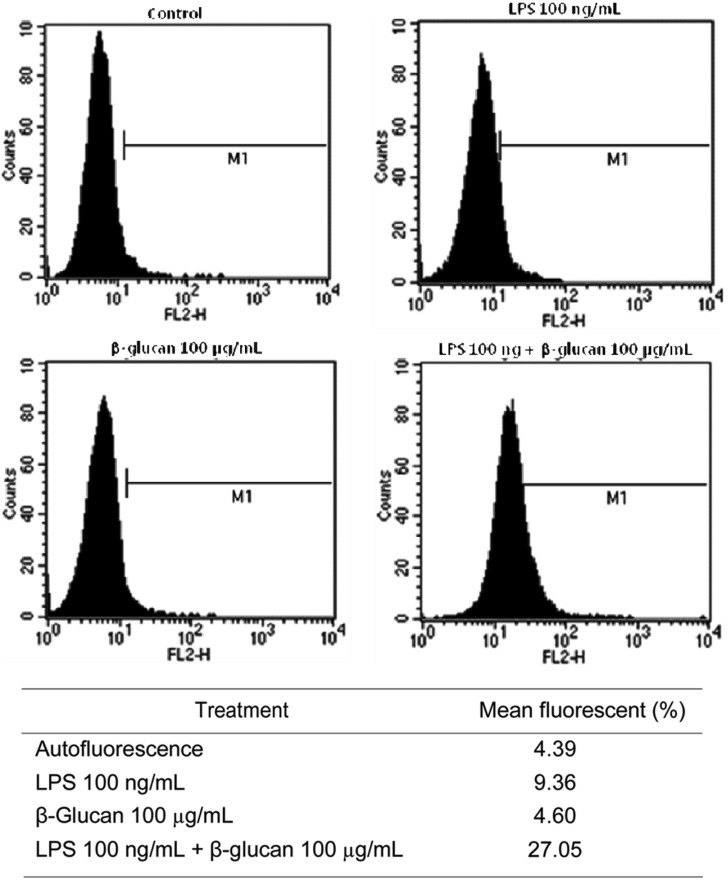
Expression of TNFR by FACS analysis
The cDNA microarray result showed that TNF or were induced by the treatment with P. ostreatus β-glucan in Raw 264.7 macrophage cell line. So we confirmed the induction of the TNFR at protein level by staining with PE-conjugated hamster anti-mouse TNFR monoclonal antibody using FACS analysis. Raw 264.7 cells were treated with LPS, P. ostreatus β-glucan, or co-treatment with LPS and P. ostreatus β-glucan for 6 hr. The arbitrary mean value of autofluorescence was 4.39 by FACS analysis. When LPS or β-glucans was treated singly, mean fluorescent were 9.36 and 4.60, respectively. When both LPS and β-glucans were added simultaneously, a strong synergistic effect was observed with respect to TNFR expression increasing mean fluorescent to 27.05 (Fig. 2). It was obvious that a TNFR was synergistically induced by the co-treatment with LPS and β-glucan, but not with LPS or β-glucan alone.
Fig. 2.
Expression of tumor necrosis factor receptor induced by lipopolysaccharide (LPS) and β-glucan detected by FACS analysis. Raw 264.7 cell were treated with LPS (100 ng/mL), Pleurotus ostreatus β-glucan (100 µg/mL), and LPS (100 ng/mL) and P. ostreatus β-glucan (100 µg/mL) for 6 hr.
Discussion
Glucans have been identified as having diverse immunomodulating activities in vitro and in vivo, as well as clinically. In this report, we identified induction of cytokines and immunomodulating molecules by the murine macrophage cell line as a transcriptional level by RT-PCR and cDNA microarray. Our results from microarray having 7,000 known genes provided an analysis of the cellular response to β-glucan treatment. The microarray analysis showed that β-glucan of P. ostreatus increased genes of immunomodulating molecules, kinases, lectin-associated families, complements and TNF families in the Raw 264.7 cell line. Based on microarray analysis, we have examined the activities of β-glucans on the cytokine production. When treated with β-glucan and LPS, induction of gene expression of TNF-α, and IL-6 was confirmed by RT-PCR analysis. Induction of TNFR type II expression was confirmed by FACS analysis. It was suggested that immunomodulation with β-glucan might stimulate the expression of cytokine genes. Systemic analysis of cytokines suggested that β-glucans could promote T cell-specific responses, perhaps through triggering the secretion of interferon-γ, IL-6, IL-8, and IL-12 from macrophages [11], neutrophils [12], and natural killer cells to kill sensitive tumor cells [13]. Exposure to β-glucan can induce the production of a cascade pro-immflammatory cytokines, where TNF-α can regulate the production of IL-6. Both TNF-α and IL-6 play key roles in the modulation of apoptosis [14]. Regulation of apoptosis has also been proposed to play an important role in the modulation of the inflammatory response [15]. Cytokines not only modulate apoptosis but also can enhance the capacity of the macrophage to ingest apoptotic neutrophils [16, 17].
In conclusion, our results can be one of the mechanisms of immunopotentiation by orally administered β-glucan, which can modulate innate immunity by inducing TNF-α, TNFR, and IL-6 from macrophages resident in intestinal organs together with LPS that derived from intestinal floral.
Acknowledgements
This work was supported financially by a grant from the sabbatical research program from the University of Seoul (UOS) to H.W. Kim. The authors appreciate this support.
References
- 1.Shim JH, Sung KJ, Cho MC, Choi WA, Yang Y, Lim JS, et al. Antitumor effect of soluble beta-1,3-glucan from Agrobacterium sp. R259 KCTC 1019. J Microbiol Biotechnol. 2007;17:1513–1520. [PubMed] [Google Scholar]
- 2.Azevedo Cde M, Marques SG, Resende MA, Gonçalves AG, Santos DV, da Silva RR, et al. The use of glucan as immuno-stimulant in the treatment of a severe case of chromoblastomycosis. Mycoses. 2008;51:341–344. doi: 10.1111/j.1439-0507.2007.01485.x. [DOI] [PubMed] [Google Scholar]
- 3.Patchen ML, MacVittie TJ, Solberg BD, Souza LM. Survival enhancement and hemopoietic regeneration following radiation exposure: therapeutic approach using glucan and granulocyte colony-stimulating factor. Exp Hematol. 1990;18:1042–1048. [PubMed] [Google Scholar]
- 4.Reyna-Villasmil N, Bermúdez-Pirela V, Mengual-Moreno E, Arias N, Cano-Ponce C, Leal-Gonzalez E, et al. Oat-derived beta-glucan significantly improves HDLC and diminishes LDLC and non-HDL cholesterol in overweight individuals with mild hypercholesterolemia. Am J Ther. 2007;14:203–212. doi: 10.1097/01.pap.0000249917.96509.e7. [DOI] [PubMed] [Google Scholar]
- 5.Ariizumi K, Shen GL, Shikano S, Xu S, Ritter R, 3rd, Kumamoto T, et al. Identification of a novel, dendritic cell-associated molecule, dectin-1, by subtractive cDNA cloning. J Biol Chem. 2000;275:20157–20167. doi: 10.1074/jbc.M909512199. [DOI] [PubMed] [Google Scholar]
- 6.Figdor CG, van Kooyk Y, Adema GJ. C-type lectin receptors on dendritic cells and Langerhans cells. Nat Rev Immunol. 2002;2:77–84. doi: 10.1038/nri723. [DOI] [PubMed] [Google Scholar]
- 7.Brown GD, Gordon S. Immune recognition: a new receptor for β-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 8.Tobias PS, Tapping RI, Gegner JA. Endotoxin interactions with lipopolysaccharide-responsive cells. Clin Infect Dis. 1999;28:476–481. doi: 10.1086/515163. [DOI] [PubMed] [Google Scholar]
- 9.Wiese A, Brandenburg K, Ulmer AJ, Seydel U, Müller-Loennies S. The dual role of lipopolysaccharide as effector and target molecule. Biol Chem. 1999;380:767–784. doi: 10.1515/BC.1999.097. [DOI] [PubMed] [Google Scholar]
- 10.Kim SI, Park HG, Cho GH, Ko IS, Kim HW. Cooperative effect of the lipopolysaccharide and culinary-medicinal cauliflower mushroom Sparassis crispa (Wulf.). Fr (Aphyllophoromycetideae)-derived β-glucan on inflammatory cytokine secretion by the murine macrophage cell line. Int J Med Mushrooms. 2009;11:9–20. [Google Scholar]
- 11.Di Luzio NR, Mcnamee R, Jones E, Lassoff S, Sear W, Hoffmann EO. Inhibition of growth and dissemination of Shay myelogenous leukemic tumor in rats by glucan and glucan activated macrophages. Adv Exp Med Biol. 1976;73:397–413. doi: 10.1007/978-1-4684-3300-5_34. [DOI] [PubMed] [Google Scholar]
- 12.Morikawa K, Takeda R, Yamazaki M, Mizuno D. Induction of tumoricidal activity of polymorphonuclear leukocytes by a linear β-1,3-D-glucan and other immunomodulators in murine cells. Cancer Res. 1985;45:1496–1501. [PubMed] [Google Scholar]
- 13.Scaringi L, Marconi P, Boccanera M, Tissi L, Bistoni F, Cassone A. Cell wall components of Candida albicans as immunomodulators: induction of natural killer and macrophage-mediated peritoneal cell cytotoxicity in mice by mannoprotein and glucan fractions. J Gen Microbiol. 1988;134:1265–1274. doi: 10.1099/00221287-134-5-1265. [DOI] [PubMed] [Google Scholar]
- 14.Wollenberg GK, DeForge LE, Bolgos G, Remick DG. Differential expression of tumor necrosis factor and interleukin-6 by peritoneal macrophages in vivo and in culture. Am J Pathol. 1993;143:1121–1130. [PMC free article] [PubMed] [Google Scholar]
- 15.Savill J, Haslett C. Granulocyte clearance by apoptosis in the resolution of inflammation. Semin Cell Biol. 1995;6:385–393. doi: 10.1016/s1043-4682(05)80009-1. [DOI] [PubMed] [Google Scholar]
- 16.Ren Y, Savill J. Proinflammatory cytokines potentiate thrombospondin-mediated phagocytosis of neutrophils undergoing apoptosis. J Immunol. 1995;154:2366–2374. [PubMed] [Google Scholar]
- 17.Takeda Y, Watanabe H, Yonehara S, Yamashita T, Saito S, Sendo F. Rapid acceleration of neutrophil apoptosis by tumor necrosis factor-alpha. Int Immunol. 1993;5:691–694. doi: 10.1093/intimm/5.6.691. [DOI] [PubMed] [Google Scholar]