Synopsis
Colonoscopy is an excellent area for quality improvement 1 because it is high volume, has significant associated risk and expense, and there is evidence that variability in its performance affects outcomes. The best endpoint for validation of quality metrics in colonoscopy is colorectal cancer incidence and mortality, but because of feasibility issues, a more readily accessible metric is the adenoma detection rate (ADR). Fourteen quality metrics were proposed by the joint American Society of Gastrointestinal Endoscopy/American College of Gastroenterology Task Force on “Quality Indicators for Colonoscopy” in 2006, which are described in further detail below. Use of electronic health records and quality-oriented registries will facilitate quality measurement and reporting. Unlike traditional clinical research, implementation of quality improvement initiatives involves rapid assessments and changes on an iterative basis, and can be done at the individual, group, or facility level.
Keywords: colonoscopy, quality, metrics, quality improvement
Introduction to Quality
Keen interest in quality within medicine began after the Institute of Medicine’s report on Medical errors was published in 2000.2 This report described the landscape of medicine at the time in the US and described shocking statistics, including the fact that 44,000 to 98,000 Americans die each year from medical errors. This report along with public opinion regarding current care in the US led to a national call for reform within medicine. The report listed the major quality domains as safety, practice consistent with present medical knowledge, and customization.
Many parties have a stake in the measurement and reporting of quality. Stakeholders include patients, providers, professional societies, payors, regulatory bodies and accrediting organizations, the National Quality Forum and the Centers for Medicare and Medicaid Services. Most agree that the goal is to improve outcomes and avoid unintended consequences. There are financial incentives for providers to report quality measures and, as of 2015, there will be financial penalties for failing to report within Medicare.
Given this landscape, colonoscopy is an excellent area for quality improvement (QI) work for several reasons. First, colonoscopy is the most common endoscopic procedure in US3, 4 and there is significant associated risk and expense. Second, standardized reporting of colonoscopies has been advocated,5 yet evidence exists that performance of colonoscopy varies and this variation may impact effectiveness.6–8 Publication of the joint American Society for Gastrointestinal Endoscopy/American College of Gastroenterology “Quality Indicators for Colonoscopy” in 2006 highlighted the importance of quality issues within colonoscopy and provided tangible benchmarks for achievement.9 The importance of ensuring the highest quality in colonoscopy is further promoted by several recent studies questioning the effectiveness of colonoscopy in reducing CRC mortality, especially from right-sided lesions.10–12
Before embarking on a discussion of quality in colonoscopy, it is important to emphasize the distinction between clinical guidelines and quality indicators. The former relates to recommendations for clinical care often related to a specific condition or work-up of a particular symptom. Guidelines may be supported by varying degrees of evidence, ranging from randomized controlled trials to expert opinion. On the other hand, quality indicators are concrete, measurable items related either to the process of care in which the receipt of medical services is tracked13 (e.g. was the cecum reached and photodocumented with landmarks?) or outcome measures (e.g. was a cancer diagnosed within 3 years of colonoscopy?), which in part reflect the results of medical care.13
Quality in Colonoscopy
When quality metrics are developed for any field, consideration is given to the endpoint by which the metric(s) can be monitored and validated. What is the best endpoint for validation of quality metrics in colonoscopy? Colorectal cancer (CRC) incidence and mortality are arguably the most relevant and important outcome in performance of colonoscopy and as such, ideally would be the endpoint by which to validate metrics. These endpoints, however, are difficult to measure and because of relatively low incidence, require many person-years of follow-up to yield meaningful results. Years of data collection are not often feasible in the quality work where data collection, results and adjustments are made much more rapidly than in classical clinical research. Another endpoint is the incidence of so-called “interval cancers,” which are CRCs that arise within a certain time frame after colonoscopy. These interval cancers may arise from missed or incompletely removed lesions at initial colonoscopy, but may also be due to biologically aggressive tumors that arise de novo. Interval CRCs can be measured in two different ways.14, 15 One is a prospective evaluation of patients after colonoscopy with removal of polyps who are enrolled in a surveillance program and return for a follow-up colonoscopy. A second way uses a case-control design in which patients with CRC are identified and assessed for whether or not they had a prior colonoscopy within 3 years of diagnosis. Because of the resources, time and large numbers necessary to measure interval CRCs, a more readily accessible metric is the adenoma detection rate (ADR). ADR has gained international acceptance as a quality indicator and will be discussed below in detail. Lastly, patient satisfaction is also an important metric for consideration, but may be challenging to interpret because of variation in patient population and other factors difficult to control for at the endoscopist level.
Colonoscopy Quality Metrics
Most colonoscopy quality metrics are process measures rather than outcome measures and occur at the level of the endoscopist, rather than the endoscopy unit or hospital. The American Society of Gastrointestinal Endoscopy/American College of Gastroenterology Task Force on “Quality Indicators for Colonoscopy” proposed 14 quality metrics that occur at the pre-procedure, intra-procedure and post-procedure phase of colonoscopy (Table 1).9 Only two measures have strong recommendations (appropriate indication and use of recommended post-polypectomy and post-cancer resection surveillance intervals) and four have intermediate recommendations (cecal intubation rates, ADR, inflammatory bowel disease surveillance biopsies, and endoscopic management of post-polypectomy bleeding). We will highlight several of these indicators in decreasing order of strength of recommendation and provide background on the measure including whether the literature supports its association with improved outcomes.
Table 1.
Proposed Quality Indicators for Colonoscopy – ASGE/ACG 20069
| Quality indicator | Grade of recommendation |
|---|---|
| Pre-procedure | |
| Appropriate indication | 1C+ |
| Informed consent including discussion of risks | 3 |
| Use of post-polypectomy and post-cancer resection surveillance intervals | 1A |
| Use of IBD disease surveillance intervals | 2C |
| Documentation of quality of bowel preparation in note | 2C |
| Intra-procedure | |
| Cecal intubation rates including photo documentation of landmark in note | 1C |
| Adenoma detection during screening | 1C |
| Withdrawal time: mean >6 minutes in normals exams | 2C |
| Biopsy specimens in patients with chronic diarrhea | 2C |
| Number and distribution of biopsy specimens in IBD surveillance | 1C |
| Endoscopic resection attempted for pedunculated polyps and sessile polyps <2 cm | 3 |
| Post-procedure | |
| Incidence of perforation | 2C |
| Incidence of post-polypectomy bleeding | 2C |
| Pot-polypectomy bleeding managed non-operatively | 1C |
Adapted by permission from Macmillan Publishers Ltd: American Journal of Gastroenterology (Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol 2006;101:873–85), copyright 2006.
1A = Strong: clear benefit, supported by randomized trials without important limitations
1C+ =Strong: clear benefit, supported by overwhelming evidence from observational studies
1C = Intermediate: clear benefit, supported by observational studies
2C = Very weak: unclear benefit, supported by observational studies
3 = Weak: unclear benefit, expert opinion only
Screening/Surveillance intervals
The use of recommended screening intervals for normal colonoscopies and recommended post-polypectomy and post-cancer surveillance intervals for those with neoplasia is important in order to minimize risks of excessive procedures and maximize cost-effectiveness of population based screening.9 Ample literature suggests that patients are told to return more frequently than dictated by guidelines.16–18 Yet those who are deemed high risk who do not return for surveillance also indicate poor quality care. In one study, 34% of those with polyps >1 cm did not return for surveillance.19 Similarly, in another study of patients with adenomas or CRC, 35% had no follow-up and 38% had their follow-up delayed.20
The importance of appropriate polyp surveillance and avoidance of inappropriate use has been recognized at the national level with the endorsement of colonoscopy interval quality measures by the National Quality Forum and their adoption by the Physician Quality Reporting System (PQRS). PQRS was initiated in 2007 by the Centers for Medicare and Medicaid Services (CMS) as a reporting program that uses “a combination of incentive payments and payment adjustments to promote reporting of quality information by eligible professionals.”21 Those who report satisfactorily on the measure receive incentive payments based on their Medicare Part B Fee charges. Starting in 2015, there will also be a payment penalty for those who do not satisfactorily report data on quality measures for covered professional services.
Of the 190 measures included in the 2011 PQRS measurements, only one pertained to colonoscopy. Measure 185, “Endoscopy & Polyp Surveillance: Colonoscopy Interval for Patients with a History of Adenomatous Polyps – Avoidance of Inappropriate Use” specifies reporting of the “percentage of patients aged 18 years and older receiving a surveillance colonoscopy and a history of colonic polyp(s) in a previous colonoscopy, who had a follow-up interval of 3 or more years since their last colonoscopy documented in the colonoscopy report.” Detailed measure specifications can be found at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/downloads/2011_physqualrptg_measurespecificationsmanual_033111.pdf. There is no performance threshold yet set for this measure, and providers receive credit simply for reporting their performance scores.
Adenoma detection rate
Detecting and removing adenomas is a fundamental goal of screening colonoscopy. Missed lesions may be a potential cause of interval cancers and perhaps the lower than expected protection rate from colonoscopy. Despite its importance, there are large disparities in the detection rates of practicing endoscopists,22 which has been shown in studies of tandem colonoscopy,23, 24 small group practices,7, 8, 25, 26 missed neoplasia,27–29 and CT colonography.30 The target rate for ADR is ≥25% in men and ≥15% in women over 50 years old presenting for average-risk screening based on observed prevalence rates of neoplasia in the US.9
Measurement of ADR should be a priority. The most accepted method for measurement is calculation of the number of screening colonoscopies in which at least 1 adenoma was found divided by the total number of screening colonoscopies performed. Others have suggested the mean number of adenomas per patient as another calculation that may add additional information.31–33 To calculate mean adenomas per patient, one would divide the total number of adenomas by the total number of colonoscopies performed. While mean adenoma per patient may prove to be a good measure of adenoma detection, current data is insufficient to establish compliance rate benchmarks for its use as a quality metric.
The type of exams and the time period or volume over which ADR should be calculated has not been solidified. For now, ADR should be limited to screening colonoscopy exams, although as more data emerges on adenoma rates in surveillance colonoscopy, surveillance may be monitored as well whether in combination with screening or independently. The time period or number of cases over which ADR is calculated is not specified, which may raise difficulty in interpreting results, especially for low-volume endoscopists. One study highlights the importance of the number of procedures over which this measure is calculated because low numbers may not accurately represent the true ADR (Table 2). At least 500 procedures need to be included in the calculation to have a reliable point estimate of ADR and a 95% confidence interval can account for uncertainty and should be used in the setting of lower numbers of procedures to help interpret endoscopist performance.33
Table 2.
Adenoma detection rate and confidence intervals
| Adenoma detection rate | ||||||
|---|---|---|---|---|---|---|
| # of colonoscopies | 15% | 20% | 25% | 30% | 35% | 40% |
| 100 | 8–22 | 12–28 | 17–33 | 21–39 | 26–44 | 30–50 |
| 500 | 12–18 | 16–24 | 21–29 | 26–34 | 31–39- | 36–44 |
| 1000 | 13–17 | 18–22 | 22–28 | 27–33 | 32–38 | 37–43 |
There is some debate over whether a polyp detection rate (PDR) or polypectomy rate can be used reliably as a surrogate for ADR. The benefit would be ease of calculation by avoiding linking with pathology results facilitating tracking given the logistical hurdles when using a registry to determine ADR.34 PDR is highly correlated with ADR with proposed benchmarks of 40% in men and 30% in women.35, 36 If there is concern over “gaming the system” in which endoscopists report and/or remove polyps of which few are adenomas, sporadic audits with pathology reports could help prevent this.36 In the Clinical Outcomes Research Initiative registry, the use of polyps >9 mm as a surrogate for advanced neoplasia is 84% specific,37 suggesting that surrogate markers might facilitate calculation of metrics within registries. In addition, novel algorithms may help facilitate linking pathology with endoscopy reports,34 especially in the setting of electronic health records.
Robust data support that ADR is a valid predictor of CRC. In a large study of over 45,000 patients in a national CRC screening program in Poland, ADR and patient age were the only predictors of interval CRC, defined as CRC before the next surveillance exam was due (< 5 years).38 Patient sex, doctor specialty and cecal intubation rates were not associated with interval cancer. This study highlighted that measurement of ADR should have a role in continuous quality-improvement programs for CRC screening. Some hypothesize that ADR may be a proxy for a more thorough examination of the colon. In a large German study, ADR was associated with endoscopist CME attendance and colonoscope instrument model, but not endoscopist procedural volume or withdrawal times.39
Numerous studies have attempted to address whether or not ADR can be improved. A systematic review that included 7 studies and 10 abstracts found that improving ADR is difficult.40 This is exemplified by a study of 5 ambulatory endoscopy centers in Minnesota in which they attempted to systematically evaluate variability in ADR over time after application of specific quality improvement programs.41 None of the interventions (blinded and unblinded review of individual ADR, education about colonoscopy quality literature highlighting ADR goals and importance of withdrawal time, discussion between practice leaders and poor performing endoscopists, and financial consequences for withdrawal time < 6 minutes) resulted in a significant improvement in ADR, which they found to be associated only with patient factors (age, sex and bowel prep quality). More research needs to be conducted to determine methods for improving ADR among low performers.
Finally, while ADR is typically calculated from traditional adenomas, the importance of serrated lesions is apparent.42, 43 Whether serrated lesions should be included in the measurement of ADR, or whether they merit their own metric remains to be determined.
Cecal intubation rates
Complete colonoscopy to the cecum is necessary for detection of a substantial fraction of proximal colon lesions.44 Quality indicators encourage documentation of cecal intubation with naming of the identified cecal landmarks (appendiceal orifice and ileocecal valve) and photographs. 9 Intubation of the cecum should be achieved in ≥95% of healthy subjects and ≥90% of all colonoscopies. Cecal intubation rates are associated with post-colonoscopy CRC. In a large database study from Canada higher cecal intubation rates were associated with protection from CRC in the right side. Similarly, in a study in the UK, higher cecal intubation rates were associated with higher ADRs.31, 45
Withdrawal times
Withdrawal time, defined as the time from the colonoscope reaching cecum to removal of the instrument from the patient, is considered a quality indicator in colonoscopy. Expert opinion suggests that the “mean withdrawal time should be ≥6 minutes in colonoscopies with normal results performed in patient with intact colons.”9 This recommendation was qualified by the statement that for those with adequate ADR, secondary measures, such as withdrawal time are of less importance.9 It must be emphasized that withdrawal time should not be applied to individual patient cases but must be averaged over many cases for an individual endoscopist.
The importance of withdrawal time and establishment of a threshold of 6 minutes came after publication of a study of 12 gastroenterologists in a community based practice.7 The investigators compared the differences in ADR between endoscopists with ≥6 minutes and <6 minutes withdrawal times. They found a strong correlation between mean withdrawal time on cases without polypectomy and detection of lesions (rs = 0.90, p<0.001). Endoscopists with mean withdrawal time ≥6 minutes also detected more advanced lesions (6.4% versus 2.3%, p=0.005).
However, adopting a minimum withdrawal time alone is not likely to result in a substantially improved ADR.46 Simply recording withdrawal time did not improve ADR in one study.46 After the institution of using a cecal time stamp to record withdrawal time, PDR did not change significantly. Another study in Peru found that adopting a 6-minute or longer withdrawal time minimum did not change PDR.47 This study should be interpreted with caution because of overall low rates of PDR in the range of 15–16%. Confidential feedback on withdrawal time with follow-up monitoring resulted in an increase in withdrawal time and in PDR, but not ADR. Mean withdrawal time without polyps went from 6.6 minutes to 8.1 minutes (p<.0001), PDR went from 33% to 38% (p=0.04), without a significant change in ADR (20% to 23%, p=0.17).48 Similarly, in a study of 42 endoscopists at an academic medical center, an institutional wide policy requiring a minimum of 7 minute withdrawal time per case increased the proportion of exams with mean withdrawal time 7 minutes (65% to 100%), but did not affect PDR.49 Lastly, a study of a large German colonoscopy registry found that withdrawal time was not associated with ADR.39
Ultimately, one has to recognize that withdrawal time is simply a surrogate marker for ADR and thus has limited value if endoscopists are already performing careful inspections. However, for those with low ADR, withdrawal time should be measured to see if very short inspection time might be a modifiable factor for improvement.
Bowel preparation quality
The diagnostic accuracy of colonoscopy depends on the cleanliness of the colon and ability to visualize lesions. Poor preparation leads not only to missed lesions but also prolonged duration of procedure, repeated exams at earlier intervals, increased complications and excessive costs.50–53 The quality of bowel preparation should be documented in every procedure note.9 Qualitative descriptives (“excellent” “good” “fair “poor”) used in clinical practice lack standardized definitions and the Quality Assurance Task Force and Multi-Society Task Force on CRC advocates the use of “inadequate to exclude polyps >5 mm in size” as a cut off for repeating colonoscopy.5 The Ottawa and Aronchick bowel preparation rating scales were created as potential standardized bowel preparation scales, but were limited by lack of extensive validation and reliability testing. Futhermore, the Ottawa scale is complex, limiting practical use, and the Aronchick scale relies on global assessments that do not address individual colonic segments.54, 55 The Boston Bowel Preparation Scale has been shown to be reliable and was validated prospectively, showing correlation with important outcomes like polyp detection and has been incorporated in the CORI and Provation™ endowriters.56, 57 However, there are no rating scales that receive universal acceptance as a gold standard.
If bowel preparation is inadequate in >10% of exams, this may reflect quality-control issues and special attention may need to be given to patient education and type of preparation.5 Bowel preparation considerations are covered in more detail in chapter 10.
Complications
Of the known complications of colonoscopy,58 only the incidences of perforation and post-polypectomy bleeding are proposed as quality measures.9 A benchmark has been set such that if perforation rates are greater than 1/500 for all colonoscopies or 1/1000 for screening colonoscopies, evaluation should be pursued.9 Bleeding rates >1% should prompt evaluation of whether inappropriate practices are taking place.9 Some data suggests that perforation rates may be higher among low volume endoscopists.59 In an administrative database study of over 97,000 patients undergoing outpatient colonoscopy in Canada, older age, male sex, having a polypectomy, and having the colonoscopy performed by a low-volume endoscopist (<300/year) were associated with increased odds of bleeding or perforation.59
The performance benchmarks established by the Joint American Society of Gastrointestinal Endoscopy/American College of Gastroenterology Task Force on “Quality Indicators for Colonoscopy” were based on available literature; however, without proper documentation or systems for capturing delayed complications, rates may be under reported60, 61 rendering the rate of “acceptable complications” inaccurate. A structured assessment tool was used to survey patients who had undergone colonoscopy in the Netherlands to determine major adverse events (hospital visit required), minor adverse events, and days missed from work. Adverse events were further categorized as definite-, possible-, or unrelated adverse events. They found the rate of definite-major and minor events to be 1% and 29% respsectively.62 Development of a proper lexicon for reporting complications should also help promote the consistent and accurate classification of adverse events.63
Colonoscopy-related complications are covered in detail in chapter 12.
Documentation
While proper documentation may not at first glance seem to impact patient outcomes related to colonoscopy, it plays an essential role in the ability to track indicators and develop benchmarks for performance. The absence of key information hinders communication with other physicians and may impede appropriate follow-up, whether too soon or delayed.
Historically, there has been variability in the quality of colonoscopy reports and documentation. A study of colonoscopy reports as part of a statewide screening program in Maryland looked at 25 data elements and found that 27% of reports did not document bowel preparation quality, 18% did not use specific cecal landmarks, and in cases with polyps, size and morphology were missing 13% and 47% of the time, respectively.64 A different study of 4800 colonoscopies by 116 Dutch endoscopists also found considerable variability in documentation of quality of bowel prep and cecal landmarks.65 A study of 135 reports from the U.S. community setting also demonstrated low completeness rates.66
Even with the use of a computer generated standardized endoscopy report within the CORI network, 13.9% did not include bowel-preparation quality, and cecal landmarks were not recorded in 14%. In addition, 10% did not include comorbidity classification and key polyp descriptors were often missing.67
This variability in reporting led to the development of the standardized colonoscopy reporting and data system (“CO-RADS”) in 2007.5 CO-RADS suggests key areas for documentation (Box 1). The literature suggests that colonoscopy reporting has improved since CO-RADS, whether due to the publication itself or temporal trends. A quality audit of 250 reports in 2009 showed improvement in reports, with 100% listing indication and informed consent, 99.6% prep quality, 78.8% cecal landmarks, 67% photo-documentation of the cecum, 67%, and 65.8% and 62.2% polyp size and morphology, respectively.68 Targeted modifications to electronic colonoscopic reporting systems can improve quality of reports. Changes to software such as drop-down menus and visual cues to optimize completion of required fields have helped improve reporting.69
Box 1. Standardized colonoscopy reporting and data system (CO-RADS) to improve the quality of colonoscopy5.
| Key subject areas for colonoscopy report |
|---|
| Patient demographics and history |
| Assessment of patient risk and co-morbidity |
| Procedure indication(s) |
| Procedure: technical description |
| Colonoscopic findings |
| Assessment |
| Interventions/unplanned events |
| Follow-up plan |
| Pathology |
Reprinted from Gastrointestinal Endoscopy 65(6), Lieberman D, Nadel M, Smith RA, et al., Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable, page 759, Copyright 2007, with permission from Elsevier.
Databases and Registries
Measurement of quality is facilitated by use of electronic health records (EHR), administrative data, and quality-oriented registries. When using such data, it is important to accurately identify the denominator of reference for the metric being assessed. Inclusion of cases in the denominator of a measure that do not accurately represent the condition being measured can result in a falsely low quality score and conversely, exclusion of cases from the denominator that do represent the condition being measured can result in a falsely high score. Understanding the source of data and its potential inherent biases can help in interpreting results.70
Electronic Health Record
The electronic health record (EHR) can be used to enhance quality.71–74 The main advantages are that the data is already collected and represents the full spectrum of care without limitations. The disadvantages are that the data content or format may not meet standards necessary for other uses because of imprecise terminology, missing or inconsistent data or may be difficult to extract from narrative text. If specific data elements can be captured from well-defined, discrete, coded fields then the EHR can be useful for secondary purposes like quality research and reporting. Using the EHR has the additional advantage of allowing for discovery of better quality measures that reflect differing degrees of disease severity.70
Administrative data
Administrative data collected from insurance companies, billing records or national databases are another source of data that can be used for evaluating quality in colonoscopy. These data are already collected and typically inexpensive to use. Large numbers of events provide sufficient power for statistical analyses, and there is the unique ability to measure samples of geographically dispersed patients. One may also potentially assemble longitudinal records across providers and settings (e.g. inpatient vs. outpatient care). One disadvantage of administrative data is that it is not collected specifically for the purpose of measuring quality and may be of questionable validity depending on the outcomes and variables being measured. Coding for a specific condition may not be specific or sensitive enough to meet the standards for quality measurement, with resultant under-detection, and there may be incomplete physician documentation.
An example of using administrative data for CRC screening comes from The National Healthcare Quality Report (NHQR) from the Agency for Healthcare Research and Quality (AHRQ) that was developed in coordination with the Department of Health and Human Services.13 The project drew upon over 33 databases in order to report on process and outcome measures at the national and state level. Reports were based on recommendations from the Institute of Medicine’s Future Directions for the National Healthcare Quality and Disparities Reports.75 The NHQR evaluated 3 core measures, of which prevention of CRC was one. They found that the overall percentage of adults age 50 and over who reported having received colorectal cancer screening significantly increased from 52 % in 2005 to 56% in 2008 with some disparities noted (e.g. higher screening rates in large fringe metropolitan areas than adults in large central metropolitan areas). The 5 states with the highest rates of screening reached a benchmark of 67%.
Performance measures related to colonoscopy can be measured from administrative data. A study of the Ontario Cancer Registry from 2000 to 2005 looked at patients who underwent colonoscopy 7 to 36 months prior to diagnosis of CRC.45 The authors found that endoscopist specialty (GI vs. other), setting (hospital vs. non hospital), procedure completion rates (≥95% vs. <80%) and polypectomy rates ((≥30% vs. <10%), but not volume were associated with post-colonoscopy CRC.
Electronic reporting software
The Clinical Outcomes Research Initiative (CORI) is a network of practice-based endoscopists who use a standardized endoscopic reporting software program to generate reports. Data from these reports are uploaded to a central repository, where they can be used to measure and enhance quality. Data from multiple practices can also be pooled for benchmarking. While quality can be measured in such an electronic setting, even with this structured reporting system, clinicians may omit important quality data. In a study of 430,000 colonoscopy reports from 74 practice sites many quality metrics were incomplete.67 For example, 14% failed to report cecal landmarks and 14% failed to report quality of bowel preparation.
Registries
Registries are repositories of data derived specifically for the disease registry itself or from a health survey. A registry can be specifically designed for research and quality reporting and as such, can avoid many of the biases of administrative data. One disadvantage is that it requires significant investment from participants for data collection and quality control. If there is automated data extraction, for example directly from electronic reporting software, there may be an initial upfront investment in time and money and systematic errors may occur throughout. Manual abstraction has ongoing costs due to time and expense and can lead to random errors at any point.
The balance between the burden of data collection and the benefits of participation is delicate. AHRQ has a handbook for registries performing outcomes and quality reseatch.76 This handbook addresses topics in registry methodology and relevant legislative issues.
States may develop their own registries to help with quality improvement. For example, New Hampshire has its own colonoscopy registry that is supported by federal funding. Typically such registries are not available for participation outside that state.34
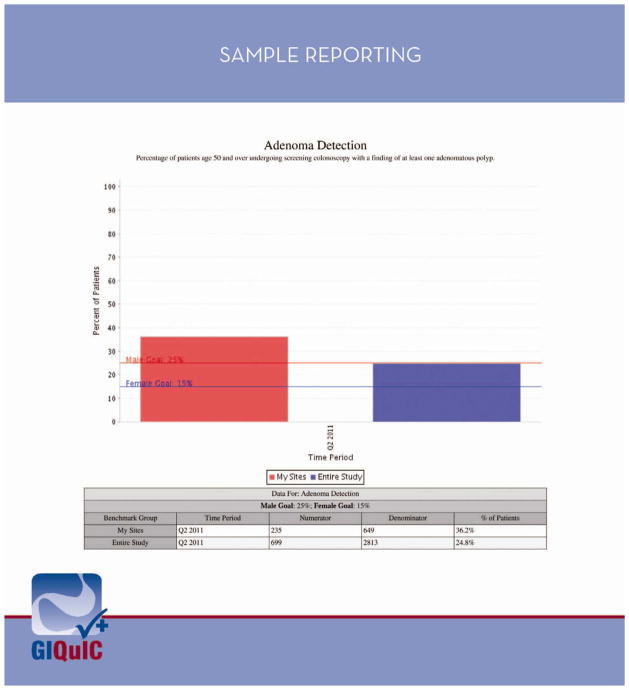
Two gastroenterology specific registries have been developed for monitoring of endoscopy quality. The GI Quality Improvement Consortium, Ltd. (GIQuIC) (http://giquic.gi.org) is a national registry for individual physicians and groups to determine performance on selected quality measures and to benchmark against other participants.77 This was created by a non-profit partnership between ACG and ASGE. Reports can be submitted manually to the registry website or automatically from certain electronic reporting software programs. Endowriters certified with GIQuIC include Amkai, CORI, eMerge Health Solutions, Endosoft, gMed, MD Reports, Olympus (version 7.4), Pentax (endoPRO iQ) and ProVation (Version 5.0). There are 84 data fields and 10 quality measures benchmarked including rate of cecal intubation, adenoma detection rate, prep assessment, and appropriate indications for procedure, among others. Participants can submit any individual PQRS measure to GIQuIC’s CMS-certified registry partner, Outcome Sciences. Figure 1 shows a sample report on ADR generated by GIQuIC.
Figure 1.
A sample report on the adenoma detection rate for an individual site generated within the GI Quality Improvement Consortium, Ltd Registry (GIQuIC). The metric for the individual site is compared to all sites within GIQuIC and national benchmarks are indicated. Used with permission from The GI Quality Improvement Consortium, Ltd, 2013.
The American Gastroenterology Association Digestive Health Outcomes Registry® (AGA Registry) has been certified by CMS as an official PQRS registry (http://www.gastro.org/practice/digestive-health-outcomes-registry). AGA Registry requires a clinical profile, office location profile, data entry either online or via EHR and patient sampling for CRC prevention or IBD. It currently supports gMED’s gastro v4 or custom integration with FIGMD. AGA Registry monitors 6 colorectal cancer prevention measures: identification of CRC risk, endoscopic examination interval, use of anesthesia professionals, procedure-related complications, colonoscopy assessment and adenoma detection rate. In April 2012, AGA Registry developed a collaboration with United Healthcare for quality reporting. Figure 2 shows a sample report on appropriate endoscopic examination intervals.
Figure 2.

A sample report on appropriate endoscopic examination intervals for an individual site generated within the AGA Digestive Health Outcomes Registry (AGA Registry). Used with permission from the American Gastroenterology Association, 2013.
Quality Improvement Initiatives
Unlike traditional biomedical research in which a protocol will be designed and data collection will occur over years prior to analysis or results, quality improvement work revolves around rapid assessments and implementation of changes on an iterative basis.
Implementation
The first step in implementing a quality improvement project is to select an outcome for your intervention. This outcome should be clearly defined with strict parameters. For example, if you want to improve your ADR, you must first decide which colonoscopies to include in your measurement. Typically this would include screening procedures only, but would you include first-time screenings or a repeat screening 10 years after an initial colonoscopy? Would you include all patients, or exclude those with a strong family history of CRC and/or partial colectomies? If you want to compare your outcome to national benchmarks, you should follow published quality measure guidelines for your criteria for inclusion of cases in the measure calculation’s numerator and denominator. If, however, you seek to simply improve your own ADR, consistency in your measurement algorithm over time may be more important than matching national standards for which cases to include.
The next step is to collect measurements to determine your baseline starting point. At this stage you may find the quality is already high, such as a near-100% cecal intubation rate. If there is little room for improvement, there is no reason to focus on that quality measure. This is also the stage where you may find your method for data measurement needs refinement. Was it too cumbersome or time consuming? Is there a way to automate the process? Is there variation in how providers are reporting the key information?
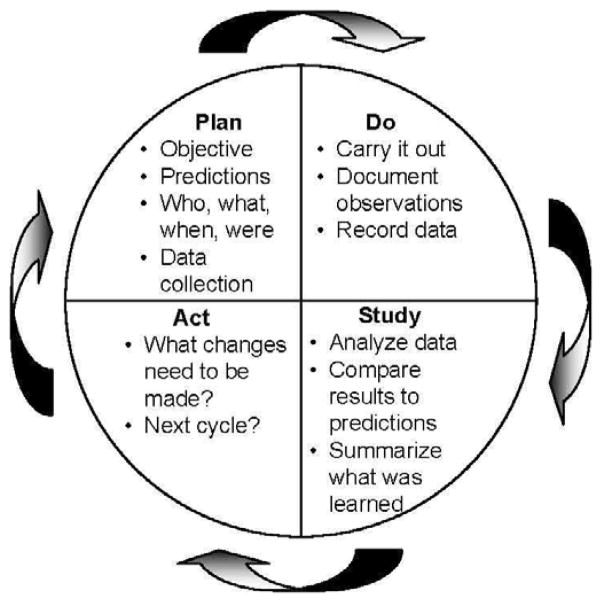
Once your data collection methods are developed, you should brainstorm how to improve compliance with the quality measure. If it is ADR, you could consider being even more meticulous in inspection of the colonic mucosa. Are bowel preparations adequate? Perhaps purchasing an automated water pump for improved flushing during procedures could be considered. In quality improvement projects, it is not necessary to collect data for extended periods of time before analyzing results. One method is the Plan-Do-Study-Act (PDSA) process, which is part of the Institute for Healthcare Improvement Model for Improvement.78 In a PDSA cycle, you plan your intervention, roll it out, study the outcomes for a brief period (e.g. one month) and note what happens (Figure 3). During the “study” phase you are looking for any problems in implementation (which may include lack of provider “buy in”) or unintended consequences. For example, if you are now spending 30 minutes on the withdrawal phase of your procedures, are patients backing up in the waiting room? After the “study” phase, you may identify some changes needed to your protocol. This leads to the “act” phase where you consider how to alter the quality improvement project to try to address any shortcomings. You then plan how to roll out these changes as you begin the next PDSA cycle.
Figure 3.
Schematic of the Plan-Do-Study-Act Cycle, a simple and powerful scientific method for quality improvement whereby a team plans a test or observation, carries it out on a small scale, studies the results, and refines the change.
It may be wise to limit the first few PDSA cycles to a few providers, endoscopy rooms, or nursing staff. This helps iron out problems and allows your quality improvement project to be optimized before exposing a large group to the new protocol. There will often be some providers or nurses who are reluctant to adopt change. Launching a full-blown quality improvement project while there are still obstacles to work through will embolden nay-sayers and make it difficult to continue. On the other hand, once those in a pilot project begin to see progress, their enthusiasm and empirical observations can often help encourage all parties to get involved.
Colonoscopy-specific initiatives
Quality improvement for colonoscopy can span the continuum of care, from access and utilization,79 patient education regarding the pre-procedure80, 81 or post-procedure time, patient recall systems82, bowel preparation,83, 84 sedation/monitoring and adverse events recognition and reporting. Whether or not specific interventions can improve performance or outcomes related to colonoscopy is variable; documentation, minimum standard requirements, education, and other forms of monitoring with videotaping have been tried. Below, we highlight a few of many studies in the literature.
Withdrawal time
A study compared historical data on 2053 screening colonoscopies to 2325 screening colonoscopies using a minimum 8-minute withdrawal time.49 The intervention was a timer beeping every 2 minutes to alert the endoscopist of the passage of time. Post-intervention patients had higher rates of neoplasia (35% vs. 24%, p<0.0001) and endoscopists with mean withdrawal times ≥ 8 minutes had higher ADRs (38% vs. 23%, p <0.0001).
Documentation
An educational initiative was undertaken to improve documentation in an unstructured dictation system for colonoscopy reporting.85 The intervention included lectures sharing baseline quality indicator compliance for recommended quality indicators. Individual compliance rates were also shared directly with endoscopists. An email was sent to remind endoscopists about documentation and an educational poster was displayed in each procedure room. Lastly, a standardized procedure data sheet was developed to help with dictations. The investigators noted a significant improvement in quality measures. Bowel preparation documentation increased from 64% to 83%, identification of the appendiceal orifice increased from 53% to 68%, photography of the cecum increased from 20% to 63%, and documentation of polyp morphology increased from 17% to 54%. Whether these improvements were sustainable remains unknown.
Complications
In order to better manage perforations after endoscopy, one center formed a multi-disciplinary team (GI, surgery, and radiology) to review the existing literature and data from the previous 10 years at their site.86 They then developed an algorithm for management of endoscopic perforations. Implementation of the algorithm and monitoring of its impact on outcomes is ongoing.
Conclusion
In summary, quality in colonoscopy is essential to maintaining the trust of patients and the public. There is a need to continually define and refine quality metrics that can help improve our care of patients and optimize outcomes. Measurement and reporting of quality, as well as achieving performance benchmarks will become increasingly relevant as more financial incentives and penalties surrounding particular metrics from organizations and payors emerge. Endoscopy-unit level quality measures will likely become increasingly important as well. Future research should focus on bolstering evidence to support how particular metrics correlate with clinically relevant outcomes, as well as on identifying types of interventions that improve these metrics (e.g. How can we improve ADR? How can we decrease inappropriate use of surveillance colonoscopy?). Further adoption of EHRs will make registry reporting easier and will permit greater automation of quality measurement.
Key points.
Quality in colonoscopy is essential to maintaining the trust of patients and the public
Several metrics have been suggested as quality indicators, including appropriate screening/surveillance intervals, adenoma detection rates (ADR), cecal intubation rates, and appropriate documentation
Implementation of quality improvement projects requires identification of a problem and outcome for measurement and development of an intervention followed by frequent short-term assessments and adjustments
The electronic health records and other registries will facilitate quality reporting and measurement
Footnotes
The authors have no financial or other conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dulai GS, Farmer MM, Ganz PA, et al. Primary care provider perceptions of barriers to and facilitators of colorectal cancer screening in a managed care setting. Cancer. 2004;100:1843–52. doi: 10.1002/cncr.20209. [DOI] [PubMed] [Google Scholar]
- 2.America CoQoHCi, Medicine Io. To Err Is Human: Building a Safer Health System. The National Academies Press; 2000. [PubMed] [Google Scholar]
- 3.Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136:741–54. doi: 10.1053/j.gastro.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Seeff LC, Richards TB, Shapiro JA, et al. How many endoscopies are performed for colorectal cancer screening? Results from CDC’s survey of endoscopic capacity. Gastroenterology. 2004;127:1670–7. doi: 10.1053/j.gastro.2004.09.051. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman D, Nadel M, Smith RA, et al. Standardized colonoscopy reporting and data system: report of the Quality Assurance Task Group of the National Colorectal Cancer Roundtable. Gastrointest Endosc. 2007;65:757–66. doi: 10.1016/j.gie.2006.12.055. [DOI] [PubMed] [Google Scholar]
- 6.Cotton PB, Connor P, McGee D, et al. Colonoscopy: practice variation among 69 hospital-based endoscopists. Gastrointest Endosc. 2003;57:352–7. doi: 10.1067/mge.2003.121. [DOI] [PubMed] [Google Scholar]
- 7.Barclay RL, Vicari JJ, Doughty AS, Johanson JF, Greenlaw RL. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–41. doi: 10.1056/NEJMoa055498. [DOI] [PubMed] [Google Scholar]
- 8.Chen SC, Rex DK. Endoscopist can be more powerful than age and male gender in predicting adenoma detection at colonoscopy. Am J Gastroenterol. 2007;102:856–61. doi: 10.1111/j.1572-0241.2006.01054.x. [DOI] [PubMed] [Google Scholar]
- 9.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–85. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 10.Baxter NN, Goldwasser MA, Paszat LF, Saskin R, Urbach DR, Rabeneck L. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. doi: 10.7326/0003-4819-150-1-200901060-00306. [DOI] [PubMed] [Google Scholar]
- 11.Brenner H, Hoffmeister M, Arndt V, Stegmaier C, Altenhofen L, Haug U. Protection from right- and left-sided colorectal neoplasms after colonoscopy: population-based study. J Natl Cancer Inst. 2009;102:89–95. doi: 10.1093/jnci/djp436. [DOI] [PubMed] [Google Scholar]
- 12.Singh H, Nugent Z, Demers AA, Kliewer EV, Mahmud SM, Bernstein CN. The Reduction in Colorectal Cancer Mortality After Colonoscopy Varies by Site of the Cancer. Gastroenterology. 2010;139:1128–37. doi: 10.1053/j.gastro.2010.06.052. [DOI] [PubMed] [Google Scholar]
- 13.AHRQ Publication No 11–0004. Rockville, MD: Agency for Healthcare Research and Quality; Feb, 2011. National Healthcare Quality Report 2010. [Google Scholar]
- 14.Lieberman D. Progress and challenges in colorectal cancer screening and surveillance. Gastroenterology. 2010;138:2115–26. doi: 10.1053/j.gastro.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Lieberman D. How good is your dentist? How good is your endoscopist? The quality imperative. Gastroenterology. 2012;142:194–6. doi: 10.1053/j.gastro.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 16.Saini SD, Nayak RS, Kuhn L, Schoenfeld P. Why don’t gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol. 2009;43:554–8. doi: 10.1097/MCG.0b013e31818242ad. [DOI] [PubMed] [Google Scholar]
- 17.Mysliwiec PA, Brown ML, Klabunde CN, Ransohoff DF. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141:264–71. doi: 10.7326/0003-4819-141-4-200408170-00006. [DOI] [PubMed] [Google Scholar]
- 18.Schoen RE, Pinsky PF, Weissfeld JL, et al. Utilization of surveillance colonoscopy in community practice. Gastroenterology. 2010;138:73–81. doi: 10.1053/j.gastro.2009.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brueckl WM, Fritsche B, Seifert B, et al. Non-compliance in surveillance for patients with previous resection of large (> or = 1 cm) colorectal adenomas. World J Gastroenterol. 2006;12:7313–8. doi: 10.3748/wjg.v12.i45.7313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulder SA, Van Leerdam ME, Ouwendijk RJ, Bac DJ, Giard RW, Kuipers EJ. Attendance at surveillance endoscopy of patients with adenoma or colorectal cancer. Scand J Gastroenterol. 2007;42:66–71. doi: 10.1080/00365520600780601. [DOI] [PubMed] [Google Scholar]
- 21.Physicians Quality Reporting System (PQRS) U.S. Department of Health & Human Services, Centers for Medicare and Medicaid Services; Sep 27, 2012. [(Accessed November 23, 2012]. at http://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/PQRS/index.html?redirect=/pqri. [Google Scholar]
- 22.Rex DK, Hewett DG, Snover DC. Editorial: Detection targets for colonoscopy: from variable detection to validation. Am J Gastroenterol. 2010;105:2665–9. doi: 10.1038/ajg.2010.330. [DOI] [PubMed] [Google Scholar]
- 23.van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–50. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 24.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–8. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 25.Atkin W, Rogers P, Cardwell C, et al. Wide variation in adenoma detection rates at screening flexible sigmoidoscopy. Gastroenterology. 2004;126:1247–56. doi: 10.1053/j.gastro.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 26.Bretthauer M, Skovlund E, Grotmol T, et al. Inter-endoscopist variation in polyp and neoplasia pick-up rates in flexible sigmoidoscopy screening for colorectal cancer. Scand J Gastroenterol. 2003;38:1268–74. doi: 10.1080/00365520310006513. [DOI] [PubMed] [Google Scholar]
- 27.Hosokawa O, Shirasaki S, Kaizaki Y, Hayashi H, Douden K, Hattori M. Invasive colorectal cancer detected up to 3 years after a colonoscopy negative for cancer. Endoscopy. 2003;35:506–10. doi: 10.1055/s-2003-39665. [DOI] [PubMed] [Google Scholar]
- 28.Leaper M, Johnston MJ, Barclay M, Dobbs BR, Frizelle FA. Reasons for failure to diagnose colorectal carcinoma at colonoscopy. Endoscopy. 2004;36:499–503. doi: 10.1055/s-2004-814399. [DOI] [PubMed] [Google Scholar]
- 29.Schoen RE, Pinsky PF, Weissfeld JL, et al. Results of repeat sigmoidoscopy 3 years after a negative examination. JAMA. 2003;290:41–8. doi: 10.1001/jama.290.16.2123-b. [DOI] [PubMed] [Google Scholar]
- 30.Pickhardt PJ, Nugent PA, Mysliwiec PA, Choi JR, Schindler WR. Location of adenomas missed by optical colonoscopy. Ann Intern Med. 2004;141:352–9. doi: 10.7326/0003-4819-141-5-200409070-00009. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJ, Rutter MD, Blanks RG, et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–7. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 32.Denis B, Sauleau EA, Gendre I, Piette C, Bretagne JF, Perrin P. Measurement of adenoma detection and discrimination during colonoscopy in routine practice: an exploratory study. Gastrointest Endosc. 2011;74:1325–36. doi: 10.1016/j.gie.2011.07.038. [DOI] [PubMed] [Google Scholar]
- 33.Do A, Weinberg J, Kakkar A, Jacobson BC. Reliability of adenoma detection rate is based on procedural volume. Gastrointest Endosc. 2012 doi: 10.1016/j.gie.2012.10.023. [DOI] [PubMed] [Google Scholar]
- 34.Greene MA, Butterly LF, Goodrich M, et al. Matching colonoscopy and pathology data in population-based registries: development of a novel algorithm and the initial experience of the New Hampshire Colonoscopy Registry. Gastrointest Endosc. 2011;74:334–40. doi: 10.1016/j.gie.2011.03.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams JE, Holub JL, Faigel DO. Polypectomy rate is a valid quality measure for colonoscopy: results from a national endoscopy database. Gastrointest Endosc. 2012;75:576–82. doi: 10.1016/j.gie.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams JE, Le TD, Faigel DO. Polypectomy rate as a quality measure for colonoscopy. Gastrointest Endosc. 2011;73:498–506. doi: 10.1016/j.gie.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman DA, Holub JL, Moravec MD, Eisen GM, Peters D, Morris CD. Prevalence of colon polyps detected by colonoscopy screening in asymptomatic black and white patients. JAMA. 2008;300:1417–22. doi: 10.1001/jama.300.12.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaminski MF, Regula J, Kraszewska E, et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med. 2010;362:1795–803. doi: 10.1056/NEJMoa0907667. [DOI] [PubMed] [Google Scholar]
- 39.Adler A, Wegscheider K, Lieberman D, et al. Factors determining the quality of screening colonoscopy: a prospective study on adenoma detection rates, from 12 134 examinations (Berlin colonoscopy project 3, BECOP-3) Gut. 2012 doi: 10.1136/gutjnl-2011-300167. [DOI] [PubMed] [Google Scholar]
- 40.Corley DA, Jensen CD, Marks AR. Can we improve adenoma detection rates? A systematic review of intervention studies. Gastrointest Endosc. 2011;74:656–65. doi: 10.1016/j.gie.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Shaukat A, Oancea C, Bond JH, Church TR, Allen JI. Variation in detection of adenomas and polyps by colonoscopy and change over time with a performance improvement program. Clin Gastroenterol Hepatol. 2009;7:1335–40. doi: 10.1016/j.cgh.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 42.Kahi CJ, Hewett DG, Norton DL, Eckert GJ, Rex DK. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–6. doi: 10.1016/j.cgh.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 43.Kahi CJ, Li X, Eckert GJ, Rex DK. High colonoscopic prevalence of proximal colon serrated polyps in average-risk men and women. Gastrointest Endosc. 2011;75:515–20. doi: 10.1016/j.gie.2011.08.021. [DOI] [PubMed] [Google Scholar]
- 44.Rabeneck L, Souchek J, El-Serag HB. Survival of colorectal cancer patients hospitalized in the Veterans Affairs Health Care System. Am J Gastroenterol. 2003;98:1186–92. doi: 10.1111/j.1572-0241.2003.07448.x. [DOI] [PubMed] [Google Scholar]
- 45.Baxter NN, Sutradhar R, Forbes SS, Paszat LF, Saskin R, Rabeneck L. Analysis of administrative data finds endoscopist quality measures associated with postcolonoscopy colorectal cancer. Gastroenterology. 2011;140:65–72. doi: 10.1053/j.gastro.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 46.Taber A, Romagnuolo J. Effect of simply recording colonoscopy withdrawal time on polyp and adenoma detection rates. Gastrointest Endosc. 2010;71:782–6. doi: 10.1016/j.gie.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Velasquez J, Espinoza-Rios J, Huerta-Mercado J, et al. Impact assessment of increasing the time of withdrawal of colonoscopy in the detection rate of polyps in our midst. Rev Gastroenterol Peru. 2009;29:321–5. [PubMed] [Google Scholar]
- 48.Lin OS, Kozarek RA, Arai A, et al. The effect of periodic monitoring and feedback on screening colonoscopy withdrawal times, polyp detection rates, and patient satisfaction scores. Gastrointest Endosc. 2010;71:1253–9. doi: 10.1016/j.gie.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clin Gastroenterol Hepatol. 2008;6:1091–8. doi: 10.1016/j.cgh.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 50.Burke CA, Church JM. Enhancing the quality of colonoscopy: the importance of bowel purgatives. Gastrointest Endosc. 2007;66:565–73. doi: 10.1016/j.gie.2007.03.1084. [DOI] [PubMed] [Google Scholar]
- 51.Froehlich F, Wietlisbach V, Gonvers JJ, Burnand B, Vader JP. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European Panel of Appropriateness of Gastrointestinal Endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–84. doi: 10.1016/s0016-5107(04)02776-2. [DOI] [PubMed] [Google Scholar]
- 52.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–9. doi: 10.1067/mge.2003.294. [DOI] [PubMed] [Google Scholar]
- 53.Thomas-Gibson S, Rogers P, Cooper S, et al. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy. 2006;38:456–60. doi: 10.1055/s-2006-925259. [DOI] [PubMed] [Google Scholar]
- 54.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–6. doi: 10.1016/s0016-5107(03)02875-x. [DOI] [PubMed] [Google Scholar]
- 55.Aronchick CA, Lipshutz WH, Wright SH, Dufrayne F, Bergman G. A novel tableted purgative for colonoscopic preparation: efficacy and safety comparisons with Colyte and Fleet Phospho-Soda. Gastrointest Endosc. 2000;52:346–52. doi: 10.1067/mge.2000.108480. [DOI] [PubMed] [Google Scholar]
- 56.Calderwood AH, Jacobson BC. Comprehensive validation of the Boston Bowel Preparation Scale. Gastrointest Endosc. 2010;72:686–92. doi: 10.1016/j.gie.2010.06.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lai EJ, Calderwood AH, Doros G, Fix OK, Jacobson BC. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–5. doi: 10.1016/j.gie.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fisher DA, Maple JT, Ben-Menachem T, et al. Complications of colonoscopy. Gastrointest Endosc. 2011;74:745–52. doi: 10.1016/j.gie.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 59.Rabeneck L, Paszat LF, Hilsden RJ, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology. 2008;135:1899–906. 906, e1. doi: 10.1053/j.gastro.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 60.Warren JL, Klabunde CN, Mariotto AB, et al. Adverse events after outpatient colonoscopy in the Medicare population. Ann Intern Med. 2009;150:849–57. W152. doi: 10.7326/0003-4819-150-12-200906160-00008. [DOI] [PubMed] [Google Scholar]
- 61.Ko CW, Riffle S, Michaels L, et al. Serious complications within 30 days of screening and surveillance colonoscopy are uncommon. Clin Gastroenterol Hepatol. 2009;8:166–73. doi: 10.1016/j.cgh.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Jonge V, Sint Nicolaas J, van Baalen O, et al. The incidence of 30-day adverse events after colonoscopy among outpatients in the Netherlands. Am J Gastroenterol. 2011;107:878–84. doi: 10.1038/ajg.2012.40. [DOI] [PubMed] [Google Scholar]
- 63.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 64.Li J, Nadel MR, Poppell CF, Dwyer DM, Lieberman DA, Steinberger EK. Quality assessment of colonoscopy reporting: results from a statewide cancer screening program. Diagn Ther Endosc 2010. 2010 doi: 10.1155/2010/419796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.de Jonge V, Sint Nicolaas J, Cahen DL, et al. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75:98–106. doi: 10.1016/j.gie.2011.06.032. [DOI] [PubMed] [Google Scholar]
- 66.Palmer LB, Abbott DH, Hamilton N, Provenzale D, Fisher DA. Quality of colonoscopy reporting in community practice. Gastrointest Endosc. 2010;72:321–7. 7 e1. doi: 10.1016/j.gie.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lieberman DA, Faigel DO, Logan JR, et al. Assessment of the quality of colonoscopy reports: results from a multicenter consortium. Gastrointest Endosc. 2009;69:645–53. doi: 10.1016/j.gie.2008.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beaulieu D, Barkun A, Martel M. Quality audit of colonoscopy reports amongst patients screened or surveilled for colorectal neoplasia. World J Gastroenterol. 2012;18:3551–7. doi: 10.3748/wjg.v18.i27.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Beaulieu D, Martel M, Barkun AN. A prospective intervention study of colonoscopy reporting among patients screened or surveilled for colorectal neoplasia. Can J Gastroenterol. 2012;26:718–22. doi: 10.1155/2012/623402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Logan JR, Lieberman DA. The use of databases and registries to enhance colonoscopy quality. Gastrointest Endosc Clin N Am. 2010;20:717–34. doi: 10.1016/j.giec.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 71.Einbinder JS, Scully K. Using a clinical data repository to estimate the frequency and costs of adverse drug events. Proc AMIA Symp. 2001:154–8. [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald CJ, Overhage JM, Dexter P, Takesue BY, Dwyer DM. A framework for capturing clinical data sets from computerized sources. Ann Intern Med. 1997;127:675–82. doi: 10.7326/0003-4819-127-8_part_2-199710151-00049. [DOI] [PubMed] [Google Scholar]
- 73.Owen RR, Thrush CR, Cannon D, et al. Use of electronic medical record data for quality improvement in schizophrenia treatment. J Am Med Inform Assoc. 2004;11:351–7. doi: 10.1197/jamia.M1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tang PC, Ralston M, Arrigotti MF, Qureshi L, Graham J. Comparison of methodologies for calculating quality measures based on administrative data versus clinical data from an electronic health record system: implications for performance measures. J Am Med Inform Assoc. 2007;14:10–5. doi: 10.1197/jamia.M2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.National Research Council. Future Directions for the National Healthcare Quality and Disparities Reports. Washington, D.C: The National Academies Press; 2010. [PubMed] [Google Scholar]
- 76.Gliklich RE, Dreyer NA, editors. Registries for Evaluating Patient Outcomes: A User’s Guide. 2. 2010. (Prepared by Outcome DEcIDE Center [Outcome Sciences, Inc. dba Outcome] under Contract No. HHSA29020050035I TO1.) [PubMed] [Google Scholar]
- 77.Deas T, Pike IM. Using Quality Indicators to Improve Performance. EndoEconomics Spring. 2010:19–20. [Google Scholar]
- 78.Langley GL, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. 2. San Francisco: Jossey-Bass Publishers; 2009. [Google Scholar]
- 79.Christie J, Itzkowitz S, Lihau-Nkanza I, Castillo A, Redd W, Jandorf L. A randomized controlled trial using patient navigation to increase colonoscopy screening among low-income minorities. J Natl Med Assoc. 2008;100:278–84. doi: 10.1016/s0027-9684(15)31240-2. [DOI] [PubMed] [Google Scholar]
- 80.Shaw MJ, Beebe TJ, Tomshine PA, Adlis SA, Cass OW. A randomized, controlled trial of interactive, multimedia software for patient colonoscopy education. J Clin Gastroenterol. 2001;32:142–7. doi: 10.1097/00004836-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 81.Friedman M, Borum ML. Colorectal cancer screening of African Americans by internal medicine resident physicians can be improved with focused educational efforts. J Natl Med Assoc. 2007;99:1010–2. [PMC free article] [PubMed] [Google Scholar]
- 82.Leffler DA, Neeman N, Rabb JM, et al. An alerting system improves adherence to follow-up recommendations from colonoscopy examinations. Gastroenterology. 2011;140:1166–73. e1–3. doi: 10.1053/j.gastro.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 83.Siddiqui AA, Yang K, Spechler SJ, et al. Duration of the interval between the completion of bowel preparation and the start of colonoscopy predicts bowel-preparation quality. Gastrointest Endosc. 2009;69:700–6. doi: 10.1016/j.gie.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 84.Calderwood AH, Lai EJ, Fix OK, Jacobson BC. An endoscopist-blinded, randomized, controlled trial of a simple visual aid to improve bowel preparation for screening colonoscopy. Gastrointest Endosc. 2011;73:307–14. doi: 10.1016/j.gie.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 85.Coe SG, Panjala C, Heckman MG, et al. Quality in colonoscopy reporting: an assessment of compliance and performance improvement. Dig Liver Dis. 2012;44:660–4. doi: 10.1016/j.dld.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 86.Kowalczyk L, Forsmark CE, Ben-David K, et al. Algorithm for the management of endoscopic perforations: a quality improvement project. Am J Gastroenterol. 2011;106:1022–7. doi: 10.1038/ajg.2010.434. [DOI] [PubMed] [Google Scholar]