Abstract
63 strains of Aspergillus section Fumigati were isolated from 17 samples of arable soil in a central province of Korea. Based on the results of genotypic and phenotypic analyses, they were identified as Aspergillus fumigatus, A. lentulus, Neosartorya coreana, N. fennelliae, N. fischeri, N. glabra, N. hiratsukae, N. laciniosa, N. pseudofischeri, N. quadricincta, N. spinosa and N. udagawae. Among these, N. fennelliae, N. hiratsukae, N. quadricincta, and N. udagawae had not been previously recorded in Korea. The diversity of Aspergillus section Fumigati species from arable soil in Korea is also addressed.
Keywords: Aspergillus section Fumigati, Korea, Neosartorya, Soil
Aspergillus section Fumigati (AsF) is an economically important fungus and teleomorphic species of this section belong to the genus Neosartorya. Eight strictly mitotic species and 17 Neosartorya species in AsF are generally accepted [1].
The diversity of AsF species in Korean soil remains poorly characterized. Aspergillus fumigatus has generally been reported in paddy fields [2-6] and forest soils [7-9] and Neosartorya fischeri has been detected primarily in paddy fields [2, 6]. On the contrary, A. fumigatus has been frequently studied in clinical environments in Korea [10-12]. Recently, Hong et al. [13] reported two new species--Neosartorya coreana and N. laciniosa--in Korean soil, with Korean strains of N. fischeri, N. glabra, N. pseudofischeri, and N. spinosa. Hong et al. [14] also isolated Aspergillus lentulus from Korean soil.
In this study, we isolated AsF strains from arable soil in a central province of Korea, and identified their species by genotypic and phenotypic characteristics in order to evaluate the diversity of AsF in Korean soils.
Materials and Methods
Soil samples
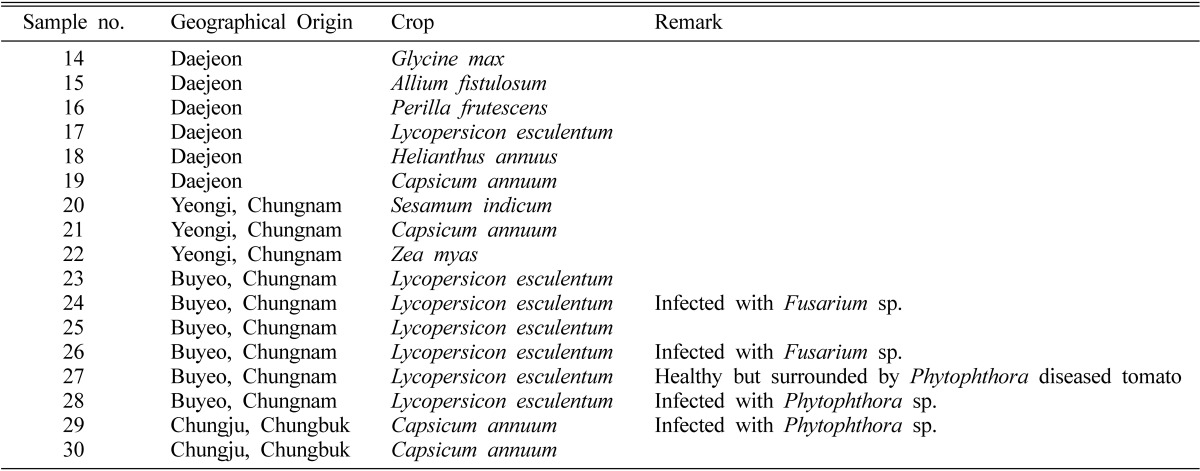
Soil samples were collected from 17 arable soil sites. Their geographical origins and crops are listed in Table 1. The soil samples were preserved at 4℃ until analysis.
Table 1.
List of arable soil samples in Korea used in this study
Isolation of Aspergillus strains
From each soil sample, 10 g of soil was suspended in 90 mL of sterilized water in a 250 mL flask and was shaken for 30 min at 200 rpm. Two isolation methods were utilized for each soil sample. To screen the xerophilic strains, the suspensions were diluted further by factors of 10. One mL aliquots of the suspensions (10-1 to 10-3) were pipetted into 9 cm Petri dishes and mixed with 20 mL of undercooled (ca. 45℃) Dichloran 18% Glycerol Agar (31.5 g Dichloran-Glycerol Agar Base [Oxoid CM0729], 220 g glycerol, 0.1 g chloramphenicol, 1 L distilled water). To screen thermo-tolerant strains (for ascospores of Neosartorya), the suspensions were incubated in water baths at 75℃ for 30 min. 25 mL heat-treated suspensions were pipetted into 14.5 mm Petri dishes and mixed with equal volumes of undercooled (ca. 45℃) MEA ds-chloramphenicol (50 mg/L). The plates were incubated for 2~4 days for the screening of xerophilic strains, and for 7~21 days for thermotolerant strains. Fungal colonies which showed typical Aspergillus, Penicillium and their teleomorphic morphology under a stereo-microscope were transferred into malt extract agar (MEA), and incubated for 7~21 days, after which their genera were determined on the basis of macromorphology. Among them, strains of AsF and its teleomorph Neosartorya were inoculated at three points on MEA, Czapeks agar (CZA), and oatmeal agar (OA), then incubated for 7~21 days. Their morphological features were evaluated in detail by stereo- and compound microscopy, and strains that evidenced characteristics identical to the other strains were removed. Finally, 63 strains were selected, and are listed in Table 2. They were transferred to MEA slants, incubated, and stored at 15℃ until use. The strains were then lyophilized and preserved in the Korean Agricultural Culture Collection (KACC in National Agrobiodiversity Center, NAAS, RDA, Korea), and are publicly available for research.
Table 2.
Aspergillus section Fumigati strains used in this study
aFront two digits in the numbers correspond to the sample number in Table 1.
bIn random amplified polymorphic DNA (RAPD)-PCRs, the strain showed the same band patterns with the strain written in RAPD column.
cGenBank accession number of β-tubulin gene.
CBS, Centraalbureau voor Schimmelcultures, Utrecht, the Netherlands; KACC, Korean Agricultural Culture Collection, Suwon, Korea; T, type strain; NP, not performed.
Species identification
For morphological identification, micro- and macro-morphology analyses were conducted and SEM was also carried out to identify teleomorphic species, according to the method of Hong et al. [14]. To evaluate heterothallism, conidial strains were crossed with one another on OA and MEA media and in all combinations with heterothallic strains of Neosartorya fennelliae, CBS 598.74T (MT A) and CBS 599.74T (MT a), N. udagawae, CBS 114217T and CBS 114218T, N. spathulata, CBS 408.89T (MT A) and CBS 406.89T (MT a), with 28 days of incubation at 25℃.
For molecular identification, modified versions of the methods of Hong et al. [14] were utilized. Random amplified polymorphic DNA (RAPD)-PCRs with the primers PELF and URP1F were conducted for 63 strains, and 38 genetically different strains were selected. Their β-tubulin genes were sequenced and their sequences were analyzed with the type strains of related species to determine the taxonomic positions of the Korean strains.
Results and Discussion
Mycoflora of arable soil in Korea
Mycoflora in arable soil were analyzed via the dilute plate technique with DG-18 and heat treatments. 440 strains were isolated from 17 arable soil samples. The composition of the strains is presented in Table 3. Strains of AsF and its teleomorph, Neosartorya were screened via macromorphology on MEA, CZA, and OA and micromorphology, and 63 strains were ultimately selected (Table 2). The phenotypic (Figs 1 and 2) and genotypic (Fig. 3) analyses allowed the 63 strains to be identified as Aspergillus fumigatus, A. lentulus, Neosartorya coreana, N. fennelliae, N. fischeri, N. glabra, N. hiratsukae, N. laciniosa, N. pseudofischeri, N. quadricincta, N. spinosa and N. udagawae.
Table 3.
Composition of fungal strains isolated from 17 samples of arable soil in Korea

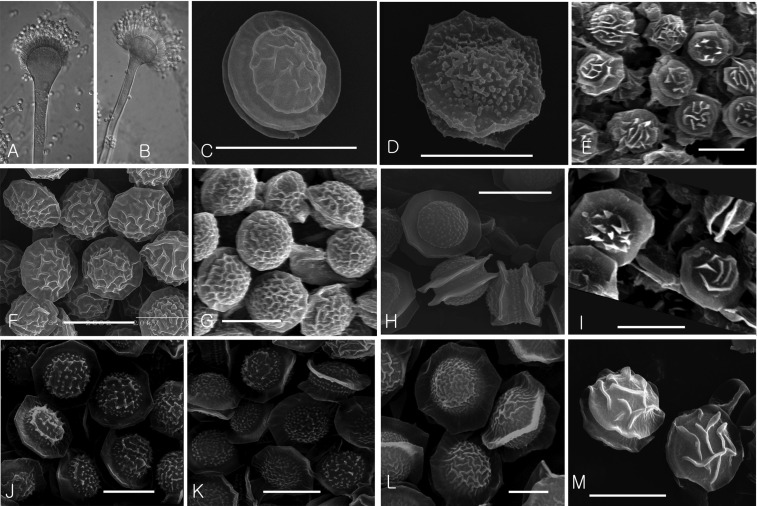
Fig. 1.
Aspergillum (A, B) of strict anamorphic species and ascospores (C~M) of Neosartorya spp. in Aspergillus section Fumigati from arable soil in Korea (scale bar = 5 µm). A, Aspergillus fumigatus (Korean Agricultural Culture Collection [KACC]) 41388); B, A. lentulus (KACC 41642); C, Neosartorya fennelliae (KACC 41675 X Centraalbureau voor Schimmelcultures [CBS] 598.74T); D, N. udagawae (KACC 41683 X CBS 114218T); E, N. quadricincta (KACC F3810); F, N. fischeri (KACC 41664); G, N. hiratsukae (KACC 41688); H, N. glabra (KACC 41654); I, N. pseudofischeri (KACC 41661); J, N. spinosa (KACC 41663); K, N. laciniosa (KACC 41658); L, N. coreana (KACC 41659); M, Neosartorya sp. (KACC 41691).
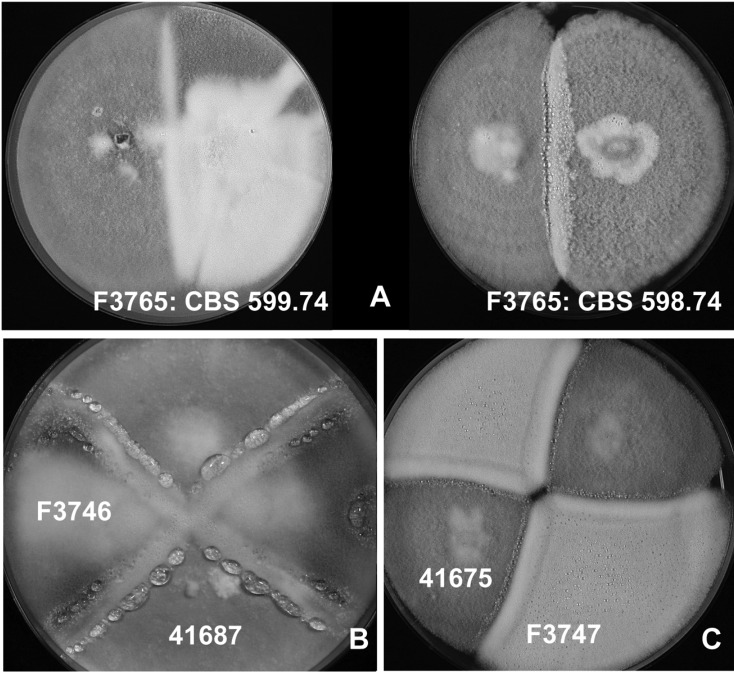
Fig. 2.
Mating behavior of Korean strains of Neosartorya fennelliae. A, Mating of Korean Agricultural Culture Collection (KACC) F3765 with type strains of N. fennelliae CBS 598.74T (MT A) and Centraalbureau voor Schimmelcultures (CBS) 599.74T (MT a), KACC F3765 produced ascomatal with CBS 598.74 (MT A); B, Ascomata formation between KACC F3746 and 41687, C. ascomata formation between KACC 41675 and F3747.
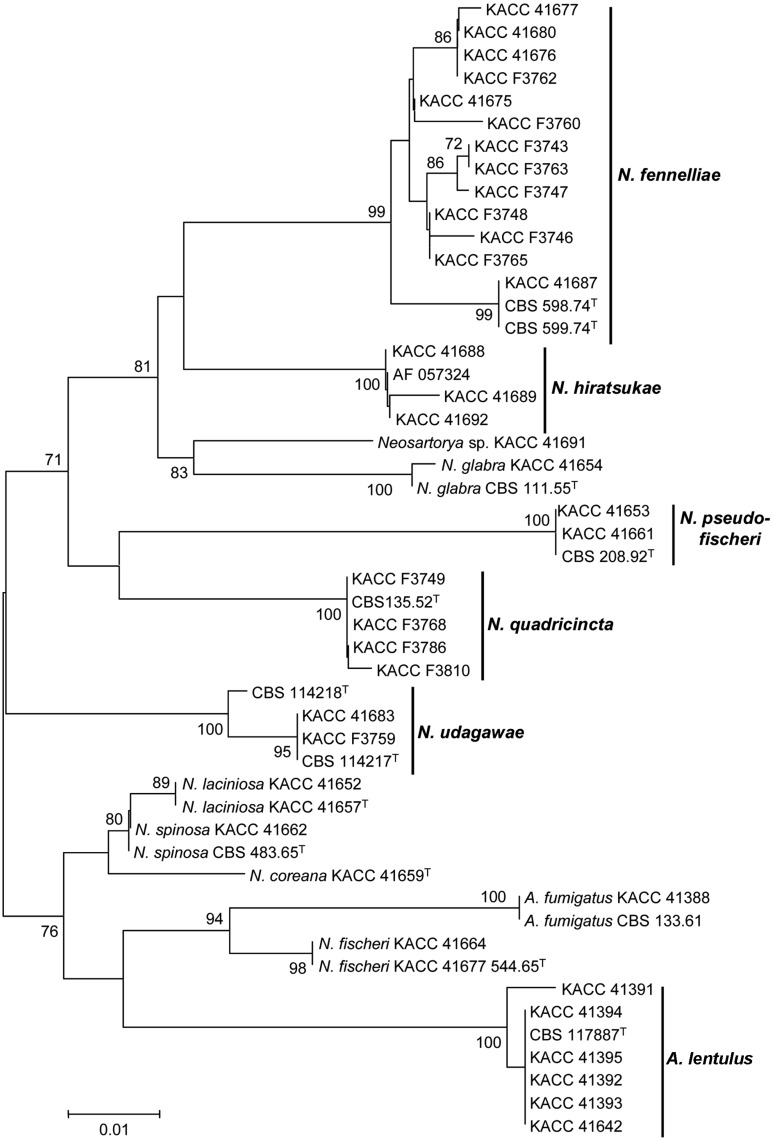
Fig. 3.
Taxonomic position of Korean strains of Aspergillus section Fumigati based on partial β-tubulin phylogeny. Partial β-tubulin gene (primers bt2a and bt2b) sequences were first analyzed using the Tamura-Nei parameter distance calculation model with gamma-distributed substitution rates, which was then used to construct the Neighbor-Joining tree with MEGA version 3.1. The numbers above or below the nodes represent bootstrap values of > 70% (out of 1,000 bootstrap replications). KACC strains were isolated from arable soil in Korea.
Strict anamorph species in AsF
Aspergillus fumigatus and A. lentulus were isolated in this study. A. fumigatus was readily identified on the basis of its dark green to turquoise color and velutinous colony, and fast and abundant conidiation on MEA and Czapek yeast extract agar (CYA). Vesicles (15~23[25] µm) and stipe (6~8[10] µm) widths of species from Korean soils were generally larger than those of the A. lentulus strains. The species were isolated from almost all of the examined soil samples.
A. lentulus strains from Korea evidenced slower growth, slower conidiation, thinner stipes (4~6 µm), and more globose vesicles than were observed in A. fumigatus (Fig. 1). Seven strains of A. lentulus isolated from Korean soil grew at 10℃, but did not grow at 50℃. In our analysis of β-tubulin phylogeny, the strains were found to be clustered with the type strain of A. lentulus, CBS 11787 (Fig. 3). The species has been reported only from clinical environments [14]. Interestingly, it was isolated from 7 of 17 soil samples, with a very high frequency (Table 2). This result indicates that A. lentulus can frequently be isolated from soil as well as the clinical environment, and its ecological niche could be in soil. Differentiation between A. fumigatus and A. lentulus was previously described well by Hong et al. [14].
Heterothallic species in AsF
Twenty one strains produced ascomata only when mated with compatible strains (Fig. 2). Seventeen strains from 11 soil samples were identified as N. fennelliae on the basis of their mating behavior and β-tubulin gene sequence, and their mating types were denoted in Table 2. N. fennelliae was characterized by its ascospores, which evidenced two equatorial crests and shallow rugose to reticulate convex surfaces (Fig. 1). Colonies of the species on CYA and MEA evidenced diverse patterns depending on the strains, usually with minimal to sparse conidiation, and the strains often evidenced a light yellowish color on the obverse and reverse of the CYA and MEA plates. Although 17 strains produced ascomata when mated with compatible strains in N. fennelliae, the intraspecific heterogeneity was high. KACC 41687, CBS 598.74T, and CBS 599.74T were separated from the other strains, evidencing a minimum of 97.8% similarity with the others (Fig. 3). However, the intraspecific group of the three strains was not supported by calmodulin gene phylogeny (data not shown).
Four strains from Korean soil generated ascomata when mated with N. udagawae CBS 114218T (= KACC 41156), thereby suggesting an identification of N. udagawae. They were also clustered into the N. udagawae group in the β-tubulin tree (Fig. 3). The species evidenced lenticular ascospores with two equatorial or several irregular crests and tuberculate convex surfaces (Fig. 1). The species were found in only two soil samples and the strains of this study were composed of only one mating type.
Homothallic species in AsF
N. quadricincta was well separated from the other homothallic species in AsF, as it evidenced ascospores with four distinct equatorial crests. Nine strains from four soil samples were identified as N. quadricincta on the basis of ascospore morphology (Fig. 1) and β-tubulin gene sequence (Fig. 3). The species was characterized by comparatively slower growth on CZA (< 30 mm 7 days at 25℃) than on MEA, and ascospores with 4 prominent equatorial crests and convex surfaces harboring raised flaps of tissue or long ridgelines.
Neosartorya fischeri and N. hiratsukae in homothallic AsF species in Korea had ascospores with two closely appressed crests and reticulate convex surfaces. Two strains were identified as N. fischeri based on their ascospore morphology (Fig. 1) and β-tubulin gene sequence (Fig. 3). The species was characterized by ascospores with convex surfaces bearing coarse and irregular height networks. Five strains from three soil samples were identified as N. hiratsukae based on their ascospore morphology (Fig. 1) and β-tubulin gene sequences (Fig. 3). The species was characterized by very restricted growth on CZA (< 12 mm) and ascospores with convex surfaces bearing fine and irregular height networks.
Species evidencing ascospores with two distinctly separated equatorial crests were N. glabra, N. pseudofischeri, N. spinosa, N. laciniosa, and N. coreana in AsF in Korea. Their species differentiation was described by Hong et al. [13]. Four strains were identified as N. glabra, because they had ascospores with smooth convex surfaces and with relatively rigid equatorial crests (Fig. 1). β-Tubulin phylogeny supported this identification (Fig. 3). The five strains from five soil samples were identified as N. pseudofischeri, because they evidenced ascospores ornamented with raised flaps of tissue, in the form of triangular projections or long ridgelines (Fig. 1). This convex surface ornamentation was similar to that of N. quadricincta, and both shapes were somewhat roseate. However, N. pseudofischeri had two equatorial crests, whereas N. quadricincta had four crests. The identification of N. pseudofischeri was confirmed by the β-tubulin gene sequence tree (Fig. 3). N. spinosa, N. laciniosa, and N. coreana were isolated from four, two, and one soil samples, respectively, and the morphological and molecular characteristics of the Korean strains were previously described in detail by Hong et al. [13]. KACC 41691 was similar to N. pseudofischeri in terms of its ascospore morphology, but its phylogenetic position differed from that of N. pseudofischeri (Fig. 3). This may possibly represent a new species in AsF, but further examination will be required in order to clarify the taxonomic position of the strain.
AsF flora in arable soil
AsF species usually arrest attention in the fields of medical and food mycology. Only two species in AsF--A. fumigatus and N. fischeri--were described in the Compendium of Soil Fungi [16]. In this study, it is interesting to note that 13 extraordinary AsF species were isolated with high frequency from 17 arable soil samples. A. fumigatus, A. lentulus, N. fennelliae, and N. quadricincta were isolated with particularly high frequency. This may suggest that AsF species are widely distributed in arable soils in Korea and might perform important roles in crop growth.
References
- 1.Samson RA, Hong SB, Frisvad JC. 2006. Old and new concepts of species differentiation in Aspergillus. Med Mycol. 2006;44:S133–S148. doi: 10.1080/13693780600913224. [DOI] [PubMed] [Google Scholar]
- 2.Cho DH, Chun JK. Studies on the mold flora in the several degraded paddy soils. Agric Chem Biotechnol. 1962;3:17–18. [Google Scholar]
- 3.Lee BH, Kim SJ, Lee HW. The taxonomic studies of Korean Aspergilli. Korean J Microbiol. 1968;6:6–11. [Google Scholar]
- 4.Kim SJ. Taxonomic studies of Korean Aspergilli. Korean J Microbiol. 1971;9:1–26. [Google Scholar]
- 5.Lee YN, Kim NJ, Suh HW. Isolation and identification of Aspergillus. Korean J Microbiol. 1976;14:105–116. [Google Scholar]
- 6.Min KH, Ito T, Yokoyama T. Fungal flora of paddy field in Korea (IV), filamentous fungi isolated by heat treatment. Korean J Mycol. 1987;15:187–195. [Google Scholar]
- 7.Kim KS. Soil borne fungi of Phyllosticta reticulata forests in Korea (I) Korean J Mycol. 1979;7:91–116. [Google Scholar]
- 8.Min KH, Hong SW, Yokoyama T. Hyphomycetes from Korean soil (II): the genus Aspergillus and some other microfungi. Korean J Microbiol. 1980;18:104–114. [Google Scholar]
- 9.Song HS, Min KH. Microfungal flora of Tricholoma matsutake producing and nonproducing sites in the forest of Pinus densiflora. Korean J Mycol. 1991;19:109–119. [Google Scholar]
- 10.Park HS, Lim MK, Lee HS, Lee SS, Kim C, Lee KM, et al. A case of rheumatoid arthritis with eosinophilia and aspergilloma within the lung nodule resulting in bronchopleural fistula. Korean J Med. 1997;53:720–726. [Google Scholar]
- 11.Lee J, Cho B, Lee HJ, Kim SY, Chung NG, Kim HK. Successful treatment of cerebral aspergillosis in a child with acute lymphoblastic leukemia. Korean J Pediatr Hematol Oncol. 2000;7:121–128. [Google Scholar]
- 12.Shin JH, Lee CJ, Lee JY, Song JW, Kee SJ, Suh SP, et al. Random amplified polymorphic DNA (RAPD) analysis for Aspergillus species isolated from clinical specimens. Korean J Clin Microbiol. 2001;4:33–39. [Google Scholar]
- 13.Hong SB, Cho HS, Shin HD, Frisvad JC, Samson RA. Novel Neosartorya species isolated from soil in Korea. Int J Syst Evol Microbiol. 2006;56:477–486. doi: 10.1099/ijs.0.63980-0. [DOI] [PubMed] [Google Scholar]
- 14.Hong SB, Go SJ, Shin HD, Frisvad JC, Samson RA. Polyphasic taxonomy of Aspergillus fumigatus and related species. Mycologia. 2005;97:1316–1329. doi: 10.3852/mycologia.97.6.1316. [DOI] [PubMed] [Google Scholar]
- 15.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell. 2005;4:625–632. doi: 10.1128/EC.4.3.625-632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domsch KH, Gams W, Anderson TH. Compendium of soil fungi. Eching: IHW-Verla; 1993. [Google Scholar]