Abstract
The aim of this study was to determine the in vitro anti-inflammatory effect of hot water extract from Cordyceps militaris fruiting bodies (CMWE) on lipopolysaccharide (LPS)-stimulated nitric oxide (NO) production, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) release in RAW 264.7 cells. The treatment of macrophages with various concentrations of hot CMWE significantly reduced LPS-induced production as well as NO, TNF-α and IL-6 secretion in a concentration-dependent manner. These results suggest that CMWE have potent inhibitory effects on the production of these inflammatory mediators.
Keywords: Anti-inflammatory activity, Cordyceps militaris, Lipopolysaccharide, Nitric oxide, Pro-inflammatory cytokines, RAW 264.7 cell
Traditionally, various mushrooms have been used in many countries for maintianing health and for the prevention and treatment of variety diseases such as hepatitis, hypertension, hypercholesterolemia and gastric cancer [1]. These mushrooms include many different genera such as Cordyceps, Paecilomyces, Torrubiella and Podonectria. Cordyceps militaris, which belongs to the class Ascomycetes, has been long used as a folk medicine in Korea, China and Japan, and has more recently been used in western medicine due to its various physiological activities [2]. Especially, the chemical components of C. militaris, such as cordycepin [3], nucleoside [4] and various polysaccharides [5] have been widely studied. Cordycepin (3-deoxyadenosine), a metabolite of C. militaris, has been shown to inhibit the growth of various tumor cells [6]. Extracellular biopolymers from a mycelial liquid culture of C. militaris were reported to have anti-fibrotic effects on fibrotic rats induced by bile duct ligation and scission operation [7]. Recent studies have shown that mushroom extracts contain active components that suppress tissue injury associated with the pathological processes of many inflammatory diseases [8, 9]. In addition, 70% ethanol extracts prepared from fruiting bodies and cultured mycelia of C. militaris on croton oil-induced ear edema in mice were reported to show topical anti-inflammatory activity [10]. However, the pharmacological activities of C. militaris have not been well documented despite its increasing usage.
Inflammation is a beneficial host response to foreign challenge or tissue injury that ultimately leads to the restoration of normal tissue structure and function. A normal inflammatory response is self-limiting and involves the downregulation of pro-inflammatory protein expression, the increased expression of anti-inflammatory proteins, and a reversal in the vascular changes that facilitated the initial immune cell recruitment process [11]. Prolonged inflammation contributes to the pathogenesis of many inflammatory diseases, including bronchitis [12], gastritis [13], inflammatory bowel disease [14], multiple sclerosis [15] and rheumatoid arthritis [16].
Nitric oxide (NO), prostaglandin E2 (PGE2) and cytokines such as interleukin-1 beta (IL-1β), IL-6 and tumor necrosis factor-α (TNF-α), are well known for their involvement in the development of inflammation [17]. Macrophages play an important role in the regulation of inflammation and the immune response by releasing pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) and inflammatory factors (NO and PGE2) that recruit additional immune cells to sites of infection or tissue injury [18]. Following exposure to immune stimulants, including bacterial toxins such as lipopolysaccharide (LPS) and lipoteichoic acid, the production of these mediators by macrophages has been found in many inflammatory tissues along with increased expressions of their mRNAs [19]. Although NO and pro-inflammatory cytokines are involved in the host defense mechanism, their overproduction contributes to the pathogenesis of several diseases, such as sepsis, rheumatoid arthritis, atherosclerosis, pulmonary fibrosis and chronic hepatitis [20]. Thus, inhibiting the production of these inflammatory mediators may prevent or suppress various inflammatory diseases. In this study, we prepared hot water extract of C. militaris fruiting bodies and also investigated the effects of NO and pro-inflammatory cytokines from LPS-stimulated RAW 264.7 cells.
Materials and Methods
Materials and chemicals
RPMI 1640 media and fetal bovine serum were purchased from Gibco Ltd. (Grand Island, NY, USA). MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazolium bromide), LPS, L-NMMA (NG-Methyl-L-arginine acetate salt), polymyxin B, arachidonic acid and indomethacin were purchased from Sigma Chemicals Co. (St. Louis, MO, USA). TNF-α and IL-6 were obtained from BD science (San Jose, CA, USA) and all other solvents/chemicals were of reagent or analytical grade.
Preparation of hot water extraction of C. militaris
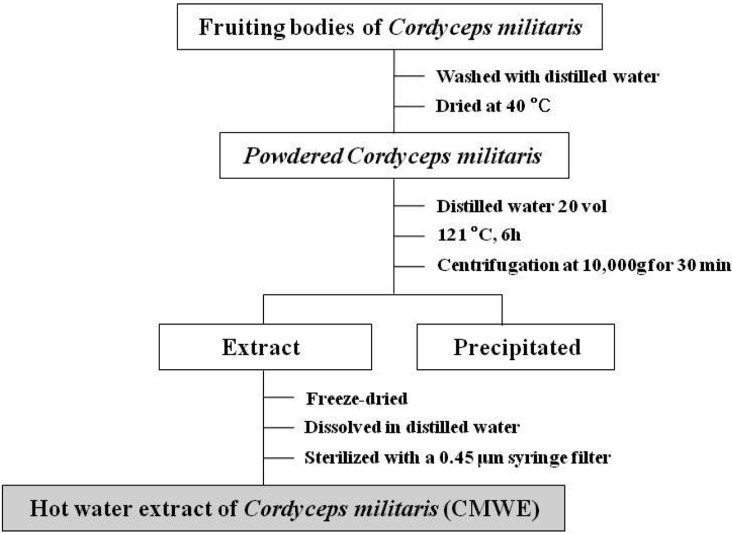
We used C. militaris strain that was previously identified by phylogenetic analysis [21] and prepared hot water extract from fruiting bodies. In brief, the fruiting bodies of C. militaris were washed with distilled water and dried at 40℃. Then, powdered C. militaris (50 g) was boiled with 1 L of water at 121℃ for 6 hr. The insoluble materials were removed by centrifugation at 10,000 g for 30 min at 4℃, and the resulting supernatants were freeze-dried. The dried hot water extract of C. militaris fruiting bodies (CMWE) was dissolved in distilled water and sterilized with a 0.45 µm syringe filter before being used for cell culture (Fig. 1).
Fig. 1.
The procedures for hot water extraction of Cordyceps militaris fruiting bodies (CMWE).
Cell culture and treatment
RAW 264.7 cells, a murine macrophage cell line, were grown in monolayer culture using RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 units/mL of penicillin, and 100 µg/mL of streptomycin. Cell cultures were grown at 37℃ in a humidified CO2 incubator (5% CO2). Cells were passaged prior to confluence by removing cells with trypsin/ehylenediaminetetra acetic acid solution, followed by centrifugation and reseeding. After 10~15 passages, RAW 264.7 cells were no longer used for these assays. The effect of CMWE on cytotoxicity was tested by treating cells with different concentrations of CMWE in RPMI-1640 medium.
MTT assay for cell viability
The number of viable cells was determined by the ability of mitochondria to convert MTT (3[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) to formazan dye. RAW 264.7 cells were cultured overnight in 96-well plates at a density of 3 × 104 cells/200 µL in each well. The next day, cells were coincubated with CMWE (78~1,250 µg/mL) for 24 hr. After 24 hr, the medium was removed and 10 µL of 10 mg/mL MTT was then added to each well, followed by incubation for another 4 hr at 37℃ under humidified 5% CO2 atmosphere. The MTT was removed and cells were lysed with 150 µL of DMSO. The absorbance was then measured at 550 nm using a microplate reader (OpsysMR, DYNEX, Ltd., Frankfurt, Germany).
NO assay
Nitrite that accumulated in the culture medium was measured as an indicator of NO production based on the Griess reaction. In brief, RAW264.7 cells were plated in a 24-well plate at a density of 2 × 105 cells/well in 1 mL of culture medium followed by incubation for 18 hr. Cells were then coincubated with CMWE (78~1,250 µg/mL) in 500 ng/mL of LPS for 24 hr. After 24 hr, the culture supernatant was collected for nitrite measurement. One-hundred µL of the spent medium were plated in a 96-well plate and an equal amount of Griess reagent (0.1% N-1-[naphthyl]ethylenediamine-diHCl and 1% sulfanilamide and 5% H3PO4) was added. The plate was incubated for 10 min, and the absorbance was measured at 550 nm using a microplate reader (OpsysMR, DYNEX. Ltd). The amount of NO was calculated using asodium nitrite standard curve.
Cytokine assays
The inhibitory effects of CMWE on the production of TNF-α and IL-6 were measured by enzyme-linked immunosorbent assay (ELISA) using culture supernatants collected from treated cells. RAW 264.7 cells were plated in a 24-well plate at a density of 2 × 105 cells/well in 1 mL of culture medium, followed by incubation for 18 hr and coincubation with 312.5~19.5 µg/mL of CMWE in 500 ng/mL of LPS for 24 hr. Cell-free culture supernatants were collected and assayed according to the instructions of the ELISA kit (OptiEIA, BD science) manufacturer to determine the amount of TNF-α and IL-6 released from the cell. Concentrations for two wells of each sample were determined and this experiment was performed in triplicate.
Statistical analysis of data
All values were expressed as mean ± the standard deviation (± SD). Statistical differences between the treatments and the control were evaluated by ANOVA (Dunnett's test) and Students-t tests. p < 0.05 was considered to be significant (*p < 0.05).
Results
Cell viability
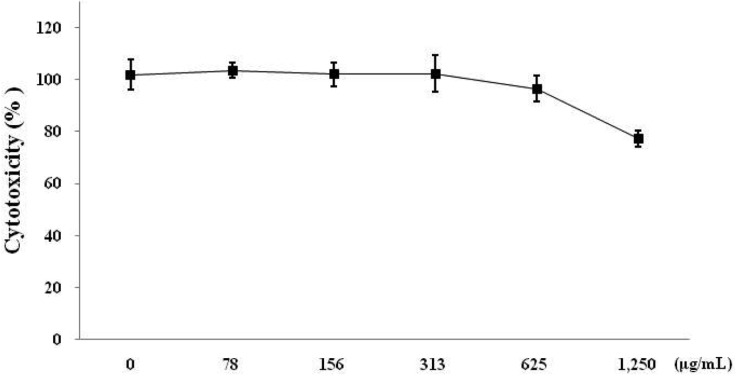
In order to determine whether CMWE causes toxicity in RAW 264.7 cells, cell viability was tested with various concentrations of CMWE by MTT assay. CMWE showed 77% cell viability at 1,250 µg/mL and little cytotoxic effects at concentrations from 78 to 1,250 µg/mL in RAW 264.7 cells (Fig. 2). Therefore, we tested the anti-inflammatory properties of CMWE under concentrations of 1,250 µg/mL and found that CMWE had no effect on cell cytotoxicity.
Fig. 2.
Cytotoxicity of hot water extract of Cordyceps militaris fruiting bodies (CMWE) in RAW 264.7 cells. RAW 264.7 cells were treated with 0, 78, 156, 313, 625, and 1250 µg/mL of CMWE for 24 hr. Cell viability was determined by MTT assay. Three independent assays were performed in triplicate and the data shown are the mean ± SD.
Inhibition of NO production by CMWE in LPS-stimulated RAW 264.7 cells
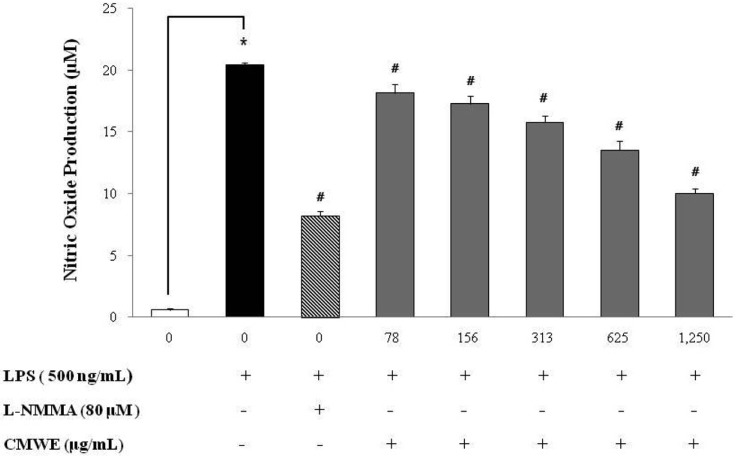
To analyze the potential anti-inflammatory properties of CMWE, we used RAW 264.7 murine macrophage cells, which can produce NO upon stimulation with LPS. As shown in Fig. 3, CMWE inhibited LPS-induced NO production in a dose-dependent manner. Unstimulated cells secreted basal levels of NO while LPS stimulation resulted in an increase in NO production (20.5 ± 0.18 µM; inhibition 0%). CMWE significantly inhibited the production of LPS-stimulated NO production in a concentration-dependent manner. NO production was reduced (10 ± 0.39 µM; inhibition 51%) at the highest concentration of CMWE tested (1,250 µg/mL), and CMWE had an inhibitory effect on NO production identical to that exhibited by L-MMNA (8.2 ± 0.37 µM; inhibition 57%) on NO synthase. This inhibition of NO production by LPS-stimulated murine macrophages upon L-NMMA treatment indicates an absolute requirement for L-arginine in NO production. Indeed, pegmatite showed strongly inhibited NO production the same as positive control.
Fig. 3.
Effects of hot water extract of Cordyceps militaris fruiting bodies (CMWE) on lipopolysaccharide (LPS)-induced nitirc oxide (NO) in murine macrophages. RAW 264.7 cells were treated with varying concentrations of CMWE in the presence of 500 ng/mL of LPS or LPS alone for 24 hr. L-NMMA was used as a positive control. Three independent assays were performed in triplicate and the data shown are the mean ± SD. *p < 0.05 vs. media alone and #p < 0.05 vs. LPS-treated group (control); significance of difference between treated groups by Students-t tests (*) and ANOVA followed by Dunnett's test (#).
Reduced production of pro-inflammatory cytokines in LPS-stimulated RAW 264.7 cells
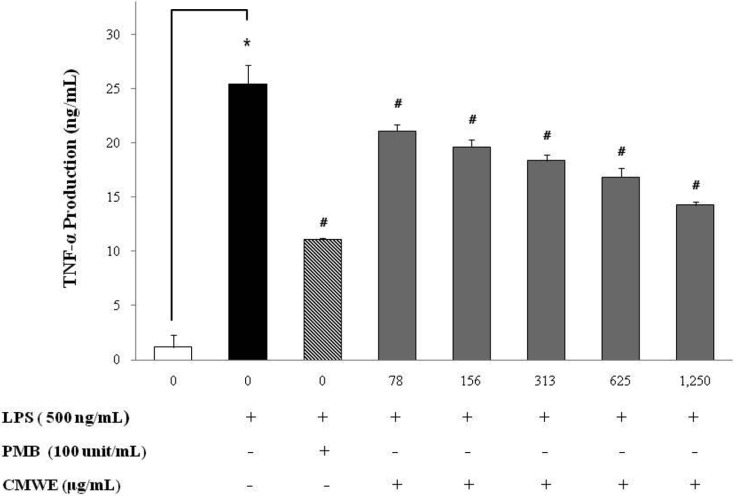
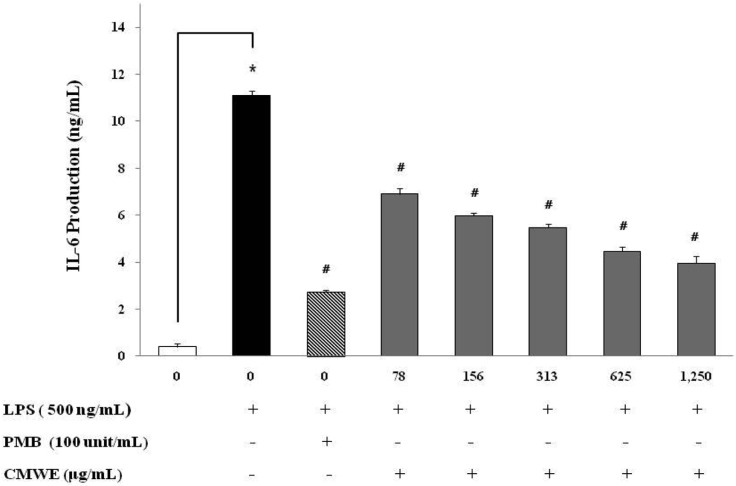
To examine the inhibitory activity of CMWE on the production of the pro-inflammatory cytokines TNF-α and IL-6, RAW 264.7 cells were incubated with various concentrations of CMWE in the presence of LPS for 24 hr. The levels of TNF-α and IL-6 in the cell-free culture supernatants as evaluated by ELISA were significantly increased in RAW 264.7 cells in response to LPS stimulation alone (Figs 4 and 5). LPS-induced TNF-α secretion was reduced by 14 ng/mL (inhibition; 44%) at a CMWE concentration of 1,250 µg/mL (Fig. 4), whereas LPS alone stimulated TNF-α secretion to 25.4 ng/mL (inhibition; 0%) in RAW 264.7 cells. We also found that treatment with 1,250 µg/mL of CMWE inhibits IL-6 secretion (4 ng/mL; inhibition 65%) when compared to untreated control (11.1 ng/mL; inhibition 0%) in LPS-stimulated RAW 264.7 cells (Fig. 5). In addition, treatment with polymixn B (100 U/mL) as a LPS inhibitor reduced TNF-α secretion by 11.1 ng/mL (inhibition; 57%) and IL-6 secretion by 2.7 ng/mL (inhibition; 76%). These results indicate that CMWE reduced the production of pro-inflammatory cytokines such as TNF-α and IL-6 in a concentration-dependent manner.
Fig. 4.
Effects of hot water extract of Cordyceps militaris fruiting bodies (CMWE) on lipopolysaccharide (LPS)-induced tumor necrosis factor-α (TNF-α) production by RAW 264.7 cells. Cells were treated with the indicated concentrations of CMWE in the presence of 500 ng/mL of LPS or LPS alone for 24 hr. The concentration of TNF-α in condition medium was analyzed by ELISA. Three independent assays were performed in triplicate and the data shown are the mean ± SD. *p < 0.05 vs. media alone and #p < 0.05 vs. LPS-treated group (control); significance of difference between treated groups by Students-t tests (*) and ANOVA followed by Dunnett's test (#).
Fig. 5.
Effects of hot water extract of Cordyceps militaris fruiting bodies (CMWE) on lipopolysaccharide (LPS)-induced interleukin 6 (IL-6) production by RAW 264.7 cells. Cells were treated with the indicated concentrations of CMWE in the presence of 500 ng/mL of LPS or LPS alone for 24 hr. The concentration of IL-6 in condition medium was analyzed by ELISA. Three independent assays were performed in triplicate and the data shown are the mean ± SD. *p < 0.05 vs. media alone and #p < 0.05 vs. LPS-treated group (control); significance of difference between treated groups by Students-t tests (*) and ANOVA followed by Dunnett's test (#).
Discussion
C. militaris is one of the most well known medicinal entomopathogenic fungi and as such has been widely used for the treatment of various diseases. However, the pharmacological and biochemical activities of C. militaris have not been clearly elucidated. To identify the anti-inflammatory effects of CMWE in traditional herbal medicine, the effects of CMWE on the production of NO, TNF-α and IL-6 in LPS-stimulated RAW 264.7 cells were investigated. These results showed that CMWE inhibits the production of major macrophage-derived inflammatory mediators in a dose-dependent manner.
NO synthesized by inducible nitric oxide synthesis (iNOS) has been reported as a major inflammatory mediator and seems to be involved in both acute and chronic inflammation [22]. The physiological or normal generation of NO mediates the bactericidal and tumoricidal actions of macrophages. However, the aberrant release of NO can lead to amplification of inflammation as well as tissue injury [23]. Large amounts of pro-inflammatory mediators, such as NO and PGE2, are generated by the inducible isoforms of NO synthase (iNOS) and COX-2 [24]. The increased production of these inflammatory mediators may result in severe tissue damage and septic shock. NO is a signaling molecule that plays a key role in the pathogenesis of inflammation. In addition, NO is a potent neurotransmitter at neuron synapses and contributes to the regulation of apoptosis. NO is involved in the pathogenesis of inflammatory disorders of the joint, gut and lungs. Therefore, since the overproduction of NO can be harmful and result in various inflammatory and autoimmune diseases [25], pharmacological alteration of NO production presents a promising strategy for the development of medicinal foods to cure inflammatory disorders. Here, it has been demonstrated that CMWE significantly inhibits NO production in LPS-stimulated RAW 264.7 cells (Fig. 3).
LPS is a component of the outer cell wall of Gram-negative bacteria. Systemic injection with LPS in experimental animals is a widely used in vivo or in vivo models for the study of endotoxic shock and acute systemic inflammation. LPS is a potent activator of macrophages and activates the immune system, leading to the release of endogenous pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 [26]. Pro-inflammatory cytokines such as TNF-a, IL-1 and IL-6 have been shown to control inflammation in vitro as well as in vivo [27, 28], and moreover these cytokines are thought to be interlinked in a cascade, being produced serially by macrophages during the inflammatory response. Furthermore, the development of hyperalgesic states during inflammation is thought to be mediated by pro-inflammatory cytokines [29]. Indeed, RAW 264.7 macrophages provide us with an excellent model for anti-inflammatory drug screening.
In the present study, CMWE treatment of LPS-stimulated RAW 264.7 cells suppressed secretion of pro-inflammatory cytokines. LPS was used as the prototypical inflammatory stimulus in our experiments due to its ability to initiate a range of pro-inflammatory mediators [30]. It was obvious that CMWE was efficient in restraining the secretion of TNF-α and IL-6 in a concentration-dependent manner in LPS-stimulated cells (Figs 4 and 5). Additionally, the CMWE showed biological activity at low dosage despite being a water extract.
These findings suggest that CMWE may prevent inflammation by suppressing LPS-induced inflammatory mediators. Based on our data, the presence of various components in CMWE might imply the existence of effective anti-inflammatory compounds. Moreover, these data suggest that C. militaris may be considered a potential medicinal food for the prevention of inflammatory disease when hot water extract from C. militaris fruiting bodies is consumed. Therefore, usage of CMWE as a source of natural drugs requires that the mechanisms of the protective and anti-inflammatory properties of CMWE be further investigated.
Acknowledgments
This study was supported by research funds from Dong-A University.
References
- 1.Shiao MS, Wang ZN, Lin LJ, Lien JY, Wang JJ. Profiles of nucleosides and nitrogen bases in Chinese medicinal fungus Cordyceps sinensis and related species. Bot Bull Acad Sin. 1994;35:261–267. [Google Scholar]
- 2.Sung JM, Lee HK, Yoo YJ, Choi YS, Kim SH, Kim YO, et al. Classification of Cordyceps species based on protein banding pattern. Korean J Mycol. 1998;26:1–7. [Google Scholar]
- 3.Melling J, Belton FC, Kitching D, Stones WR. Production of pure cordycepin (3'-deoxyadenosine) from Cordyceps militaris. J Pharm Pharmacol. 1972;24(Suppl):125P. [PubMed] [Google Scholar]
- 4.Sun YJ, Lü P, Ling JY, Zhang HX, Chen C, Zhang CK. Nucleoside from Cordyceps kyushuensis and the distribution of two active components in its different parts. Yao Xue Xue Bao. 2003;38:690–694. [PubMed] [Google Scholar]
- 5.Yu R, Wang L, Zhang H, Zhou C, Zhao Y. Isolation, purification and identification of polysaccharides from cultured Cordyceps militaris. Fitoterapia. 2004;75:662–666. doi: 10.1016/j.fitote.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Kodama EN, McCaffrey RP, Yusa K, Mitsuya H. Antileukemic activity and mechanism of action of cordycepin against terminal deoxynucleotidyl transferase-positive (TdT+) leukemic cells. Biochem Pharmacol. 2000;59:273–281. doi: 10.1016/s0006-2952(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 7.Nan JX, Park EJ, Yang BK, Song CH, Ko G, Sohn DH. Anti-fibrotic effect of extracellular biopolymer from submerged mycelial cultures of Cordyceps militaris on liver fibrosis induced by bile duct ligation and scission in rats. Arch Pharm Res. 2001;24:327–332. doi: 10.1007/BF02975101. [DOI] [PubMed] [Google Scholar]
- 8.Kim BC, Choi JW, Hong HY, Lee SA, Hong S, Park EH, et al. Heme oxygenase-1 mediates the anti-inflammatory effect of mushroom Phellinus linteus in LPS-stimulated RAW264.7 macrophages. J Ethnopharmacol. 2006;106:364–371. doi: 10.1016/j.jep.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Park YM, Won JH, Kim YH, Choi JW, Park HJ, Lee KT. In vivo and in vitro anti-inflammatory and anti-nociceptive effects of the methanol extract of Inonotus obliquus. J Ethnopharmacol. 2005;101:120–128. doi: 10.1016/j.jep.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 10.Won SY, Park EH. Anti-inflammatory and related pharmacological activities of cultured mycelia and fruiting bodies of Cordyceps militaris. J Ethnopharmacol. 2005;96:555–561. doi: 10.1016/j.jep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Cook-Mills JM, Deem TL. Active participation of endothelial cells in inflammation. J Leukoc Biol. 2005;77:487–495. doi: 10.1189/jlb.0904554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vernooy JH, Dentener MA, van Suylen RJ, Buurman WA, Wouters EF. Long-term intratracheal lipopolysaccharide exposure in mice results in chronic lung inflammation and persistent pathology. Am J Respir Cell Mol Biol. 2002;26:152–159. doi: 10.1165/ajrcmb.26.1.4652. [DOI] [PubMed] [Google Scholar]
- 13.Sakagami T, Vella J, Dixon MF, O'Rourke J, Radcliff F, Sutton P, et al. The endotoxin of Helicobacter pylori is a modulator of host-dependent gastritis. Infect Immun. 1997;65:3310–3316. doi: 10.1128/iai.65.8.3310-3316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fichtner-Feigl S, Fuss IJ, Preiss JC, Strober W, Kitani A. Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides. J Clin Invest. 2005;115:3057–3071. doi: 10.1172/JCI24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klotz L, Schmidt M, Giese T, Sastre M, Knolle P, Klockgether T, et al. Proinflammatory stimulation and pioglitazone treatment regulate peroxisome proliferator-activated receptor gamma levels in peripheral blood mononuclear cells from healthy controls and multiple sclerosis patients. J Immunol. 2005;175:4948–4955. doi: 10.4049/jimmunol.175.8.4948. [DOI] [PubMed] [Google Scholar]
- 16.Walsh NC, Crotti TN, Goldring SR, Gravallese EM. Rheumatic diseases: the effects of inflammation on bone. Immunol Rev. 2005;208:228–251. doi: 10.1111/j.0105-2896.2005.00338.x. [DOI] [PubMed] [Google Scholar]
- 17.Sautebin L. Prostaglandins and nitric oxide as molecular targets for anti-inflammatory therapy. Fitoterapia. 2000;71(Suppl 1):S48–S57. doi: 10.1016/s0367-326x(00)00181-7. [DOI] [PubMed] [Google Scholar]
- 18.Boscá L, Zeini M, Través PG, Hortelano S. Nitric oxide and cell viability in inflammatory cells: a role for NO in macrophage function and fate. Toxicology. 2005;208:249–258. doi: 10.1016/j.tox.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 19.Penglis PS, Cleland LG, Demasi M, Caughey GE, James MJ. Differential regulation of prostaglandin E2 and thromboxane A2 production in human monocytes: implications for the use of cyclooxygenase inhibitors. J Immunol. 2000;165:1605–1611. doi: 10.4049/jimmunol.165.3.1605. [DOI] [PubMed] [Google Scholar]
- 20.Coker RK, Laurent GJ. Pulmonary fibrosis: cytokines in the balance. Eur Respir J. 1998;11:1218–1221. doi: 10.1183/09031936.98.11061218. [DOI] [PubMed] [Google Scholar]
- 21.Lee KW, Nam BH, Jo WS, Oh SJ, Kang EY, Cui Y, et al. Collection, identification and hepatic effect of native Codyceps militaris. Korean J Mycol. 2006;34:7–14. [Google Scholar]
- 22.de las Heras B, Abad MJ, Silván AM, Pascual R, Bermejo P, Rodríguez B, et al. Effects of six diterpenes on macrophage eicosanoid biosynthesis. Life Sci. 2001;70:269–278. doi: 10.1016/s0024-3205(01)01402-3. [DOI] [PubMed] [Google Scholar]
- 23.Evans CH. Nitric oxide: what role does it play in inflammation and tissue destruction? Agents Actions Suppl. 1995;47:107–116. doi: 10.1007/978-3-0348-7343-7_9. [DOI] [PubMed] [Google Scholar]
- 24.Vane JR, Mitchell JA, Appleton I, Tomlinson A, Bishop-Bailey D, Croxtall J, et al. Inducible isoforms of cyclooxygenase and nitric-oxide synthase in inflammation. Proc Natl Acad Sci USA. 1994;91:2046–2050. doi: 10.1073/pnas.91.6.2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tergaonkar V. NFkappaB pathway: a good signaling paradigm and therapeutic target. Int J Biochem Cell Biol. 2006;38:1647–1653. doi: 10.1016/j.biocel.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 26.Ando H, Takamura T, Ota T, Nagai Y, Kobayashi K. Cerivastatin improves survival of mice with lipopolysaccharide-induced sepsis. J Pharmacol Exp Ther. 2000;294:1043–1046. [PubMed] [Google Scholar]
- 27.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 28.Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. 1994;56:559–564. [PubMed] [Google Scholar]
- 29.Watkins LR, Maier SF, Goehler LE. Immune activation: the role of pro-inflammatory cytokines in inflammation, illness responses and pathological pain states. Pain. 1995;63:289–302. doi: 10.1016/0304-3959(95)00186-7. [DOI] [PubMed] [Google Scholar]
- 30.Abreu MT, Arditi M. Innate immunity and toll-like receptors: clinical implications of basic science research. J Pediatr. 2004;144:421–429. doi: 10.1016/j.jpeds.2004.01.057. [DOI] [PubMed] [Google Scholar]