Abstract
Evidence suggests that respiratory sinus arrhythmia (RSA) may be an important indicator of physiological flexibility. However, few studies have examined the relation between RSA and defensive habituation, a process contingent on physiological flexibility. In three independent samples, habituation was defined as the time course of 9 startle responses. In Sample one and two, startle was recorded (1) while shock electrodes were attached to participants’ and (2) before a threat-of-shock task. In Sample three, startle was recorded without these two components. In the first two samples, startle magnitude significantly decreased over time but in Sample three, startle only decreased at a trend level. Further, low RSA was associated with less of a reduction in startle magnitude over time within the first two samples, but was unrelated to startle reduction in the third. This suggests that low RSA is associated with less habituation to contextual anxiety, which may reflect difficulties regulating anxiety.
Keywords: Respiratory sinus arrhythmia, Habituation, Startle
1. Introduction
For the past several decades, researchers have been interested in understanding the psychophysiological mechanisms underlying habituation. Habituation is defined as the gradual decrease in physiological responding to a stimulus over time, and is considered to be an adaptive response style to an ongoing, non-threatening stimulus (Groves and Thompson, 1970; Herry et al., 2010; Rankin et al., 2009). Within the affective literature, there has been a particular emphasis on understanding deficits in habituating to aversive stimuli. A slower rate (or lack) of response reduction to a non-threatening stimulus has been considered to be maladaptive and an index of heightened vigilance (Oken et al., 2006), difficulty in flexibly responding to environmental challenges (Raskin, 1975; Siddle, 1991), and/or deficits in inhibitory learning (Craske et al., 2008).
According to Foa and Kozak (1986) classic “information model,” in response to an aversive stimulus, neural structures that underlie fear learning are initially activated. Subsequently, information signaling that the aversive stimulus does not pose actual danger or threat is learned. Studies have suggested that this subsequent learning occurs through interactions within the medial prefrontal-amygdala circuit (Myers and Davis, 2007) as well as activation of the temporal lobe memory system and structures involved in conscious awareness (LeDoux, 1996). Although researchers are beginning to challenge aspects of Foa and Kozak’s theory (Craske et al., 2008), it is thought that the result of habituation processes include diminished defensive responding, decreased scanning of the environment for threatening cues, and lower levels of anxiety.
Some empirical evidence indicates that individuals with anxiety disorders exhibit lower rates of habituation. For example, individuals with panic disorder have exhibited decreased habituation of startle responses and skin conductance in response to aversive auditory tones (Ludewig et al., 2002; Roth et al., 1990). Similarly, it has been demonstrated that individuals with posttraumatic stress disorder (PTSD) have less skin conductance habituation to trauma cues compared with individuals without PTSD (Rothbaum et al., 2001), and socially anxious individuals have evidenced less skin conductance and heart rate habituation while giving impromptu speeches compared with low-anxious controls (Eckman and Shean, 1997). Although these studies suggest that individuals with anxiety disorders indeed display habituation deficits, it is important to note that methodological and operational heterogeneity within the habituation literature has lead to some mixed findings (Hoenig et al., 2005; Ross et al., 1989).
Another physiological indictor associated with flexible responding to the environment is respiratory sinus arrhythmia (RSA). RSA is the rhythmic fluctuation of heart rate during the respiratory cycle and is considered a non-invasive measure of the extent to which the vagus nerve mediates parasympathetic influences on the heart (Porges, 1995, 1997, 2007). Importantly, RSA is often conceptualized as an individual difference factor that reflects one’s ability to maintain homeostasis and adaptively respond to environmental demands (Porges, 1995, 2007; Thayer and Lane, 2000). Consistent with this conceptualization, low RSA has been shown to be associated with difficulty regulating emotional states and attention (Berntson et al., 1998; Demaree et al., 2004; Frazier et al., 2004; Porges et al., 1994; Weinstein and Quigley, 2006).
It is also important to note that a large body of evidence indicates that individuals with anxiety disorders exhibit low levels of RSA (see Friedman, 2007 for a review). Numerous studies have shown that individuals with PTSD, panic disorder, and generalized anxiety disorder all exhibit lower RSA during resting periods as well as during symptom provocation (e.g., trauma cues for PTSD; Blom et al., 2010; Cohen et al., 1998; Jovanovic et al., 2009; Thayer et al., 1996; Yeragani et al., 1993).
Given that the startle eyeblink response (i.e., the rapid contraction of the orbicularis oculi muscle below the eye) is a useful measure of aversive emotional states (Bradley et al., 1999; Lang, 1995), several studies have begun to explore the relation between individual differences in RSA and startle reactivity. For instance,Ruiz-Padial et al. (2003) demonstrated that low RSA is associated with greater startle reactivity during the viewing of affective pictures, and Melzig et al. (2009) found that low RSA was related to greater startle reactivity during threat-of-shock. Notably, however, these studies did not examine whether RSA was related to the pattern of startle responding over time. Instead, both of these prior studies (and much of the affective science literature at large) collapsed across startle responses to create an average startle reactivity measure. Therefore, it is currently unclear whether low RSA is associated with greater average reactivity, less habituation over time, or both. This distinction is noteworthy given that dysfunctional reactivity and responding over time may reflect different mechanisms.
In sum, extant research and theory would predict that low RSA would be associated with less habituation. However, to our knowledge, no study has specifically examined this question. The primary aim of the current study was to examine whether individual differences in RSA were associated with habituation, defined as the decrease in startle responding over time. To explore this question, we used data from three independent samples, during which participants’ reflexive eyeblinks were measured in response to nine acoustic startle probes presented over the course of 2.5 min.
There were several important differences among the samples that allowed for a thorough analysis of the RSA-habituation association. The first two samples of participants had shock electrodes attached to their wrists and subjects were told that right after the 2.5 min period, they would partake in a threat-of-shock task. As these two factors likely increased the contextual anxiety of the startle assessment (Baas et al., 2002; Grillon and Ameli, 1998) and likely confounded data interpretation, we conducted a third experiment, with the aim of reducing contextual anxiety and isolating the relation between RSA and habituation to startle probes.
Additionally, although the protocols for the first two samples were almost identical, the second employed a different decibel level of the startle probe than the first sample. As the magnitude of startle response may vary as a function of decibel level (Blumenthal, 1988, 1996; Blumenthal et al., 2005), the second sample provided evidence as to the robustness of the findings from Sample 1. Lastly, in Sample 1, RSA was exclusively collected during the startle habituation task. Although one could argue that RSA may best be understood as interactions between autonomic regulation and environmental demands (Bertsch et al., 2012; Casadei et al., 1996), it is possible that RSA may have been confounded by the state effects of the task. As such, within Sample 2 and Sample 3, RSA was collected during a pre-task resting period and during the startle task. In sum, these important differences between sample methodologies were designed to elucidate the relation between RSA and habituation, with and without contextual anxiety.
2. Methods
2.1. Participants
Data for the first two samples came from two independent, larger studies examining factors associated with startle potentiation to predictable and unpredictable threat-of-shock (Nelson and Shankman, 2011; Sarapas et al., under review). Data for the third sample was collected exclusively to elucidate findings from Samples 1 and 2. All participants were undergraduates and data collection took place between 2007 and 2012 at a midsize urban university. Demographic information including age, sex, and ethnicity were collected from all participants. In Samples 2 and 3, psychiatric medication was an exclusion criterion as prior evidence has shown that many psychotropic medications affect startle modulation to threat (e.g., Grillon et al., 2006) and RSA (Licht et al., 2009). Medication use was not assessed in Sample 1. Eligibility for all three samples included right handedness and no history of head trauma. Each sample protocol was approved by the University Institutional Review Board and informed consent was obtained prior to participation. The N’s for the three samples were 69, 110, and 51, respectively. However, to ensure data quality, within Sample 1 three individuals were excluded for not producing at least 5 of 9 possible startle responses (final N = 66). Within Sample 2, two were excluded for missing resting RSA data, three for missing RSA data during the task, three for current psychotropic medication use, and eight for not producing at least 5 of 9 possible startle responses (final N = 94). Within Sample 3, two individuals were excluded due to technical equipment failure (final N = 49). Participant characteristics for the final three samples are presented in Table 1.
Table 1.
Demographics and descriptive statistics for each sample.
| Sample 1 (N = 66) |
Sample 2 (N = 94) |
Sample 3 (N = 49) |
|
|---|---|---|---|
| % Female | 76.9%a | 59.6%b | 77.6%a |
| Age (SD) | 19.83 (2.76)a | 19.39 (2.25)a,b | 18.82 (1.41)b |
| Task RSA (SD) | 6.44 (1.38)a | 6.91 (1.01)b | 6.74 (1.34)a,b |
| Rest RSA (SD) | – | 6.66 (1.05)a | 6.93 (1.46)a |
| Ethnicity | |||
| African American | 12.3%a | 8.5%a | 12.2%a |
| Caucasian | 41.5%a | 34.0%a | 26.5%a |
| Asian | 23.1%a | 21.3%a | 22.4%a |
| Hispanic/Latino | 15.4%a | 27.7%a | 30.6%a |
| Other | 7.7%a | 8.5%a | 6.1%a |
Note. Means or percentages with different subscripts across rows were significantly different in pairwise comparisons (p < .05, chi-square test for categorical variables and Tukey’s honestly significant difference test for continuous variables). SD = standard deviation; RSA = respiratory sinus arrhythmia; Task RSA = RSA during the startle task; Rest RSA = RSA collected at rest prior to the startle task.
2.2. Procedure
The procedures were largely the same for Sample 1 and Sample 2. First, participants provided written informed consent and were told that they would receive electric shocks during a computerized task later in the laboratory visit. Next, shock electrodes were placed on the participants’ left wrist and they were seated in an electrically shielded, sound-attenuated booth where they completed the startle task. Participants were told that during this task, they would only hear loud tones through headphones and not receive any shocks. They were directed to sit still and look at the computer monitor in front of them, which displayed a fixation cross. Participants were not told when the task would end or how many startle probes they would hear. Over the course of 2.5 min, participants were administered 9 acoustic startle probes with probe-to-probe intervals of 15–20 s (average time between startle probes = 17.22 s [SD = 2.22]). Eyeblink reflexes in response to each startle probe were recorded. Electrocardiogram (ECG) data was simultaneously collected throughout the task.
Given that the designs of Sample 1 and Sample 2 likely elicited contextual anxiety, Sample 3 was designed to examine the relation between RSA and habituation in the absence of (or at least reduced) contextual anxiety. To this end, Sample 3 did not include a shock task (and thus, participants were not informed of an upcoming shock task) and shock electrodes were not attached participants’ wrist during data collection. Sample 3 participants did, however, complete the exact same startle task as the prior two samples (e.g., same probe-to-probe intervals, etc.). The task instructions provided to participants was also the same as the prior two samples.
In addition to the collection of ECG data during the task in all three samples, within Sample 2 ECG data was collected during a rest period prior to the startle task. This period occurred after informed consent in which they were informed of the upcoming shock task but prior to the attachment of the shock electrodes. Participants were instructed to relax and sit still while viewing a fixation cross presented on the computer monitor in front of them. Heart rate was collected during alternating 90 second, eyes open versus eyes closed recording conditions, for a total of 6 min (counterbalanced: OCCO versus COOC). Participants were instructed to alternate between eyes open and eyes closed conditions because resting EEG data was being simultaneously collected as part of a larger protocol. During this time, participants were sitting in an upright position and no instructions were provided on respiration.
Within Sample 3, resting RSA data was also collected. Specifically, individuals completed a 90-s resting data collection period prior to the startle task. Participants were seated in an upright position and instructed to sit still and relax.
2.3. Stimulus delivery
All stimuli were administered using PSYLAB (Contact Precision Instruments, London, UK) and psychophysiological data were acquired using Neuroscan 4.4 (Compumedics, Charlotte, NC). The acoustic startle probes used in Sample 1 were 40-ms duration, 95 dB bursts of white noise with near instantaneous rise time presented binaurally through headphones. In Sample 2 and Sample 3, startle probes were increased to 103 dB. Startle probes were generated using a sound generator and were assessed and corrected every few months for potential drift. Both decibel levels are considered within the recommended range for startle research (Blumenthal et al., 2005). In all three samples, startle response was measured from two 4-mm Ag/AgCl electrodes placed over the orbicularis oculi muscle below the right eye per published guidelines (Blumenthal et al., 2005). For Sample 1 and Sample 3, the ground electrode was placed in the center of the forehead along the midline and another electrode was placed on the back of the neck along the midline as part the 60-cycle noise cancellation procedures employed by the Neuroscan data acquisition system. For Sample 2, the ground electrode and noise cancellation reference were part of an EEG cap and located along the midline of the anterior scalp and between electrodes Cz and CPz, respectively. In all three studies, ECG was recorded from two electrodes placed on the sternum and below the left clavicle. Both EMG and ECG data were collected using a bandpass filter of DC-200 Hz at a sampling rate of 1000 Hz. Although the upper end of this frequency band is below the Blumenthal et al. (2005) recommendation of 500 Hz, the missing bandwidth (200–500 Hz) was not likely to effect the experimental manipulation or the reliability of the results (A. Van Boxtel and T. Blumenthal, personal communications, December 14, 2009).
2.4. RSA and startle data processing
ECG data were initially processed using QRSTool (Allen et al., 2007) and all artifacts were identified and corrected by hand. After initial correction, inter-beat-interval (IBI) series were extracted for each recording block (i.e., blocks of 2.5 min for the task data and blocks of 90 s for the resting data). Each block was input into CardioEdit (Brain-Body Center, University of Illinois at Chicago) for further artifact correction. After data were processed, average RSA was calculated based on methods developed by Porges and Bohrer (1990), using CardioBatch (Brain-Body Center, University of Illinois at Chicago). It is important to note that respiration was not recorded in any of the three protocols and thus, the potential impact of respiration in the present study is unclear.
Startle blinks were scored according to guidelines provided by Blumenthal et al. (2005). Startle data were first rectified and then smoothed using a FIR filter with a band pass of 28–40 Hz. Peak amplitude of the blink reflex was defined within the 20–150 ms time frame following the startle probe onset relative to baseline (average baseline EMG level for the 50 ms preceding the startle probe onset). Blinks were scored as non-responses if EMG activity during the 20–150 ms post-stimulus time frame did not produce a blink peak that was visually differentiated from baseline activity. Non-responses were coded as zero-magnitude in all three samples. Blinks were scored as missing if the baseline period was contaminated with noise, movement artifact, or if a spontaneous or voluntary blink began before minimal onset latency and thus interfered with the startle probe-elicited blink response. Missing blinks were not included in analyses or in the calculation of average blink magnitude across studies.
2.5. Data analysis plan
Descriptive analyses indicated that each startle blink was skewed in Sample 1 (range 1.1–3.5), Sample 2 (range 1.8–3.0), and Sample 3 (range 1.0–2.0), so each was normalized using a square-root transformation. Post-transformation, all blink skew values were below 1.0. RSA did not require transformation as the skewness and kurtosis values were all below 1.0. Chi-squared analyses and analyses of variance (ANOVA) were conducted to determine if any of the samples differed on important demographic and study variables.
Within the literature, habituation has been defined in multiple ways (LaRowe et al., 2006; Rothbaum et al., 2001). Most commonly, researchers have used a difference score of initial response (or block of responses) minus last response (Ellwanger et al., 2003). Given that these definitions do not capture the slope of responding over time, we used random coefficients effects to model the pattern (or slope) of startle responding during the task.
All random coefficients models were run using the PROC MIXED procedure in SAS 9.2 (SAS Institute Inc., 2008). Blink number (i.e., time) was a random effect and RSA was a fixed effect. RSA was mean-centered in both samples and gender was included as a covariate in all analyses given its consistent association with general affective responding in the literature (Feingold, 1994). Separate models were run for each index of RSA, collected within each sample. All models were also re-run with response to the first probe as an additional covariate in order to account for potential intercept (i.e., initial startle reactivity) effects. If an individual had a missing value for their first blink (Sample 1, N = 4; Sample 2, N = 5; Sample 3, N = 0), the average blink 1 magnitude of the sample was used to impute the startle response for that subject. If there was a significant time by RSA interaction, follow-up random coefficients modeling analyses were run to examine the simple slopes of startle responding as a function of RSA at high versus low levels of RSA (i.e., 1 SD above and below the mean; Aiken and West, 1991).
Currently, there are no universally accepted procedures for calculating effect sizes in random coefficient models (Scherbaum and Ferreter, 2009). However, we chose to use standardized betas (which can be interpreted as correlation coefficients) to measure the strength of the relationship between RSA and change in startle response over the course of the task (see Klein et al., 2011 for a similar approach). Specifically, we regressed the difference score of first blink minus last blink onto RSA, while controlling for gender. Seven subjects from Sample 1, ten from Sample 2, and one from Sample 3 were missing from these effect size calculations as they were missing either the first or last blink (but these subjects were included in the random coefficients models).
3. Results
3.1. Sample comparisons and descriptive data
Within all three samples, there were no significant differences between those that were included versus excluded on age, gender, ethnicity, and RSA (at rest and during the task; all p’s > .22). However, in Sample 1 and Sample 2, those that were included exhibited a significantly higher average startle magnitude1 than those that were excluded [Sample 1 = F(1, 68) = 5.26, p < .05; Sample 2 = F(1, 109) = 8.63, p < .05].
There were no sample differences in ethnicity or resting RSA. However, there were more males in Sample 2 than in Sample 1 ( χ2 = 5.48, p < .05) and Sample 3 (χ2 = 4.62, p < .05). The mean age of Sample 1 was significantly higher than the mean age of Sample 3 [F(1, 114) = 6.32, p < .05]. Mean RSA during the startle task was significantly higher in Sample 2 than in Sample 1 [F(1, 159) = 6.10, p < .05].
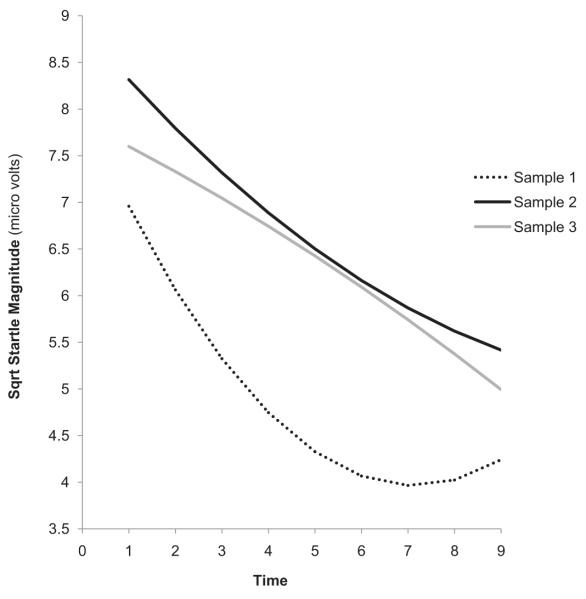
Preliminary random coefficients analyses indicated that when controlling for gender, there was a significant linear (b = −0.94, t(64) = −7.22, p < .01) and quadratic (b = 0.08, t(64) = 6.23, p < .01) effect for time in Sample 1. In Sample 2, there was also a significant linear (b = −0.53, t(92) = −5.41, p < .01) and quadratic (b = 0.02, t(64) = 2.12, p < .05) effect for time. Thus, in the first two samples, the magnitude of participants’ blinks significantly decreased over time and the rate of this decrease decelerated across data points. In Sample 3, however, there was only a trend for a linear effect for time (b = −0.25, t(47) = −1.75, p = .09) and no significant quadratic effect (b = −0.01, t(47) = −0.57, p = .57). As can be seen in Fig. 1, the average magnitude across all blinks was lower in Sample 1 than in Sample 2 [F(1, 159) = 9.34, p < .01] and Sample 3 [F(1, 114) = 7.61, p < .01] (Samples 2 and 3 did not differ on average blink magnitude). The sample differences in average blink magnitude were expected given the use of a lower dB startle probe in Sample 2 (Blumenthal et al., 2005).
Fig. 1.
Slope of habituation for each sample. Sqrt Startle Magnitude = square-root transformed startle magnitude values.
Resting RSA and task RSA were not correlated with average blink magnitude (i.e., startle reactivity) in any of the three samples (all p’s > .26). The magnitude of the first or last blink was not correlated with any measure of RSA across all samples (all p’s > .13).
3.2. Sample 1: RSA collected during task
When RSA during the task was included in the model, there continued to be significant linear (b = −0.96, t(63) = −7.39, p < .01) and quadratic effects (b = 0.08, t(63) = 6.20, p < .01) for time. Notably, results indicated a significant time by RSA interaction (b = −0.05, t(427) = −1.91, p = .05, ES = .27) signifying that participants’ blink magnitudes decreased over time as a function of their RSA (see Table 2). In addition, this interaction remained significant when controlling for blink 1 magnitude (b = −0.05, t(365) = −2.05, p < .05).
Table 2.
Results from the random coefficients analyses by sample and measure of RSA.
| Variable | Sample 1 Task RSA |
Sample 2 Task RSA |
Sample 2 Rest RSA |
Sample 3 Task RSA |
Sample 3 Rest RSA |
|---|---|---|---|---|---|
| RSA × Time | b = −0.05; t = 1.91* | b = −0.06; t = −2.03* | b = −0.07; t = 2.60* | b = 0.02; t = 0.43 | b = 0.03; t = 0.87 |
| RSA | b = 0.09; t = 0.32* | b = 0.20; t = 0.53 | b = 0.20; t = 0.57 | b = 0.27; t = 0.65 | b = 0.21; t = 0.54 |
| Gender | b = 0.97; t = 1.11 | b = 0.80; t = 1.05 | b = 0.85; t = 1.12 | b = 2.77; t = 2.10* | b = 2.29; t = 1.68 |
| Linear | b = −0.96; t = −7.39** | b = −0.52; t = −5.30** | b = −0.51; t = −5.26** | b = −0.14; t = −0.90 | b = −0.15; t = −0.91 |
| Quadratic | b = 0.08; t = 6.20** | b = 0.02; t = 1.98* | b = 0.02; t = 2.02* | b = −0.01; t = −0.69 | b = −0.01; t = −0.67 |
| Time × Gender | b = −0.11; t = −1.26 | b = −0.03; t = −0.43 | b = −0.04; t = −0.70 | b = −0.14; t = −1.22 | b = −0.13; t = −1.06 |
Note. RSA = respiratory sinus arrhythmia; Task RSA = RSA collected during the startle task; Rest RSA = RSA collected during the resting period.
p < .05.
p < .01.
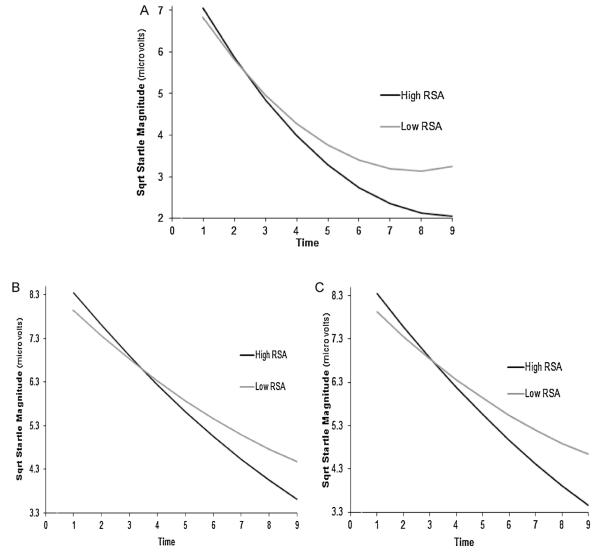
Follow-up analyses indicated that RSA was associated with the slope of responding among individuals with both high and low RSA. However, those with high RSA (b = −1.03, t(63) = −7.51, p < .01) evidenced a steeper slope than those with low RSA (b = −0.88, t(63) = −6.65, p < .01; see Fig. 2A). Additionally, examination of the figure suggests that those with low and high RSA had similar slopes until approximately the 3rd or 4th startle probe, at which point the low RSA individuals began to ‘level off’ at a greater rate than those with higher RSA.2
Fig. 2.
Slope of habituation as a function of RSA in Sample 1 (A), Sample 2 during the task (B), and Sample 2 during rest (C). Sqrt Startle Magnitude = square-root transformed startle magnitude values.
3.3. Sample 2: RSA collected during task
When RSA during the task was included in the Sample 2 model, there continued to be a significant linear (b = −0.52, t(91) = −5.30, p < .01) and quadratic effect (b = 0.02, t(91) = 1.98, p < .05) for time. More importantly, there was a significant time by RSA interaction (b = −0.06, t(635) = −2.03, p < .05, ES = .25; see Table 2). As with Sample 1, this pattern of results remained when controlling for blink 1 magnitude (b = −0.06, t(546) = −1.89, p = .05). Follow-up analyses in Sample 2 were also similar to that of Sample 1 as those with high RSA (b = −0.58, t(91) = −5.63, p < .01) exhibited a steeper slope of responding than those with low RSA (b = −0.46, t(91) = −4.50, p < .01; see Fig. 2B).
3.4. Sample 2: RSA collected during rest
First, we examined whether there were any significant differences in resting RSA during the eyes open versus the eyes closed recording blocks in Sample 2 by conducting a repeated measures ANOVA with the average of the two eyes open and the two eyes closed blocks entered as a within-subjects factor. Results indicated no significant differences in RSA [F(1, 93) = 2.21, p = .14, ]. As such, the eyes open versus closed variable was not included as a covariate in subsequent analyses.
We also examined whether there were differences in resting RSA across time using a repeated measures ANOVA with Time (90-s blocks 1, 2, 3, and 4) entered as a within-subject factor. Results indicated that RSA significantly decreased across each of the four recording blocks [F(3, 297) = 16.66, p < .01, ]. This natural decrease in RSA over the experimental session may be attributed to sustained attention or anticipation of the shock task (Hofmann et al., 2005; Porges and Raskin, 1969). Pearson’s correlations among the 4 blocks revealed high rank-order stability of RSA (all r’s > .80, p < .01) and thus, we were able to collapse all four blocks. RSA collected during the task and RSA collected at rest were highly correlated (r = .75, p < .01).
When RSA at rest was included in the startle model, there continued to be a significant linear (b = −0.51, t(91) = −5.26, p < .01) and quadratic effect (b = 0.02, t(91) = 2.02, p < .05) for time. Further, there was a significant time by RSA interaction (b = −0.07, t(635) = −2.60, p < .01, ES = .23; see Table 2). The interaction remained significant when controlling for blink 1 magnitude (b = −0.08, t(546) = −2.69, p < .01). Similar to the task RSA analyses, follow-up analyses indicated that individuals with high resting RSA (b = −0.59, t(91) = −5.80, p < .01) evidenced a steeper slope of responding than individuals with low resting RSA (b = −0.44, t(92) = −4.29, p < .01; see Fig. 2C).
3.5. Sample 3: RSA collected at rest and during task
Unlike the other two samples, when RSA was included in the startle model, there was no longer a linear trend for time: RSA at rest (b = −0.15, t(44) = −0.91, p = .37); task RSA (b = −0.14, t(44) = −0.90, p = .37). Similarly, there were no quadratic effects for time [Rest RSA: b = −0.01, t(44) = −0.67, p = .51; Task RSA: b = −0.01, t(44) = −0.69, p = .49]. Additionally, unlike the first two samples, there was also no RSA by time interaction: rest RSA (b = 0.03, t(322) = 0.87, p = .39), task RSA3 (b = 0.02, t(322) = 0.43, p = .67; Table 2). In Sample 3, there was a main effect for gender on startle responding, such that females exhibited higher average startle responding than males (b = 2.77, t(322) = 2.10, p < .05). Rest RSA and task RSA were highly correlated (r = .88, p < .01).
3.6. Sample by time by RSA interaction
When the three samples were combined into one model, there was a significant Sample (Sample 1 versus Sample 2 versus Sample 3 [dummy coded]) by time by task RSA interaction (Sample 1 versus Sample 3: b = −0.10, t[1425] = −2.58, p < .05; Sample 2 versus Sample 3: b = −0.06, t[1425] = −2.12, p < .05). Samples 1 and 2 did not differ. Therefore, the attachment of the shock electrodes and disclosure of the upcoming shock task moderated the relation between RSA and startle habituation.
4. Discussion
Recent advances in the understanding of the myelinated vagus suggest that RSA may be an important indicator of physiological flexibility (Beauchaine, 2001; Porges, 2001, 2007). However, no study to our knowledge has examined the relation between RSA and habituation, a physiological process contingent on flexible responding. Results from Sample 1 indicated that lower RSA was associated with less of a reduction in startle responding over time (i.e., less habituation). Importantly, this finding was replicated in a second, independent sample (i.e., Sample 2) that included two indices of RSA, had higher average task RSA levels, was not taking psychiatric medications, and heard louder startle probes. This consistency across samples is notable and suggests a relation between RSA and habituation. However, in both samples, startle was recorded while participants had shock electrodes attached to them and were aware of an upcoming threat-of-shock task. As such, several potentially overlapping processes may have occurred to account for the reduction in startle responses. Specifically, it is possible that individuals with low RSA had greater difficulty habituating to the actual acoustic startle probes and/or to the contextual anxiety elicited by the shock paradigm/shock electrodes. It is also possible that the RSA measurements were confounded by the same state contextual anxiety and did not capture trait-like individual differences.
We therefore collected data from a third sample in which we attempted to remove (or at least greatly reduce) contextual anxiety from the design and isolate the relation between RSA and habituation to the acoustic startle probes. To this end, the procedures for the third sample simply included a resting RSA data collection period and administration of the startle task. Interestingly, results indicated that unlike the first two samples, in Sample 3, there was only a trend for a linear decline in startle response and no quadratic effect for time. Also, when RSA was included in the model, the trend linear effect for time was no longer significant. This suggests that the effect of time on startle responses was weak, as the inclusion of a non-significant variable in the model (i.e., RSA) impacted its effect on startle. It further suggests that when external anxiogenic factors (i.e., shock electrodes and an impending shock task; Grillon and Ameli, 1998) are not present, habituation occurs to a lesser extent.
One potential explanation for this discrepancy in rates of habituation is that anxiety increases task-focused attention (Bar-Haim et al., 2007; Pacheco-Unguetti et al., 2010), which could facilitate the process by which healthy individuals’ learn that the startle probes are non-threatening and appropriately modify their startle responses. Importantly, within Sample 3, our results also indicated that there was no RSA by time interaction for either RSA collected at rest or RSA collected during the startle task. Given that the RSA values in Sample 3 did not differ from either of the other two samples, it is unlikely that these null findings are due to any qualitative differences in RSA among the different samples. Rather, these findings suggest that RSA is selectively associated with habituation to contextual anxiety.
RSA is often conceptualized as an individual difference factor reflecting one’s ability to effectively self-regulate emotional and behavioral states and maintain homeostasis (Beauchaine, 2001; Porges et al., 1994; Porges, 2007; Thayer and Lane, 2000). Numerous theoretical models (e.g., polyvagal theory, neurovisceral integration model) suggest that the vagus, in coordination with neural circuits including the prefrontal cortex and anterior cingulate cortex, plays a key role in the generation and modulation of emotion (Porges, 1995, 2007; Thayer and Lane, 2009). Consistent with this conceptualization, low RSA has been shown to be associated with more negative emotional arousal in response to daily stressors (Fabes and Eisenberg, 1997), poor impulse control (Allen et al., 2000), decreased persistence on stressful tasks (Segerstrom and Nes, 2007), increased cortisol responses to cognitive stressors (Johnsen et al., 2002), and numerous anxiety disorders (Friedman, 2007). Taken together, this literature would suggest that in the present study, those with low RSA may have had greater difficulty regulating the contextual anxiety elicited by the experimental design due to deficits in their ability to modulate inhibitory influences of the myelinated vagus on cardiac functioning.
A similar, yet slightly alternative, hypothesis is that individuals with low RSA demonstrated less habituation over time due to greater engagement in cognitive avoidance strategies, rather than deficits in the modulation of vagal influences. This hypothesis draws from recent literature suggesting that individuals with anxiety disorders often fail to habituate to feared stimuli due to their failure to engage in inhibitory learning (Craske et al., 2008; Creamer et al., 1992). Given that low RSA is associated with poor emotional coping skills (Weinstein and Quigley, 2006), it is plausible that within the present study, individuals with low RSA may have been more likely than those with high RSA to cognitively avoid negative affect by diverting their attention away from the anxiogenic experimental stimuli/task. This proposed mechanism is quite speculative (particularly given the cross-sectional nature of the analyses) so future studies are needed to directly examine mediating factors of the association between RSA and habituation.
It is important to note that in all three samples, RSA was not related to startle reactivity, defined as average blink magnitude across all 9 blinks. This was somewhat unexpected given that low RSA has previously been shown to be associated with greater emotional reactivity, including increased startle responding (Johnsen et al., 2002; Melzig et al., 2009; Ruiz-Padial et al., 2003). Nevertheless, these findings serve to highlight the important distinction between aversive responding over time (i.e., habituation) and mean levels of responding (i.e., reactivity). As was previously discussed, habituation and reactivity may reflect different mechanisms such that habituation may be the result of top-down inhibitory influences whereas reactivity may be the result of initial stimulus processing (Gross, 1999; Werner and Gross, 2009). The current pattern of results suggest that individual differences in RSA may be more robustly associated with the time course of emotional responding, relative to reactivity, at least in non-clinical samples of young adults.
Though the present study was not with a clinical sample, the current findings may have implications for the treatment of anxiety disorders. Several studies have shown that individuals with anxiety disorders exhibit lower rates of habituation (Ludewig et al., 2002; Roth et al., 1990) and lower levels of RSA (Friedman, 2007). The current findings suggest that these two clinical phenomena may be associated, either causally or due to a shared third factor (e.g., higher order neural deficits). Given that the most highly effective treatments for anxiety disorders involve exposing individuals to feared stimuli and observing their fear responses naturally decrease over time (i.e., habituation; Barlow, 2002; Deacon and Abramowitz, 2004), targeting RSA in the context of exposure-based interventions may be beneficial. Specifically, vagal enhancement treatments (e.g., Zen meditation; Murata et al., 2004) may have direct and/or indirect effects on the physiological substrates necessary for habitation, which could subsequently improve the effectiveness of exposures and increase treatment outcomes. As this is a potentially important avenue for future research, further investigation is needed to delineate these mechanisms and examine whether improvements in RSA are associated with better outcomes in exposure-based anxiety treatments.
Although these findings address important gaps within the literature, there are several limitations of note. First, individuals in each sample were not randomized and it is unknown whether unmeasured sample effects influenced the present results. Second, the impact of respiration on the calculation of RSA is an unresolved debate within the literature (see Denver et al., 2007; Hatfield et al., 1998; Porges, 2007; Sargunaraj et al., 1996; but also see Grossman et al., 1990; Grossman and Kollai, 1993; Grossman and Taylor, 2007; Houtveen et al., 2002). Several studies have demonstrated that ignoring indices of respiration leads to biased or inaccurate measures of cardiac vagal tone (e.g., Grossman and Taylor, 2007), while others studies have presented data refuting these conclusions (e.g., Denver et al., 2007). Within the present study, we did not collect respiration data and thus, we are unable to assess the potential impact of respiration on the present indices of RSA. Third, we did not collect self-reported anxiety measures in the present study to assess the degree of contextual anxiety across the three samples. However, it is likely that there was more contextual anxiety in Samples 1 and 2 versus Sample 3 given that prior studies have demonstrated that shock electrodes and anticipation of a shock task are potent elicitors of contextual anxiety (Baas et al., 2002; Grillon and Ameli, 1998). Fourth, the current study was cross-sectional and consequently, we are unable to make causal inferences as to the directionality between low RSA and deficits in habituation. Lastly, our startle task only lasted 2.5 min and it may be more meaningful to examine individual differences in responding over a longer period of time.
In sum, the results from the current study indicate that individual differences in RSA are associated with the reduction of startle responding over time, but only in the presence of contextual anxiety. Therefore, individuals with low levels of RSA may have greater difficulties regulating contextual anxiety, which results in relatively maintained levels of defensive responding over time. Given that the process of habituation is a key component of several anxiety disorder treatments (Barlow, 2002), future research is needed to elucidate the role of RSA in the time course of affective responding.
Acknowledgments
This project was supported by National Institute of Mental Health Grant R21MH080689 (Shankman) and a UIC Chancellor’s Discovery Fund for Multidisciplinary Research (Shankman and Bishop). Dr. Bishop is supported by National Institute of Mental Health Grant K08MH083888. We would like to thank Stephen W. Porges, Ph.D., for his assistance in this project and guidance on the measurement and calculation of respiratory sinus arrhythmia.
Footnotes
We also examined this relationship using startle amplitude, rather than magnitude, and found similar results (see Blumenthal et al., 2005).
It may appear that Fig. 2A indicates an ‘increase’ in startle during the last two blinks of the series. However, this pattern simply reflects an artifact of graphing curvilinear effects.
We ran additional analyses to examine whether there was a sensitization effect, defined as an increase in startle magnitude over the course of the first three blinks responses (Groves and Thompson, 1970). Specifically, we re-ran our primary analyses, but only modeled the first three blinks. Results indicated that there were no positive linear or quadratic effects for time (all p’s > .25). Additionally, there were no RSA by time interactions for the first three blinks in any of the three samples (all p’s > .29).
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Sage; Newbury Park, CA: 1991. [Google Scholar]
- Allen JJB, Chambers AS, Towers DN. The many metrices of cardiac chronotrophy: a pragmatic primer and a brief comparison of metrics. Biological Psychology. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. http://dx.doi.org/10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Allen MT, Matthews KA, Kenyon KL. The relationships of resting baroreflex sensitivity, heart rate variability and measures of impulse control in children and adolescents. International Journal of Psychophysiology. 2000;37:185–194. doi: 10.1016/s0167-8760(00)00089-1. http://dx.doi.org/10.1016/S0167-8760(00)00089-1. [DOI] [PubMed] [Google Scholar]
- Baas JMP, Grillon C, Böcker KBE, Brack AA, Morgan CA, Kenemans JL, Verbaten MN. Benzodiazepines have no effect on fear-potentiated startle in humans. Psychopharmacology. 2002;161:233–247. doi: 10.1007/s00213-002-1011-8. http://dx.doi.org/10.1007/s00213-002-1011-8. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Threat-related attentional bias in anxious and non-anxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. http://dx.doi.org/10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Barlow DH. Anxiety and Its Disorders: The Nature and Treatment of Anxiety and Panic. The Guilford Press; New York: 2002. [Google Scholar]
- Berntson GG, Sarter M, Cacioppo JT. Anxiety and cardiovascular reactivity: the basal forebrain cholinergic link. Behavioural Brain Research. 1998;94:225–248. doi: 10.1016/s0166-4328(98)00041-2. http://dx.doi.org/10.1016/S0166-4328(98)00041-2. [DOI] [PubMed] [Google Scholar]
- Beauchaine T. Vagal tone, development, and Gray’s motivational theory: toward an integrated model of autonomic nervous system functioning in psychopathology. Developmental Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. http://dx.doi.org/10.1017/S0954579401002012. [DOI] [PubMed] [Google Scholar]
- Bertsch K, Hagemann D, Naumann E, Schachinger H, Schulz A. Stability of heart rate variability indices reflecting parasympathetic activity. Psychophysiology. 2012;49:672–682. doi: 10.1111/j.1469-8986.2011.01341.x. http://dx.doi.org/10.1111/j.1469-8986.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- Blom EH, Olsson EM, Serlachius E, Ericson M, Ingvar M. Heart rate variability (HRV) in adolescent females with anxiety disorders and major depressive disorder. Acta Paediatrica. 2010;99:604–611. doi: 10.1111/j.1651-2227.2009.01657.x. http://dx.doi.org/10.1111/j.1651-2227.2009.01657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal TD. The startle response to acoustic stimuli near startle threshold: effects of stimulus rise and fall time, duration, and intensity. Psychophysiology. 1988;25:607–611. doi: 10.1111/j.1469-8986.1988.tb01897.x. http://dx.doi.org/10.1111/j.1469-8986.1988.tb01897.x. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD. Inhibition of the human startle response is affected by both prepulse intensity and eliciting stimulus intensity. Biological Psychology. 1996;44:85–104. doi: 10.1016/0301-0511(96)05214-3. http://dx.doi.org/10.1016/0301-0511(96)05214-3. [DOI] [PubMed] [Google Scholar]
- Blumenthal TD, Cuthbert BN, Filion DL, Hackley S, Lipp OV, van Boxtel A. Committee report: guidelines for human startle eyeblink electromyographic studies. Psychophysiology. 2005;42:1–15. doi: 10.1111/j.1469-8986.2005.00271.x. http://dx.doi.org/10.1111/j.1469-8986.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Affect and the startle reflex. In: Dawson ME, Schell AM, Bohmet AH, editors. Startle Modification: Implication for Neuroscience, Cognitive Science, and Clinical Science. Cambridge University Press; New York: 1999. [Google Scholar]
- Casadei B, Moon J, Johnston J, Caiazza A, Sleight P. Is respiratory sinus arrhythmia a good index of cardiac vagal tone in exercise? Journal of Applied Physiology. 1996;81:556–564. doi: 10.1152/jappl.1996.81.2.556. [DOI] [PubMed] [Google Scholar]
- Cohen H, Kotler M, Matar MA, Kaplan Z, Loewenthal U, Miodownik H, Cassuto Y. Analysis of heart rate variability in posttraumatic stress disorder patients in response to a trauma related reminder. Biological Psychiatry. 1998;44:1054–1059. doi: 10.1016/s0006-3223(97)00475-7. http://dx.doi.org/10.1016/S0006-3223(97)00475-7. [DOI] [PubMed] [Google Scholar]
- Craske MG, Kircanski K, Zelikowski M, Mystkowski J, Chowdhury N, Baker A. Optimizing inhibitory learning during exposure therapy. Behaviour Research and Therapy. 2008;46:5–27. doi: 10.1016/j.brat.2007.10.003. http://dx.doi.org/10.1016/j.brat.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Creamer M, Burgess P, Pattison P. Reaction to trauma: a cognitive processing model, Journal of. Abnormal Psychology. 1992;101:452–459. doi: 10.1037//0021-843x.101.3.452. http://dx.doi.org/10.1037/0021-843X.101.3.452. [DOI] [PubMed] [Google Scholar]
- Deacon BJ, Abramowitz JS. Cognitive and behavioral treatments for anxiety disorders: a review of meta-analytic findings. Journal of Clinical Psychology. 2004;60:429–441. doi: 10.1002/jclp.10255. http://dx.doi.org/10.1002/jclp.10255. [DOI] [PubMed] [Google Scholar]
- Demaree HA, Robinson DL, Everhart DE, Schmeichel BJ. Resting RSA is associated with natural and self-regulated responses to negative emotional stimuli. Brain and Cognition. 2004;56:14–23. doi: 10.1016/j.bandc.2004.05.001. http://dx.doi.org/10.1016/j.bandc.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Denver JW, Reed SF, Porges SW. Methodological issues in the quantification of respiratory sinus arrhythmia. Biological Psychology. 2007;74:286–294. doi: 10.1016/j.biopsycho.2005.09.00. http://dx.doi.org/10.1016/j.biopsycho.2005.09.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckman PS, Shean GD. Habituation of cognitive and physiological arousal and social anxiety. Behavior Research and Therapy. 1997;35:1113–1121. http://dx.doi.org/10.1016/S0005-7967(97)80005-8. [PubMed] [Google Scholar]
- Ellwanger J, Geyer MA, Braff DL. The relationship of age to prepulse inhibition and habituation of the acoustic startle response. Biological Psychology. 2003;62:175–195. doi: 10.1016/s0301-0511(02)00126-6. http://dx.doi.org/10.1016/S0301-0511(02)00126-6. [DOI] [PubMed] [Google Scholar]
- Fabes RA, Eisenberg N. Regulatory control and adults’ stress-related responses to daily life events. Journal of Personality and Social Psychology. 1997;73:1107–1117. doi: 10.1037//0022-3514.73.5.1107. http://dx.doi.org/10.1037/0022-3514.73.5.1107. [DOI] [PubMed] [Google Scholar]
- Feingold A. Gender differences in personality: a meta-analysis. Psychological Bulletin. 1994;116:429–456. doi: 10.1037/0033-2909.116.3.429. http://dx.doi.org/10.1037/0033-2909.116.3.429. [DOI] [PubMed] [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. http://dx.doi.org/10.1037/0033-2909.99.1.20. [PubMed] [Google Scholar]
- Frazier TW, Strauss ME, Steinhauer SR. Respiratory sinus arrhythmia as an indicator of emotional response in young adults. Psychophysiology. 2004;41:75–83. doi: 10.1046/j.1469-8986.2003.00131.x. http://dx.doi.org/10.1046/j.1469-8986.2003.00131.x. [DOI] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility-neurovisceral integration model of anxiety and cardiac vagal tone. Biological Psychology. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. http://dx.doi.org/10.1016/j.bbr.2011.03.031. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat of shock, shock electrode placement and darkness on startle. International Journal of Psychophysiology. 1998;28:223–231. doi: 10.1016/s0167-8760(97)00072-x. http://dx.doi.org/10.1016/S0167-8760(97)00072-X. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP, Pine DS, Lissek S, Lawley M, Ellis V, Levine J. The benzodiazepine alprazolam dissociates contextual fear from cued fear in humans as assessed by fear-potentiated startle. Biological Psychiatry. 2006;60:760–766. doi: 10.1016/j.biopsych.2005.11.027. http://dx.doi.org/10.1016/j.biopsych.2005.11.02. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: past, present, future. Cognition and Emotion. 1999;13:551–573. http://dx.doi.org/10.1080/026999399379186. [Google Scholar]
- Grossman P, Karemaker J, Wieling W. Prediction of tonic parasympathetic cardiac control using respiratory sinus arrhythmia: the need for respiratory control. Psychophysiology. 1990;28:201–216. doi: 10.1111/j.1469-8986.1991.tb00412.x. http://dx.doi.org/10.1111/j.1469-8986.1991.tb00412.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Kollai M. Respiratory sinus arrythymia, cardiac vagal tone, and respiration: within- and between-individual relations. Psychophysiology. 1993;30:486–495. doi: 10.1111/j.1469-8986.1993.tb02072.x. http://dx.doi.org/10.1111/j.1469-8986.1993.tb02072.x. [DOI] [PubMed] [Google Scholar]
- Grossman P, Taylor EW. Toward understanding respiratory sinus arrhythmia: relations to cardiac vagal tone, evolution and biobehavioral functions. Biological Psychology. 2007;74:263–285. doi: 10.1016/j.biopsycho.2005.11.014. http://dx.doi.org/10.1016/j.biopsycho.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Groves PM, Thompson RF. Habituation: a dual-process theory. Psychological Review. 1970;77:419–450. doi: 10.1037/h0029810. http://dx.doi.org/10.1037/h0029810. [DOI] [PubMed] [Google Scholar]
- Hatfield BD, Santa Maria DL, Porges SW, Potts JT, Spalding T, Byrne EA. Respiratory sinus arrhythmia during exercise in aerobically trained and untrained men. Medicine & Science in Sports & Exercise. 1998;30:206–214. doi: 10.1097/00005768-199802000-00006. http://dx.doi.org/10.1097/00005768-199802000-00006. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. European Journal of Neuroscience. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. http://dx.doi.org/10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hoenig K, Hochrein A, Quednow BB, Maier W, Wagner M. Impaired prepulse inhibition of acoustic startle in obsessive compulsive disorder. Biological Psychology. 2005;10:1153–1158. doi: 10.1016/j.biopsych.2005.01.040. http://dx.doi.org/10.1016/j.biopsych.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Moscovitch DA, Litz BT, Kim H, Davis LL, Pizzagalli DA. The worried mind: autonomic and prefrontal activation during worrying. Emotion. 2005;5:464–475. doi: 10.1037/1528-3542.5.4.464. http://dx.doi.org/10.1037/1528-3542.5.4.464. [DOI] [PubMed] [Google Scholar]
- Houtveen JH, Rietveld S, De Geus EJC. Contribution of tonic vagal modulation of heart rate, central respiratory drive, respiration depth, and respiratory frequency to respiratory sinus arrhythmia during mental stress and physical exercise. Psychophysiology. 2002;39:427–436. doi: 10.1017.S0048577202394022. http://dx.doi.org/10.1017/S0048577202394022. [DOI] [PubMed] [Google Scholar]
- Johnsen BH, Hansen AL, Sollers JJ, Murison R, Thayer JF. Heart rate variability is inversely related to cortisol reactivity during cognitive stress. Psychosomatic Medicine. 2002;64:289. [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovačić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009;71:264–268. doi: 10.1016/j.ijpsycho.2008.10.007. http://dx.doi.org/10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DN, Leon AC, Li C, D’Zurilla TJ, Black SR, Vivian D, Dowling F, Arnow BA, Manber R, Markowitz JC, Kocsis JH. Social problem solving and depressive symptoms over time: a randomized clinical trial of cognitive-behavioral analysis system of psychotherapy, brief supportive psychotherapy, and pharmacotherapy. Journal of Consulting and Clinical Psychology. 2011;79:342–352. doi: 10.1037/a0023208. http://dx.doi.org/10.1037/a0023208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. http://dx.doi.org/10.1037/0003-066X.50.5.372. [DOI] [PubMed] [Google Scholar]
- LaRowe SD, Patrick CJ, Curtin JJ, Kline JP. Personality correlates of startle habituation. Biological Psychology. 2006;72:257–264. doi: 10.1016/j.biopsycho.2005.11.008. http://dx.doi.org/10.1016/j.biopsycho.2005.11.008. [DOI] [PubMed] [Google Scholar]
- LeDoux J. The Emotional Brain. Touchstone Simon and Schuster; New York, NY: 1996. [Google Scholar]
- Licht CMM, de Geus JCN, van Dyck R, Penninx BWJH. Association between anxiety disorders and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Psychosomatic Medicine. 2009;71:508–518. doi: 10.1097/PSY.0b013e3181a292a6. http://dx.doi.org/10.1097/PSY.0b013e3181a292a6. [DOI] [PubMed] [Google Scholar]
- Ludewig S, Ludewig K, Geyer MA, Hell D, Vollenweider FX. Prepulse inhibition deficits in patients with panic disorder. Depression and Anxiety. 2002;15:55–60. doi: 10.1002/da.10026. http://dx.doi.org/10.1002/da.10026. [DOI] [PubMed] [Google Scholar]
- Melzig CA, Weike AI, Hamm AO, Thayer JF. Individual differences in fear-potentiated startle as a function of resting heart rate variability: implications for panic disorder. International Journal of Psychophysiology. 2009;71:109–117. doi: 10.1016/j.ijpsycho.2008.07.013. http://dx.doi.org/10.1016/j.ijpsycho.2008.07.013. [DOI] [PubMed] [Google Scholar]
- Murata T, Takahashi T, Hamada T, Omori M, Kosaka H, Yoshida H, Wada Y. Individual trait anxiety levels characterizing the properties of Zen meditation. Neuropsychobiology. 2004;50:189–194. doi: 10.1159/000079113. http://dx.doi.org/10.1159/000079113. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Molecular Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. http://dx.doi.org/10.1111/1467-8721.00166. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Shankman SA. Does intolerance of uncertainty predict anticipatory startle responses to uncertain threat? International Journal of Psychophysiology. 2011;81:107–115. doi: 10.1016/j.ijpsycho.2011.05.003. http://dx.doi.org/10.1016/j.ijpsycho.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oken BS, Salinsky MC, Elsas SM. Vigilance, alertness, or sustained attention: physiological basis and measurement. Clinical Neurophysiology. 2006;117:1885–1901. doi: 10.1016/j.clinph.2006.01.017. http://dx.doi.org/10.1016/j.clinph.2006.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety different attentional functioning under state and trait anxiety. Psychological Science. 2010;21(2):298–304. doi: 10.1177/0956797609359624. http://dx.doi.org/10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: mammalian modifications of our evolutionary heritage, a polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: an evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of New York Academy of Sciences. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. http://dx.doi.org/10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. http://dx.doi.org/10.1016/S0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. http://dx.doi.org/10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porges SW, Bohrer RE. Analyses of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Press; New York: 1990. pp. 708–753. [Google Scholar]
- Porges SW, Doussard-Roosevelt JA, Maiti AK. Vagal tone and the physiological regulation of emotion. In: Fox NA, editor. Monographs of the Society for Research in Child Development. Blackwell Publishing; Boston: 1994. pp. 167–186. [PubMed] [Google Scholar]
- Porges SW, Raskin DC. Respiratory and heart rate components of attention. Journal of Experimental Psychology. 1969;81:497–503. doi: 10.1037/h0027921. http://dx.doi.org/10.1037/h0027921. [DOI] [PubMed] [Google Scholar]
- Rankin C, Abrams T, Barry R, Bhatnagar S, Clayton D, Colombo J, Coppola G, Geyer MA, Glanzman DL, Marsland S, McSweeney FK, Wilson DA, Wu CF, Thompson R. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiology of Learning and Memory. 2009;92(2):135–138. doi: 10.1016/j.nlm.2008.09.012. http://dx.doi.org/10.1016/j.nlm.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin M. Decreased skin conductance response habituation in chronically anxious patients. Biological Psychology. 1975;2:309–319. doi: 10.1016/0301-0511(75)90039-3. http://dx.doi.org/10.1016/03010511(75)90039-3. [DOI] [PubMed] [Google Scholar]
- Ross RJ, Ball WA, Cohen ME, Silver SM, Morrison AR, Dinges DF. Habituation of the startle reflex in post-traumatic stress disorder. Journal of Neuropsychiatry and Clinical Neurosciences. 1989;1:305–307. doi: 10.1176/jnp.1.3.305. [DOI] [PubMed] [Google Scholar]
- Roth WT, Ehlers A, Taylor CB, Margraf J, Agras WS. Skin conductance habituation in panic disorder patients. Biological Psychiatry. 1990;27:1231–1243. doi: 10.1016/0006-3223(90)90421-w. http://dx.doi.org/10.1016/0006-3223(90)90421-W. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kozak MJ, Foa EB, Whitaker DJ. Posttraumatic stress disorder in rape victims: autonomic habituation to auditory stimuli. Journal of Traumatic Stress. 2001;14:283–293. doi: 10.1023/A:1011160800958. http://dx.doi.org/10.1023/A:1011160800958. [DOI] [PubMed] [Google Scholar]
- Ruiz-Padial E, Sollers JJ, Vila J, Thayer JF. The rhythm of the heart in the blink of an eye: emotion-modulated startle magnitude covaries with heart rate variability. Psychophysiology. 2003;40:306–313. doi: 10.1111/1469-8986.00032. http://dx.doi.org/10.1111/1469-8986.00032. [DOI] [PubMed] [Google Scholar]
- Sarapas C, Shankman SA, Nelson BD, Campbell ML, Bishop JR, Robison-Andrew J, Altman SE, Gorka SM, Katz AC. Are appetitive and defensive motivation related? A psychophysiological examination in two independent samples. under review. [DOI] [PMC free article] [PubMed]
- Sargunaraj D, Lehrer PM, Hochron SM, Rausch L, Edelberg R, Porges SW. Cardiac rhythm effects of.125-Hz paced breathing through a resistive load: implications for paced breathing therapy and the polyvagal theory. Applied Psychophysiology, and Biofeedback. 1996;21:131–147. doi: 10.1007/BF02284692. http://dx.doi.org/10.1007/BF02284692. [DOI] [PubMed] [Google Scholar]
- Scherbaum CA, Ferreter JM. Estimating statistical power and required sample sizes for organizational research using multilevel modeling. Organization Research Methods. 2009;12:347–367. http://dx.doi.org/10.1177/1094428107308906. [Google Scholar]
- Segerstrom SC, Nes LS. Heart rate variability reflects self-regulatory strength, effort, and fatigue. Psychological Science. 2007;18:275–281. doi: 10.1111/j.1467-9280.2007.01888.x. http://dx.doi.org/10.1111/j.1467-9280.2007.01888.x. [DOI] [PubMed] [Google Scholar]
- Siddle DA. Orienting, habituation, and resource allocation: an associative analysis. Psychophysiology. 1991;28:245–259. doi: 10.1111/j.1469-8986.1991.tb02190.x. http://dx.doi.org/10.1111/ j.1469-8986.1991.tb02190.x. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety disorder and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. http://dx.doi.org/10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders. 2000;61:201–216. doi: 10.1016/s0165-0327(00)00338-4. http://dx.doi.org/10.1016/S0165-0327(00)00338-4. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Lane RD. Claude Bernard and the heart–brain connection: further elaboration of a model of neurovisceral integration. Neuroscience & Biobehavioral Reviews. 2009;33:81–88. doi: 10.1016/j.neubiorev.2008.08.004. http://dx.doi.org/10.1016/j.neubiorev.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Weinstein SE, Quigley KS. Locus of control predicts appraisals and cardiovascular reactivity to a novel active coping task. Journal of Personality. 2006;74:911–931. doi: 10.1111/j.1467-6494.2006.00396.x. http://dx.doi.org/10.1111/j.1467-6494.2006.00396.x. [DOI] [PubMed] [Google Scholar]
- Werner KW, Gross JJ. Emotion regulation and psychopathology: a conceptual framework. In: Kring A, Sloan D, editors. Emotion Regulation and Psychopathology. The Guilford Press; New York: 2009. [Google Scholar]
- Yeragani VK, Pohl R, Berger R, Balon R, Ramesh C, Glitz D, Srinivasan K, Weinberg P. Decreased heart rate variability in panic disorder patients: a study of power-spectral analysis of heart rate. Psychiatry Research. 1993;46:89–103. doi: 10.1016/0165-1781(93)90011-5. http://dx.doi.org/10.1016/0165-1781(93)90011-5. [DOI] [PubMed] [Google Scholar]