Abstract
The lateral geniculate nucleus (LGN) of catarrhines – with the exception of gibbons – is typically described as a six-layered structure, comprised of two ventral magnocellular layers, and four dorsal parvocellular layers. The parvocellular layers of the LGN are involved in color vision. Therefore, it is hypothesized that a six-layered LGN is a shared-derived trait among catarrhines. This might suggest that in gibbons the lack of further subdivisions of the parvocellular layers is a recent change, and could be related to specializations of visual information processing in this taxon. To address these hypotheses, the lamination of the LGN was investigated in a range of catarrhine species, including several taxa not previously described, and the evolution of the LGN was reconstructed using phylogenetic information. The findings indicate that while all catarrhine species have four parvocellular leaflets, two main patterns of LGN parvocellular lamination occur: two undivided parvocellular layers in some species, and four parvocellular leaflets (with occasional subleaflets) in other species. LGN size was not found to be related to lamination pattern. Both patterns were found to occur in divergent clades, which is suggestive of homoplasy within the catarrhines in LGN morphology.
Keywords: evolution, phylogeny, catarrhines, primates, vision, lateral geniculate nucleus, parvocellular
Introduction
Catarrhines, the group of primates comprising humans, apes and Old World monkeys, are characterized by shared features of the visual system, including the shared derived feature of routine trichromacy. The lateral geniculate nucleus (that is, the dorsal, dLGN, subdivision; herein LGN) is a part of the visual thalamus that regulates the transmission of information from both eyes to the cerebral cortex, and occurs in each hemisphere of the brain. Given its appearance in histological sections stained for cell bodies, the LGN is considered “6-layered”, but this description of its structure has led to some confusion about its organization. In spite of the relative homogeneity of visual function within the catarrhines, it has been noted that the LGN is not always “6-layered”. However, currently there is no basis for concluding that species-specific variation in the number of LGN layers has any consequences for vision [Erwin et al. 1999]. The current paper focuses on the variants in catarrhine LGN laminar pattern as observed in sections stained for cell bodies, relative to LGN and brain size and phylogeny.
The LGN is well known for its role in transmitting signals along the retino-geniculo-cortical pathway, although retinal afferents are only a minority of its inputs, which also include the primary visual cortex and the thalamic reticular nucleus [Saalmann and Kastner 2011]. In primates, the LGN is comprised of easily recognizable parvocellular (P) and magnocellular (M) layers, and less conspicuous koniocellular (K) layers, each of which belong to distinct pathways processing different aspects of visual input.
Each pathway is comprised, in catarrhines, of a distinct group of nerve fibers originating from retinal ganglion cells and terminating in the LGN. The magnocellular pathway originates in the large, sensitive parasol ganglion cells of the retina, which primarily get inputs from rods, and which synapse in the magnocellular (i.e., large-celled) layers of the LGN, and then project to layer 4Cα of cortical area V1. The magnocellular pathway carries high-contrast visual information, including information about motion. The parvocellular pathway originates in the small, numerous midget ganglion cells of the retina, which primarily get inputs from cones (see below), and which synapse on the parvocellular (i.e., small-celled) layers of the LGN, which then project to layer 4Cβ of V1 [Leventhal et al. 1981; Rodiek 1988]. The parvocellular pathway carries information about color and fine structure.
Magnocellular and parvocellular pathway organization is maintained in V1 and its primary target, V2, as the P-I (P interblob), P-B (P blob), and M streams [DeYoe and Van Essen 1988]. Early visual areas V1 and V2 have feed-forward projections to higher visual areas, in which visual streams and related functions become more segregated. Areas V3 [Felleman and Van Essen 1987], V3A [Tootell et al. 1997], and MT [Albright et al. 1984] are involved in motion detection, and are associated with the M stream [DeYoe et al. 1990]. Area V4, dubbed the “color center” [Lueck et al. 1989; McKeefry and Zeki 1997; Zeki 2004], is associated with the P-B and P-I streams [DeYoe et al. 1994; Van Essen et al. 1992].
More recently, in macaques koniocellular (i.e., very small-celled) neurons have been distinguished from LGN neurons of the magnocellular and parvocellular pathways on the basis of positive immunohistochemical staining for the α subunit of type II Ca2+/calmodulin-dependent protein kinase (αCAMKII; [Hendry and Yoshioka 1994; Yoshioka and Hendry 1995], the calcium-binding protein calbindin [Goodchild and Martin 1998; Jones and Hendry 1989], and the gamma subunit of protein kinase C [Fukuda et al. 1994]. In fact, the koniocellular neurons in the LGN have been known for quite some time to exist in anthropoids [Chacko 1948, 1954, 1955a; 1949; e.g., Le Gros Clark 1941b; 1941a; Solnitzky and Harman 1943], and are sometimes referred to as “interlaminar cells” or as occurring within “intercalated layers”. The koniocellular layers of catarrhines are presumably homologous to the prominent koniocellular layers of lorises and bushbabies [Kaas et al. 1978]. Koniocellular neurons are distributed in six distinct layers in the catarrhine LGN, one located ventral to each magnocellular and parvocellular layer, and additional neurons with the koniocellular neurochemical signature are distributed within the magnocellular and parvocellular layers [Hendry and Reid 2000; Hendry and Yoshioka 1994; Yoshioka and Hendry 1995]. Displaced koniocellular neurons form perpendicular bridges that link koniocellular layers by splitting up parvocellular and magnocellular layers. In macaques, koniocellular LGN axons originating from ventral LGN layers K1 and K2 terminate in V1 layers 1 and 3A, and those originating from dorsal LGN layers K3-K6 terminate in layer 3Bα [Casagrande et al. 2007].
The koniocellular and parvocellular laminae belong to two different subsystems in which color information is processed. The ancient subsystem, which also exists in dichromats, is primarily responsible for differentiating “blue” outputs of short-wavelength-sensitive (S) cones from “yellow” outputs of the medium- (M) and long- (L) wavelength-sensitive M/L cones. In this subsystem, small bistratified retinal ganglion cells with large receptive fields draw outputs from S cones and M/L cones, and these outputs are carried to the koniocellular laminae of the LGN [Regan et al. 2001]. The recent subsystem exits only in trichromats, and is primarily responsible for differentiating green outputs of M cones from red outputs of the L cones. In this system midget ganglion cells with small receptive fields carry outputs of M and L cones to the parvocellular laminae of the LGN [Regan et al. 2001].
Other pathways in addition to the magnocellular, parvocellular, and koniocellular pathways also exist. At the level of the retina, at least ten classes of ganglion cells have been distinguished in macaques [Dacey et al. 2003]. It has been shown that there are direct inputs from the LGN to extrastriate visual areas which do not match criteria for inclusion in M, parvocellular, or koniocellular pathways [Sincich et al. 2004].
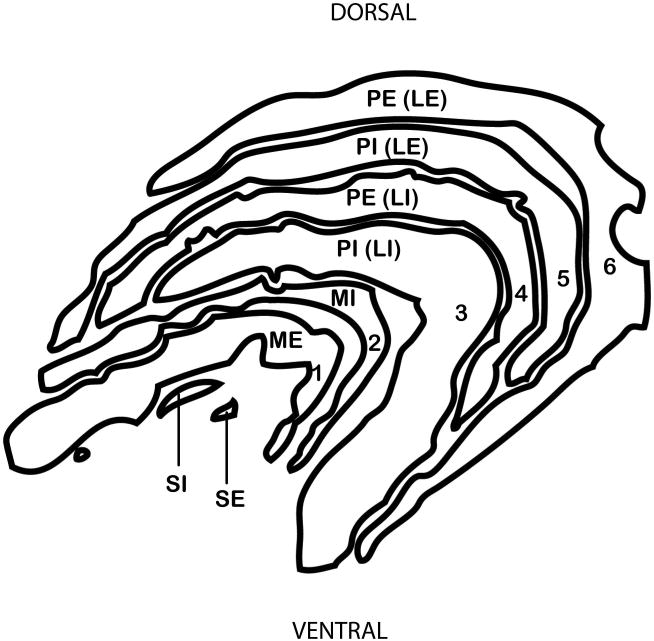
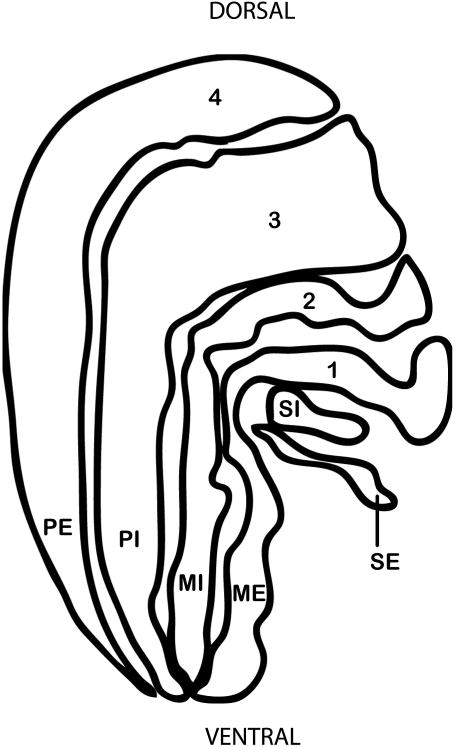
Often, the LGN of macaques and humans is described in terms of its six principal layers (Fig. 2), numbered from the ventral aspect closest to the optic tract and pia matter, to the dorsal aspect closets to the optic radiations. The two most ventral layers (layers 1 and 2) are magnocellular, being comprised of large, darkly stained cells. The next four layers (layers 3-6) are parvocellular and are comprised of medium, more lightly stained cells. The present study uses a different nomenclature which was introduced by Kaas and colleagues [1972] with the intention of recognizing homologous layers across species based on cell type and location. To envision this scheme, consider a generalized mammalian LGN, which is a 3-dimensional structure in which the “external” part near the border (on all sides) receives contralateral retinal inputs, and the “internal” part at the core receives ipsilateral retinal inputs [Huberman and Chapman 2007]. The LGNs of all primates have two magnocellular layers (ME – magnocellular external and MI -magnocellular internal) and two parvocellular layers (PE – parvocellular external and PI – parvocellular internal). Further subdivisions of the parvocellular layers are called “leaflets”. Thus, parvocellular layers 3-6 are better described as two, 3-dimensionally complex, parvocellular layers that are divided into four leaflets, as the leaflet pairs are fused rostrally [Kaas et al. 1978; Malpeli et al. 1996]. To acknowledge this, leaflets are first named according to the layer of which they are a part (PI or PE) and then to according to their location (LI - internal leaflet or LE - external leaflet). Sometimes the leaflets are further subdivided into divisions called subleaflets.
Fig. 2. Schematic diagram showing layers of the LGN in Macaca fascicularis.
Traditional (1-6) nomenclature is shown (left) and nomenclature after Kaas and Huerta (1978) is shown (right).
“PE” parvocellular external; “PI” parvocellular internal; “MI” magnocellular internal; “ME” magnocellular external; “SI” superficial internal ; “SE” superficial external
Also easily visible are other layers ventral to the magnocellular layers and dorsal to the axons of optic tract [e.g., Balado and Franke 1937; Kanagasuntheram et al. 1969]. In most publications these have been called the S layers (SI – S external and SE – S external)[Kaas et al. 1978], a term which could actually refer to either of two different things [Hendry and Reid 2000]. Typically in catarrhines S layers refer to a subset of the koniocellular layers, with the pair representing the ipsilateral and contralateral retinae [Hendry and Reid 2000]. However, displaced magnocellular layers at the same location are also called S layers [Hendry and Yoshioka 1994]. Here we retain the traditional nomenclature “S layers” to refer to the ventral layers without acknowledgement of whether they are in fact magnocellular or koniocellular, because making the distinction was beyond the scope of the study, which incorporates reports published before the koniocellular layers were well known.
In primates that have an LGN with two parvocellular layers, one layer receives retinal input from the ipsilateral eye, and the other receives retinal input from the contralateral eye. When the parvocellular layers are further subdivided into leaflets, the leaflets are interdigitated such that a leaflet receiving retinal input from the ipsilateral eye is adjacent to a leaflet receiving inputs from the contralateral eye. Therefore, the distinction Kaas et al. [1978] make between “layers” and “leaflets” is important because although in two dimensional cross-sections macaques appear to have a total of six layers, of which four are parvocellular layers and two are magnocellular layers, three-dimensional modeling of the LGN shows that pairs of parvocellular layers receiving input from the ipsilateral eye are actually continuous with each other [Erwin et al. 1999].
Physiological studies of lamination in macaques have indicated optical specificity across leaflets. Schiller and Malpeli [1978] found that, in rhesus macaques, blue-sensitive cells were found primarily in the ventral-most parvocellular leaflets, PE(LI) and PI(LI). However, this early study only considered the parvocellular and magnocellular layers, and did not distinguish the koniocellular layers from them. More recent studies in marmosets [Martin et al. 1997] and macaques [Roy et al. 2009] indicate that in fact neurons carrying signals from the blue-sensitive S cones are predominantly located in the koniocellular layers, whereas neurons carrying signals from the M and L cones are segregated in the parvocellular layers. These finding support the aforementioned distinction between ancient koniocellular and recent parvocellular subsystems of color information processing.
The ventral-most parvocellular leaflets PE(LI) and PI(LI) also have cells that are mostly off-center, where as the dorsal parvocellular leaflets PE(LE) and PI(LE) are mostly on-center [Schiller and Malpeli 1978]. Central vision is represented in the segment of the LGN having four parvocellular leaflets in macaques, whereas peripheral vision is represented in the part with just the two undivided layers [Malpeli and Baker 1975]
The morphology of the LGN varies between primate groups [Kaas and Huerta 1988; Kaas et al. 1978]. In addition, there have been some reports of variation in LGN laminar pattern within hominoids [Armstrong 1979; Chacko 1955a; Kanagasuntheram et al. 1969; Tigges and Tigges 1987]. Little is known about how these variations in LGN lamination may be related to species-specific adaptations. Although the LGNs of humans and macaques have been well studied, the laminar pattern of other catarrhines requires further documentation, especially for the colobine monkeys, most hylobatid species, orangutans, and bonobos.
The function of LGN lamination has been the object of speculation for some time. Famously, Le Gros Clark, upon pointing out that the laminae were paired for each eye, suggested that in the macaque each of the different pairs of laminae received inputs from only one of each of the three classic color receptor types [red, blue and green; 1949]. Although this idea has not held up against recorded responses of LGN cells to monochromatic light [De Valois et al. 1958], the precise reason why macaques, humans and most other catarrhines (which are all trichromatic) have parvocellular leaflets, and other closely related taxa do not, remains unclear. An hypothesis proposed by Kaas [1972; 1978] is that leaflets are related to the increased parvocellular mass of the LGN. Although Kaas suggests that size differences might be of functional relevance, this has not been specifically explored.
Here LGN laminar pattern of catarrhines was considered for the first time in relationship to allometric scaling and phylogeny. The aim of the present study was to determine the number of LGN parvocellular leaflets in catarrhine species for which the number is unknown and to provide an outgroup to better understand variation in LGN structure among hominoids. A key goal was to determine whether the laminar patterns found in humans and macaques are in fact homologous. These data were used to explore the possible evolutionary origins of different LGN laminar patterns in the catarrhines. First, the lamination of the LGN was examined in respect to LGN size. Second, the phylogenetic polarity of the observed differences was examined on a phylogenetic tree.
Materials and methods
Specimens and tissue preparation
Observations and measurements were made on coronal and sagittal histological sections from adult specimens of a total of 46 brains representing 20 catarrhine species. In order to accumulate a large and diverse sample, specimens in the study came from several different collections: the Zilles and Stephan comparative neuroanatomy collections at the C&O Vogt Institute of Brain Research in Düsseldorf, Germany, the Yakovlev-Haleem and Welker collections at the National Museum of Health and Medicine in Washington, DC, and the Great Ape Aging Project at the George Washington University, Washington, DC and the Mount Sinai School of Medicine, New York, NY.
The brains from the Zilles collection were immersion fixed with either 4% formaldehyde or Bodian's solution within a few hours after death, embedded in paraffin and serially-sectioned along the coronal plane at a thickness of 20 μm (except for one chimpanzee brain, which was horizontally sectioned at a thickness of 15 μm), and stained for cell bodies based on Gallyas' procedure [Gallyas 1971], using silver according to the technique described by Merker [1983]. The brains from the Stephan collection were perfused in situ with Bouin's fluid through the carotid arteries after the blood was washed out with physiological saline, embedded in paraffin and serially-sectioned along the coronal plane at a thickness of 20 μm, and Nissl-stained using cresyl violet. The Pan troglodytes brains from the Yakovlev-Haleem collection were sagittally sectioned at a thickness of 35 μm, and separate alternating series were stained for Nissl and for myelin. The brains from the George Washington University were immersion fixed in 10% neutral buffered formalin. Left occipital lobe and parieto-occipital lobe blocks were cryoprotected by immersion with increasing concentrations of sucrose solutions up to 30%, frozen on dry ice, and serially-sectioned on a microtome at a thickness 40 μm, and Nissl-stained with cresyl violet. For further details about the sections see Supplemental Table 1. Because multiple collections were used to include as large a sample as possible, it is prudent to keep methodological considerations in mind as the tissue preparation techniques varied across collections, which may impact volume measurements [Stephan et al. 1981].
Characterization of parvocellular lamination
For each specimen, sections stained for cell bodies with either silver or cresyl violet were examined. A minimum of one left hemisphere was investigated per species, although both right and left hemispheres were investigated for most specimens (the number of specimens per species is indicated in Table 1).
Table 1.
LGN parvocellular lamination, and species mean brain weights and LGN volumes.
| Species | Leaflets* | no. | Brain wt. (g) | no. | LGN vol. (cm3) | no. | LGN residual |
|---|---|---|---|---|---|---|---|
| Homo sapiens | Y | 10 | 303.7 | 10 | 0.335 | 10 | -13.35 |
| Pan troglodytes | Y | 6 | 406.5 | 3 | 0.344 | 3 | 5.12 |
| Pan paniscus | Y | 2 | 364.5 | 2 | 0.282 | 2 | 0.21 |
| Gorilla gorilla | Y | 1 | 376.0 | 1 | 0.300 | 1 | 1.64 |
| Pongo pygmaeus | N | 5 | 384.7 | 3 | 0.259 | 3 | -2.73 |
| Hylobates lar | N | 2 | 106.0 | 2 | 0.166 | 2 | -0.41 |
| Hylobates muelleri | N | 1 | 101.8 | 1 | 0.162 | 1 | -0.60 |
| Symphalangus syndactylus | N | 1 | 138.7 | 1 | NA | NA | |
| Cercopithecus ascanius | Y | 1 | 61.5 | 1 | 0.147 | 1 | 1.03 |
| Cercopithecus mitis | Y | 2 | 73.3 | 2 | 0.167 | 2 | 2.04 |
| Erythrocebus patas | Y | 1 | 94.2 | 1 | 0.267 | 1 | 10.45 |
| Lophocebus albigena | Y | 1 | 110.5 | 1 | 0.182 | 1 | 0.88 |
| Macaca fascicularis | Y | 1 | 57.6 | 1 | 0.092 | 1 | -4.13 |
| Macaca mulatta | Y | 1 | 78.0 | 1 | 0.158 | 1 | 0.75 |
| Miopithecus talapoin | Y | 1 | 41.1 | 1 | 0.109 | 1 | -0.72 |
| Papio anubis | Y | 1 | 206.5 | 2 | 0.395 | 1 | 17.22 |
| Nasalis larvatus | Y | 1 | 64.3 | 1 | 0.157 | 1 | 1.75 |
| Pygathrix nemaeus | N | 1 | 69.0 | 1 | 0.115 | 1 | -2.85 |
| Piliocolobus badius | N | 2 | 76.9 | 2 | 0.128 | 2 | -2.16 |
| Colobus angolensis | Y | 1 | 74.4 | 1 | 0.103 | 1 | -4.46 |
N = 2 parvocellular layers; Y = 4 parvocellular leaflets (may include subleaflets)
Because the number of layers or leaflets is not constant throughout the anterior-posterior extent of the LGN, the entire span of the LGN was investigated in each specimen to determine the maximum number of distinct parvocellular leaflets in a coronal section. Leaflets were considered distinct if they were separated by a sharp, soma-poor gap. Such interlaminar zones could be distinguished from the LGN representation of the optic disc, which is also a type of gap, although its orientation does not follow the laminar pattern. Often, there was no interlaminar space between magnocellular and parvocellular layers, but these two categories of layers were easily distinguished on the basis of location, staining intensity, and cell size. Note that potential “hidden layers” may exist (see discussion), however, because these are not known in catarrhines, and were not visible in the available material, they could not be accounted for in the present study.
Estimation of volumes
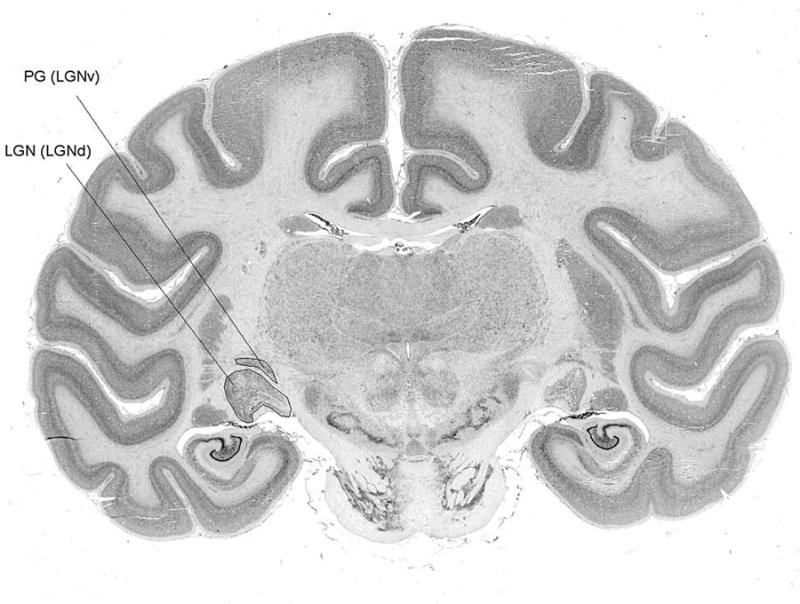
Volume measurements were taken for the dorsal part of the LGN complex, which is laminated, and which is most often just called the LGN, but is also known as the dLGN (dorsal lateral geniculate nucleus), LGBd (dorsal lateral geniculate body), or CGLd (corpus geniculatus lateralis dorsalis). The unlaminated anterior LGN, known as LGNv (ventral LGN) or PG (pregeniculate nucleus) was excluded (Fig. 1).
Fig. 1. Coronal section through brain of Macaca fascicularis howing location of LGN and PG.
LGN residual volumes were calculated to determine whether change in LGN morphology might be better attributed to changes in LGN volume relative to brain volume. The residual values were calculated from the equation,
in which the expected LGN volumes were determined from an ordinary least squares regression of log (LGN volume) as a function of log (brain weight).
All statistical analyses were assessed with an α level of 0.05. Nonparametric tests for independent samples were performed to determine whether the two groups (2 parvocellular layers and 4 parvocellular leaflets) differed significantly in LGN volume, or LGN volume residual.
Phylogenetic analysis
The results were interpreted in an evolutionary context as plotted on a catarrhine phylogenetic tree including the key monophyletic groups discussed here, based on a consensus phylogeny from the 10kTrees Website [Arnold et al. 2010]. The character plotted on the tree topology was “parvocellular laminar pattern”, and the character states in which it occurs were “leaflets” where four parvocellular leaflets were observed, or “no leaflets” where only two parvocellular layers were observed. The polarity of the character was not determined a priori because it is not clear which, of either of these, would best represent the ancestral catarrhine state. The maximum parsimony reconstruction method was used to trace the evolution of the character.
Results
Lamination
The results (Table 1) are consistent with earlier observations about LGN lamination (Table 2). However, the current study presents some clarifications and new data. In the current sample, the presence of parvocellular leaflets was not found to vary polymorphically within species. However, in those species in which leaflets occurred, it was found that some (but not all) individuals had further divisions of the parvocellular layers into subsidiary layers. Therefore, all catarrhine parvocellular laminar patterns fall into either the “four parvocellular leaflets” or the “two parvocellular layers” category.
Table 2. Previous studies reporting LGN parvocellular lamination in catarrhine species.
| Species | Leaflets* | Subleaflets | Projections | No. | Publications |
|---|---|---|---|---|---|
| HOMINOIDEA | |||||
| Homo sapiens | Y | Y | many | Balado and Franke [1937] | |
| Pan troglodytes | Y | Y | Y | 1 | Tigges et al. [1977], |
| Gorilla gorilla | Y | N | 1 | Nakagawa et al. [1998] | |
| Pongo pygmaeus | conflicting, insufficient data | N | 1 | Balado and Franke [1937], Armstrong and Frost [1988], Tigges and Tigges [1987],Chacko [1955a], Armstrong [1979], Tigges and Tigges [1987], Kanagasuntheram et al. [1969] | |
| Hylobates lar | N | Y | 4 | ||
| Hylobates moloch | N | N | 1 | Tigges and Tigges [1987] | |
| Hylobates agilis | N | N | 2 | Kanagasuntheram et al. [1969] | |
| Symphalangus syndactylus | conflicting, insufficient data | N | 1 | Kanagasuntheram et al. [1969] | |
| CERCOPITHECOIDEA | |||||
| CERCOPITHECINAE | |||||
| Cercopithecus ascanius | Y | 1 | Schulz [1967] | ||
| Cercopithecus aethiops | Y | Y | Kanagasuntheram et al. [1969] | ||
| Macaca rhesus | Y | Y | many | Kaas et al. [1978] | |
| Macaca fascicularis | Y | Y | |||
| Papio ursinus | Y | Y | |||
| Mandrillus sphinx | Y | Y | Kaas et al. [1972] | ||
| COLOBINAE | |||||
| Piliocolobus badius | N | 1 | Schulz [1967] |
N = 2 parvocellular layers; Y = 4 parvocellular leaflets (may include subleaflets)
Retinal projections were studied, for detecting ‘hidden layers’
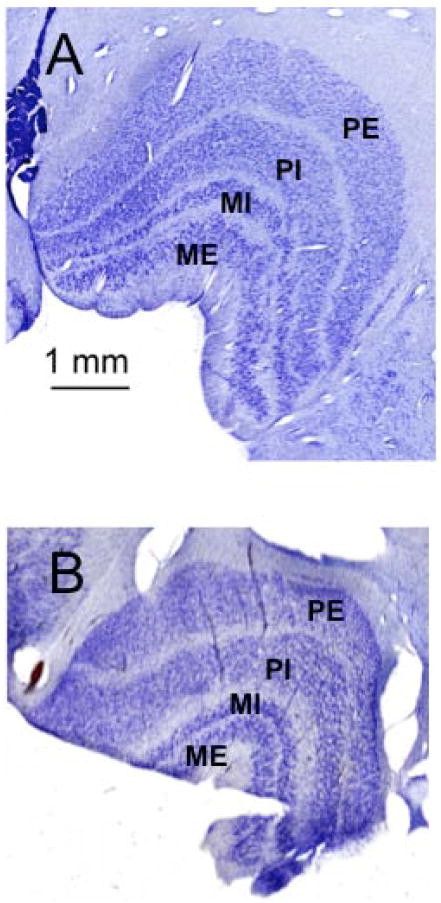
It should be noted that the observations made here on orangutans and siamangs add clarification about earlier reports: in the current study, both species were found to have two undivided parvocellular layers. Because studies of LGN lamination have been conflicting or entirely nonexistent for orangutans, bonobos, siamangs, and colobines, specimens belonging to each of these groups are illustrated here (Figs. 4-8). For Hylobates lar, tracings of the lamina are labeled using both the traditional nomenclature and that of Kaas and Huerta [1978, Fig 6.9]. In addition, examples of images from other species investigated are shown for comparative purposes.
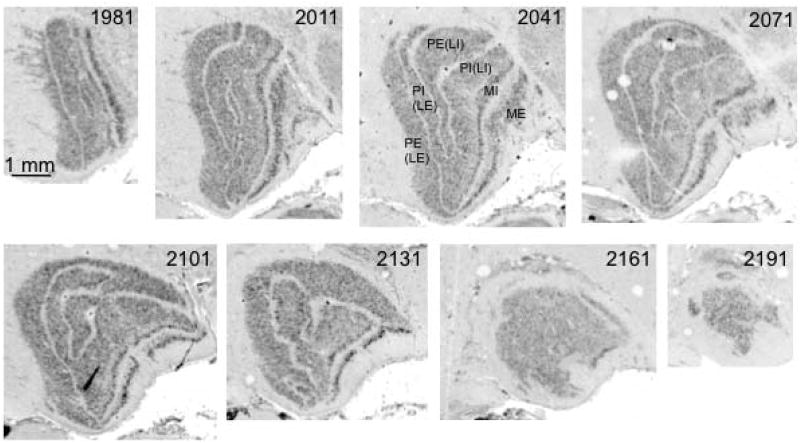
Fig. 4. Coronal sections through left LGN of a juvenile bonobo (Pan paniscus).
Sections are numbered from caudal to rostral aspect, as indicated below the images. Two magnocellular layers and four parvocellular leaflets are labeled in section 2041.
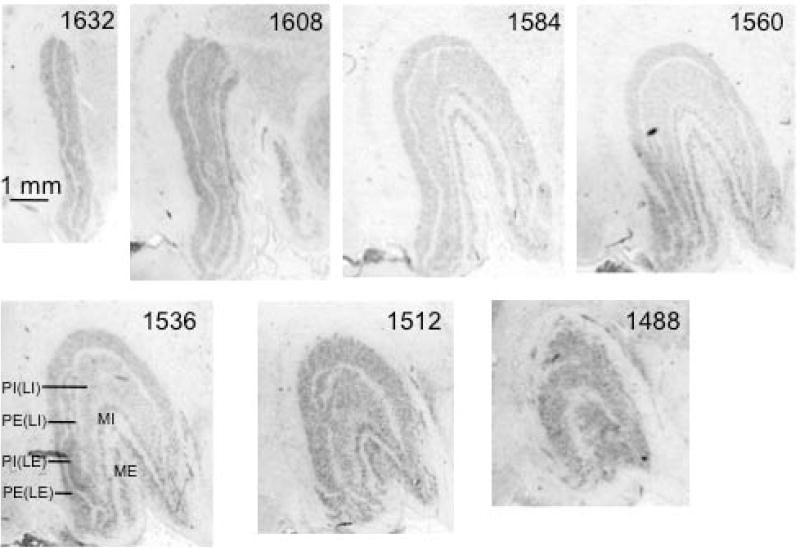
Fig. 8. Coronal sections through left LGN of a proboscis monkey (Nasalis larvatus).
Sections are numbered from rostral to caudal aspect, as indicated below the images. Two magnocellular layers and two parvocellular layers are labeled in section 1536.
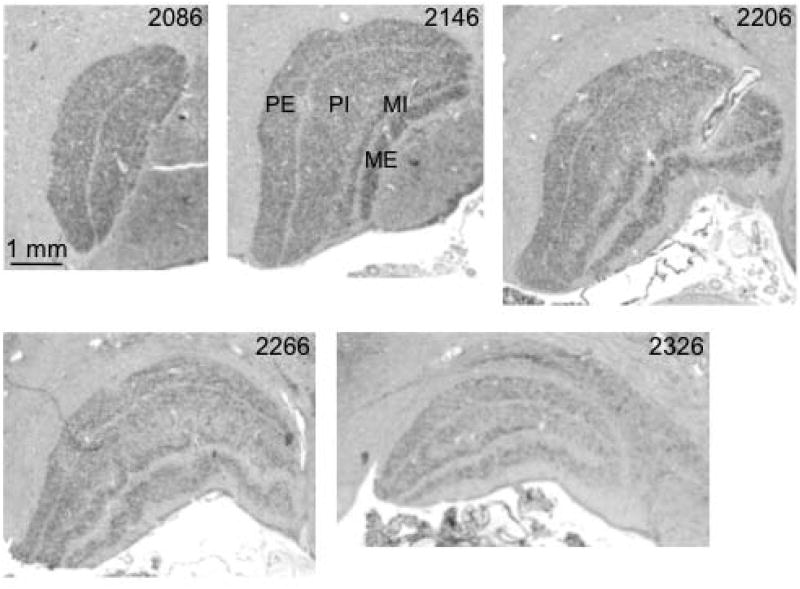
Fig. 6. Coronal sections through left LGN of an orangutan (Pongo pygmaeus).
Sections are numbered from caudal to rostral aspect, as indicated below the images. Two magnocellular layers and two parvocellular layers are labeled in section 2146.
Fig. 9. Schematic diagram showing layers of the LGN in Hylobates lar.
Traditional (1-4) nomenclature is shown (left) and nomenclature after Kaas and Huerta (1978) is shown (right).
“PE” parvocellular external; “PI” parvocellular internal; “MI” magnocellular internal; “ME” magnocellular external; “SI” superficial internal; “SE” superficial external
Volumetric analysis
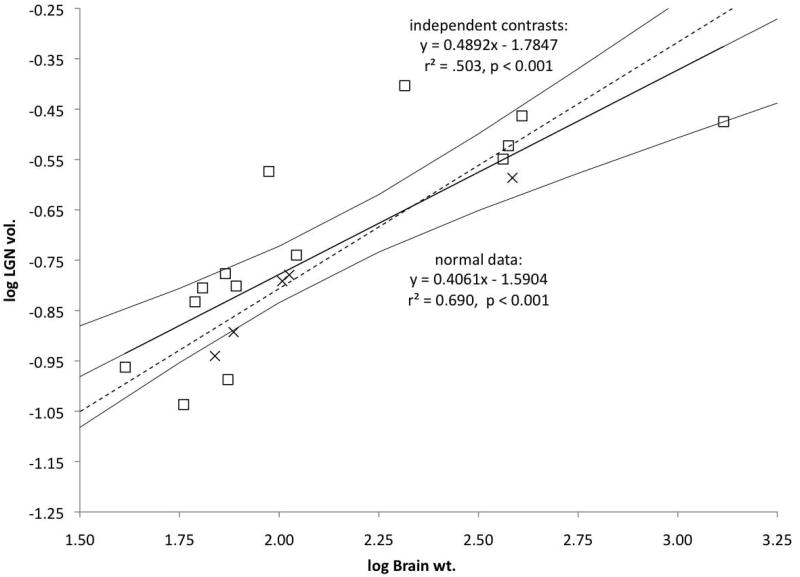
LGN volume increases with brain weight across the sample (Fig. 10). The catarrhine two parvocellular layers (n = 5) and four parvocellular leaflets (n = 14) LGN samples overlap in their brain weights (X axis) and LGN volumes (Y axis). However, larger LGNs tend to have leaflets, and the two parvocellular layers LGNs all fall below the normal LS regression line.
Fig. 10. Regression of LGN volume as a function of brain weight in catarrhines.
LS regression lines with upper and lower 95% confidence intervals (solid lines) and PIC LS regressions (dotted lines) are shown. Species mean values are plotted for species with four parvocellular leaflets (open squares) and two undivided parvocellular layers (crosses).
Nonparametric tests for two independent samples (Mann-Whitney U) were conducted to assess whether the leaflet sample had significantly larger LGNs than the no leaflet sample. In addition to comparing LGN volumes, LGN residuals were calculated to determine whether change in LGN morphology might be better attributed to changes in LGN volume relative to brain weight (Table 1). The nonparametric tests for two independent samples failed to detect any significant differences between the leaflet and no leaflet samples in absolute LGN volume (p = 0.405; U = 44). Similarly, no significant difference was detected between these samples in LGN residuals (p = 0.096; U = 53).
Phylogenetics
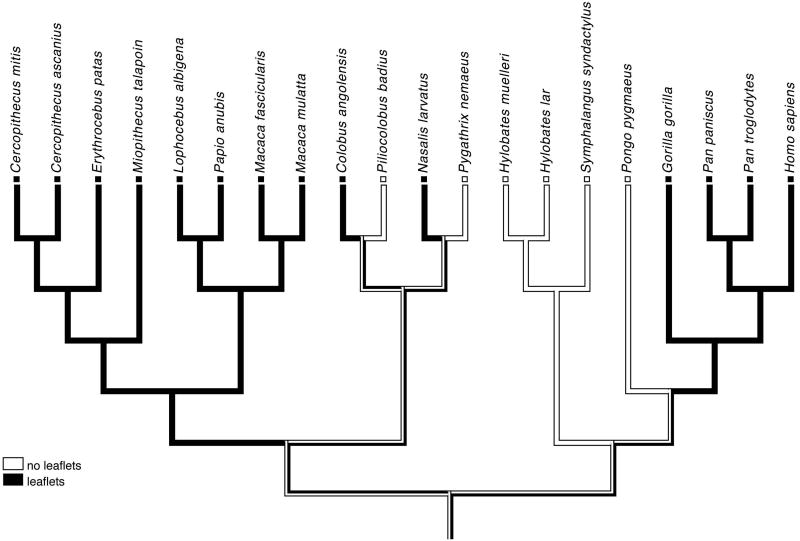
For the character “lamination pattern”, a minimum of four character state changes (steps) were required by the consensus phylogenetic tree (Fig. 3). There were two equally parsimonious hypotheses of the character state evolution which differ in character polarity: one assumes that the catarrhine ancestral condition is “leaflets”, whereas the other assumes that it is “no leaflets”. Catarrhine parvocellular patterns are summarized here per monophyletic group. In the hominoid superfamily, both patterns were observed. All four African hominoid species sampled (humans, chimpanzees, bonobos, and gorillas) had four parvocellular leaflets. Two groups had only two parvocellular layers: the orangutans and the hylobatid clade (gibbons and siamangs). Also within the cercopithecoid superfamily, both patterns were observed. All cercopithecine species had four parvocellular leaflets. The colobines varied: in the Asian clade, the proboscis monkey (Nasalis larvatus) had four parvocellular leaflets, although the red-shanked douc (Pygathrix nemaeus) had two parvocellular layers; in the African clade, the Angolan colobus monkey (Colobus angolensis) had four parvocellular leaflets, whereas the red colobus monkeys (Piliocolobus badius) had two parvocellular layers.
Fig. 3. Maximum parsimony analysis of changes in catarrhine LGN laminar pattern.
Changes in the LGN lamination pattern (character state changes) are indicated by bars.
Consensus phylogeny from 10kTrees Website [Arnold et al. 2010]. Both the first (A) and the second (B) hypotheses of character state evolution require an equal number (minimum of 4) character state changes within the catarrhine clade.
Discussion
The present study is the first to document and compare the morphology of the LGN in all living hominoid species, and to include representatives of all catarrhine subfamilies. In the current study, it was found that two main patterns of LGN parvocellular lamination occur within the catarrhines: two parvocellular layers in some species, and four parvocellular leaflets (with occasional subleaflets) in other species. The phylogenetic distribution of these two conditions on the catarrhine consensus phylogeny suggests homoplasy.
Because macaques represent the typical primate model in neuroscience research, neuroanatomical studies rarely venture further than comparisons between macaques and humans. As a result, similarities found between macaques and humans are often assumed to be homologous characters. The lamination of the LGN is a good example of the diversity that exists within the catarrhine clade.
Comparison to previous studies of catarrhine LGN lamination
In previous studies, several catarrhine species were demonstrated to have an LGN lamination pattern of two parvocellular layers further divided into four leaflets. These include humans [Balado and Franke 1937], chimpanzees [Chacko 1955b; Tigges et al. 1977], gorillas [Nakagawa et al. 1998], and several cercopithecine monkeys [Kaas et al. 1978].
The only hominoid species previously documented to have an LGN laminar pattern of two undivided parvocellular layers were the lar gibbons (Hylobates lar), and the silvery gibbons (Hylobates moloch)[Tigges and Tigges 1987]. In addition, one other catarrhine species, Piliocolobus badius, was documented as having two undivided parvocellular LGN layers in Schulz [1967; see his Figs. 5-7], but this rather obscure report has been overlooked and the laminar pattern of the cercopithecines has been generalized to all Old World monkeys [e.g., Kaas and Huerta 1988; Kaas et al. 1978].
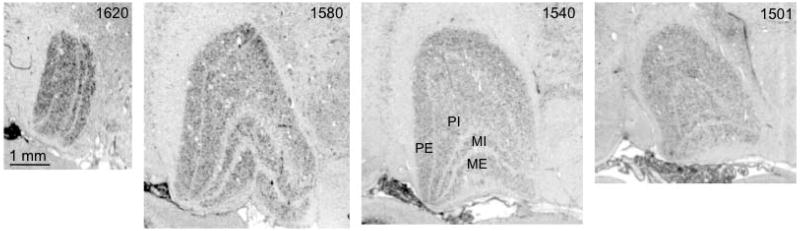
Fig. 5. Coronal sections through left LGN of a red colobus money (Piliocolobus badius).
Sections are numbered from rostral to caudal aspect, as indicated below the images. Two magnocellular layers and four parvocellular leaflets are labeled in section 1540.
Fig. 7. Coronal sections through left LGN of two different hylobatid species, Müller's gibbon (Hylobates muelleri) (A) and siamang (Symphalangus syndactylus) (B).
Two magnocellular layers and two parvocellular layers are labeled in each species.
The present study adds new information about the laminar pattern of orangutans and siamangs. The orangutan LGN has been previously described as displaying a pattern of four parvocellular leaflets and two magnocellular layers [Armstrong and Frost 1988; Balado and Franke 1937; but see Tigges and Tigges 1987]. Also, it was stated that siamangs (Symphalangus syndactylus) have two parvocellular layers, and inferior to them, four magnocellular layers [Kanagasuntheram et al. 1969; Figure 13] but the additional layers may actually be S layers [Kaas et al. 1978]. The discrepancy between these older data and the current observations of only two parvocellular layers seems to be due to differences in the identification of layer types (e.g., S layers probably identified as magnocellular layers) and examination of the full rostrocaudal extent of the LGN. However, future studies may also reveal intraspecific differences in LGN lamination not identified here.
In some species, there are reports of individual specimens in which LGN parvocellular layers are comprised of six fully developed subleaflets, or in some cases, less developed partial subsidiary lamination. Species for which there have been reports of subleaflets or other subsidiary lamination of the parvocellular layers of the LGN include Homo sapiens [Hickey and Guillery 1979], Pan troglodytes [Tigges et al. 1977], Macaca mulatta [Le Gros Clark and Penman 1934], Macaca fascicularis [O'Brien et al. 1997], Papio ursinus [Campos-Ortega and Hayhow 1970], Mandrillus sphinx [Kaas and Huerta 1988], and Cercopithecus aethiops [Kanagasuntheram et al. 1969]. The current study also found subleaflets in Erythrocebus patas, Cercopithecus mitis, and Macaca mulatta. The presence of subleaflets has been considered in so few individuals that further observations would likely increase its known prevalence, at least in some species. In fact, the high incidence of cases of subleaflets in humans has been indicated as a potential specialization, perhaps due to a general pattern of increased variability that follows an increase in brain size; or due to relaxation of selection pressures which in other species constrain LGN morphology [Hickey and Guillery 1979].
Although sections stained for cell bodies are sufficient for identifying the parvocellular layers in the LGN of most primates, the possibility of “hidden layers” can only be ruled out by tracing retinal inputs. Adjacent parvocellular layers receive opposite retinal inputs, as demonstrated by tracing studies. Although in most primate species parvocellular layers are well separated by interlaminar space, there are exceptions in the platyrrhines (New World monkeys). Retinal projection tracing studies in squirrel and saki monkeys revealed four hidden parvocellular leaflets, despite the fact that these species exhibit a large parvocellular mass that cannot be clearly divided into layers in sections stained for cell bodies [Kaas et al. 1978]. Also, owl monkeys and marmosets appear to have two undivided parvocellular layers, but hidden leaflets have been exposed in tracing studies [Kaas et al. 1978]. Although in all other primate species, retinal tracing studies have confirmed observations about parvocellular lamination made on sections stained for cell bodies, it is possible that the degree of lamination is underestimated when based only on cytoarchitectural examination.
Within the catarrhines, observations about LGN lamination patterns have been confirmed using retinal tracers in a variety of cercopithecines [Kaas and Huerta 1988; Kaas et al. 1978 and refs therein]. Studies of retinal inputs to the LGN are rare in hominoids because such investigations require invasive procedures, but two individuals have been studied: one chimpanzee [Tigges et al. 1977], and one gibbon [Hylobates lar; Tigges and Tigges 1987]. Hidden parvocellular leaflets are not known to occur within catarrhines, although they exist in the platyrrhines and might exist in taxa for which tracing studies have not been performed.
Phylogenetic implications
The current study has detailed the LGN lamination pattern among catarrhine primate species. However, it is difficult to analyze the evolution of the LGN in catarrhines, because the outgroup, the platyrrhines, have lamination patterns that are hard to fit into characters states comparable to those of the catarrhines [see Kaas et al. 1978]. For example, consider Hylobates lar and Macaca mulatta, compared to the squirrel monkey (Saimiri sciureus), a New World monkey in which retinal inputs to the LGN have also been investigated. The squirrel money has a large, continuous parvocellular mass comprised of four “hidden” parvocellular leaflets. On the one hand, the four parvocellular leaflets in Saimiri sciureus resembles the parvocellular lamination of Macaca mulatta However, the leaflets of squirrel monkeys are not all separated by interlaminar space, differing from both Hylobates lar and Macaca mulatta, and could indicate differences in parvocellular or koniocellular organization. Further research is necessary to accurately identify homologous characters beyond the scope of the current study, and thus discern meaningful character states that can be applied to a greater representation of taxa.
Kaas et al. [1978] speculated that the last common ancestor of anthropoid primates had an LGN lamination pattern that included two undivided parvocellular layers. They suggested that the occurrence of leaflets which are separated by interlaminar space is a primitive catarrhine characteristic, and that the lack of leaflets in hylobatids is most likely an autapomorphy because it is more parsimonious for leaflets to have been lost once than to have developed independently in hominoids and cercopithecoids [Kaas et al. 1978]. However, Kaas et al. also point out that hylobatids (and, based on the present study, colobines and orangutans as well) have the simplest and most primitive-looking LGN lamination pattern [Kaas et al. 1978]. Although the strepsirrhine primates (galagos, lorises, and lemurs) have only two parvocellular layers and two magnocellular layers, they exhibit two dominant koniocellular layers visible in material stained for cell bodies as well, and are therefore have more elaborately laminated LGNs than those of catarrhines that also lack leaflets. In fact, the only other primates with a similarly simple LGN lamination pattern in material stained for cell bodies are the tarsiers, which also have only two magnocellular and two parvocellular layers – although a thin konicellular layer is exposed when stained for calbindin [Collins et al. 2005]. Although peculiarities of the tarsier LGN [Le Gros Clark 1930; McDonald et al. 1993; Rosa et al. 1996; Sereno and Allman 1991] have long implicated the species' primitiveness among primates, these could alternatively be specializations for vision in low light [Collins et al. 2005].
In light of the new data presented here, it is equally parsimonious to explain the LGN laminar pattern of hylobatids as retention of the primitive catarrhine pattern, or as the ancestral condition of the hominoid clade, retained in hylobatids and orangutans but lost in the African hominoids (Fig. 3). This finding is not insignificant. Macaques are used in translational research as a model to better understand the function of the human LGN. The fact that the laminar patterns of these two taxa might not be derived from a common ancestor means that extrapolations from macaques to humans of LGN laminar function should be made with caution. The current finding of homoplasy in LGN lamination pattern lends support to the possibility that too few character states have been identified. Further research may produce more detailed descriptions of the LGN lamination pattern of catarrhines, which take into consideration additional details about neuronal populations.
Functional implications
It is possible that hylobatids, orangutans, and some colobines possess the primitive LGN lamination pattern and that greater parvocellular interdigitation appeared independently in African apes, cercopithecines, and other colobines. The alternative hypothesis, that LGN parvocellular lamination was reduced in these species, would have to be explained by relaxation of selective pressures, which might have lead to a loss of parvocellular inputs related to color vision. If an LGN with two undivided parvocellular layers were the primitive condition, why would a pattern of four parvocellular leaflets develop in the African ape and human clade, the cercopithecine clade, and in some colobine species?
While the functional significance of having leaflets is not very well understood [Campos-Ortega and Hayhow 1970; Kaas and Huerta 1988; Kaas et al. 1978], available evidence suggests that lamination and the presence of leaflets might be related to the thickening of the parvocellular mass [Kaas et al. 1972; Kaas et al. 1978]. Because probably exist to facilitate interocular interactions. A reduction in the thickness of parvocellular layers is achieved by splitting each thick parvocellular layer into two thinner leaflets. This would shorten the distance between neurons receiving inputs from different eyes, and thus facilitate inter-ocular interactions [Kaas et al. 1978]. Thus, in species with relatively increased parvocellular mass related to enhanced processing capacities for color vision, the presence of leaflets may exist as a mechanism to retain optimal inter-ocular interaction. Unfortunately there remains a dearth of evidence for this hypothesis. Among mammals, obvious lamination of the LGN occurs not only in primates, but also in carnivores. In some carnivores, the LGN layers are also subdivided into leaflets. However, unlike in primates, leaflets do not alternate with regard to retinal input [Sanderson 1974] therefore leaflets are unlikely to facilitate interocular interactions.
The amount of LGN tissue delegated to parvocellular and magnocellular inputs varies within primate species, and is related to activity pattern and ecology. Diurnal primates have a proportionally larger parvocellular part of the LGN than do nocturnal primates [Hassler 1966], presumably because diurnal species rely more on information of the parvocellular pathway, such as color vision. Similarly, it has been hypothesized that increased lamination through the presence of leaflets may be related to improved color vision [Tigges and Tigges 1987].
Although it was previously suggested that blue-sensitive cells were most concentrated in the two most ventral parvocellular cells in the macaque LGN [Schiller and Malpeli 1978], it is now clear that blue sensitive cells are predominantly located either in the koniocellular layers or in the ‘koniocellular bridges’ that fully or partially span the parvocellular layers [Roy et al. 2009]. There have also been other indications that parvocellular layers differ according to optical specificity. The ventral-most pair of parvocellular leaflets also have cells that respond mostly off-center, where as the dorsal parvocellular leaflets respond mostly on-center [Schiller and Malpeli 1978]. This is interesting because the pairing of function is patterned by location, and not retinal projection, and therefore functional groups cross parvocellular layers, such that the internal leaflets of PE and PI group together, and the external leaflets of PE and PI group together. Central vision is represented in the segment of the LGN having four parvocellular leaflets in macaques [Malpeli and Baker 1975], therefore the additional layers may reflect an increased dedication to central vision.
Prior to the present study, it had only been clear that hylobatids departed from the typical catarrhine LGN lamination pattern, so this taxon has been contrasted against other catarrhine species in this regard. In fact, because it remains the only catarrhine species for which tracing studies confirm two undivided parvocellular layers, it remains possible that the hylobatid laminar pattern is unique among catarrhines. Tigges and Tigges [1987] relate the LGN lamination of gibbons to several aspects of behavior and physiology. It has also been suggested that gibbons are missing the two ventral parvocellular layers, which are important in color vision due to the predominance of blue-sensitive cells [Schiller and Malpeli 1978]. In support of this hypothesis, there is evidence of poor blue discrimination in gibbons [Tigges 1963] and a higher rod to cone ratio in gibbons than in rhesus macaques [Polyak 1957; Rohen 1962].
Also, it has been suggested that the relatively decreased lamination of the gibbon LGN probably does not have a negative effect on other visual functions such as motion detection [Tigges and Tigges 1987]. Gibbons may have neural specializations for motion detection because they are they are arboreal brachiators which move quickly in a complex three-dimensional environment. Changes in the parvocellular layers are not expected to have a negative effect on motion detection, particularly because it is the magnocellular layers that are the primary conduit of this category of visual information.
Finally, it is interesting to consider how the difference in LGN lamination may relate to the behavioral differences among species of cercopithecoid monkeys. The parvocellular layers of the LGN are involved in color vision. It has been hypothesized that trichromatic color vision, which occurs in all catarrhine species, evolved as a result of either frugivorous [Mollon 1989; Sumner and Mollon 2000] or folivorous [Dominy and Lucas 2001; Lucas et al. 1998] behavior. In fact, the cercopithecoid species with four parvocellular leaflets include both primarily frugivorous cercopithecines and some primarily folivorous colobines. Also, it has been argued recently that all catarrhines, including colobines and gibbons [contra Tigges and Tigges 1987] are identical in terms of spectral sensitivity [Deegan and Jacobs 2001; Jacobs and Deegan 1999]. Therefore, any potential difference in color vision would have to be at a post-retinal level.
The traditional notion that the LGN is a simple relay nucleus for retinal inputs on their way to the cerebral cortex has been disputed by studies which have shown that the human LGN receives inputs from the striate cortex, the thalamic reticular nucleus, and the brainstem; in fact, only 10% of its inputs originate from the retina [Kastner et al. 2006]. Therefore, the LGN is in a strategic position to serve as “gatekeeper” which modulates attention to visual stimuli.
Further, in macaques, the LGN projects not only to primary visual area, but also to extrastriate areas. In macaques V2 receives a direct LGN input, mostly from the interlaminar zones and S layers [Bullier and Kennedy 1983], and prestriate areas receiving LGN inputs probably also include V3 and V4 [Benevento and Standage 1982; Fries 1981; Yukie and Iwai 1981]. More recently it was confirmed that MT, involved in motion detection, also receives a direct input from LGN neurons located primarily in interlaminar zones, and from others scattered throughout parvocellular and magnocellular laminae [Sincich et al. 2004]. This, taken together evidence of direct inputs from the superior colliculus to interlaminar layers of the LGN in macaques, suggests a disynaptic tecto-geniculo-cortical koniocellular visual pathway, which has been more fully documented in New World monkey species [Stepniewska et al. 1999; Stepniewska et al. 2000]. However, only some extrastriate-projecting LGN neurons immunostained for the koniocellular marker αCAMKII, indicating heterogeneity among the interlaminar neurons [Rodman et al. 2001; Sincich et al. 2004]. The conventional functional roles of LGN pathways has been further questioned by the discovery of a disynaptic pathway in which MT receives a parvocellular input after a relay in primary visual cortex [Nassi et al. 2006].
These new findings suggest that the role of the LGN is much more complicated than previously thought. It is interesting to consider that LGN lamination may be related to higher level processing of, for example, parvocellular inputs. In such an example it is possible that 1) the LGN acts as a modulator of parvocellular information, for example, a filter of information related to trichromatic color vision and/or 2) there may be taxonomic differences in the existence of intercalated neuronal populations which may even have direct inputs to higher order, more functionally specific visual cortical areas.
Methodological considerations
The foregoing conclusions should be considered preliminary because of the methodological limitations of the present study. Importantly, it was not feasible to reveal the source of retinal input to each LGN layer, and thereby reveal possible “hidden” layers. Because hidden lamination at the level of leaflets or subleaflets have not been observed in catarrhines thus far, the existence of hidden layers in the catarrhines would seem unlikely, but cannot be ruled out. Therefore, in species for which retinal tracings to the LGN have not been studied the number of leaflets should be taken to be a minimum number. Perhaps the findings here will be confirmed through the use of non-invasive methods such as diffusion tensor MRI to study LGN structure and connections in ape species.
Although the samples used in the current study are relatively small, it is important to note that they are significantly improved from previous studies of the LGN in hominoids. Although within-species variation may occur at the level of subsidiary lamination such as subleaflets, at the level of layers and leaflets LGN lamination seems to be standard within species, making it is unlikely that larger samples would have any effect on the general findings made here.
In summary, the increased lamination of parvocellular layers, a parallel occurrence in the African hominoids, cercopithecines, and some colobines, may be functionally relevant. An increased number of, or better differentiation of parvocellular layers with leaflets may be related to color vision or to improved perception of central (versus peripheral) vision. Alternatively, increased lamination may simply provide more inter-laminar space for neuronal populations that do not participate in the parvocellular and magnocellular geniculocortical pathways, but rather have direct extrastriate inputs, for example, related to motion detection.
Supplementary Material
Acknowledgments
We are grateful to Drs. Bernard Wood, Ralph Holloway, Peter Lucas, and Brian Richmond for comments on earlier versions of the manuscript. Dr. Katerina Semendeferi was instrumental in establishing the Zilles ape brain collection used in the present study. Dr. Joseph Erwin facilitated access to great ape brain specimens. The Yerkes Primate Center also provided brains. This work was supported by the National Science Foundation (BCS-9987590, BCS-0453005, BCS-0515484, BCS-0549117, BCS-0827531, DGE-0801634), the Fundação para a Ciência e a Tecnologia (SFRH/BPD/43518/2008), the National Institutes of Health (NS42867), and the James S. McDonnell Foundation (22002078 and 220020293).
Citations
- Albright TD, Desimone R, Gross CG. Columnar organization of directionally selective cells in visual area MT of the macaque. J Neurophysiol. 1984;51:16–31. doi: 10.1152/jn.1984.51.1.16. [DOI] [PubMed] [Google Scholar]
- Armstrong E. Quantitative comparison of the hominoid thalamus. I. Specific sensory relay nuclei. Am J Phys Anthropol. 1979;51:365–382. doi: 10.1002/ajpa.1330510308. [DOI] [PubMed] [Google Scholar]
- Armstrong E, Frost GT. The Diencephalon: A comparative review. In: Schwartz JH, editor. Orang-Utan Biology. Oxford: Oxford University Press; 1988. pp. 177–188. [Google Scholar]
- Arnold C, Matthews LJ, Nunn CL. The 10kTrees Website: A New Online Resource for Primate Phylogeny. Evol Anthropol. 2010;19:114–118. [Google Scholar]
- Balado M, Franke E. Das Corpus Geniculatum Externum. Berlin: J. Springer; 1937. [Google Scholar]
- Benevento LA, Standage GP. Demonstration of lack of dorsal lateral geniculate nucleus input to extrastriate areas MT and Visual 2 in the macaque monkey. Brain Res. 1982;252:161–166. doi: 10.1016/0006-8993(82)90991-x. [DOI] [PubMed] [Google Scholar]
- Bullier J, Kennedy H. Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Exp Brain Res. 1983;53:168–172. doi: 10.1007/BF00239409. [DOI] [PubMed] [Google Scholar]
- Campos-Ortega JA, Hayhow WR. A new lamination pattern in the lateral geniculate nucleus of primates. Brain Res. 1970;20:335–339. doi: 10.1016/0006-8993(70)90303-3. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Yazar F, Jones KD, Ding Y. The morphology of the koniocellular axon pathway in the macaque monkey. Cereb Cortex. 2007;17:2334–2345. doi: 10.1093/cercor/bhl142. [DOI] [PubMed] [Google Scholar]
- Chacko L. The laminar pattern of the lateral geniculate body in the primate. J Neurol Neurosurg Psychiatry. 1948;11:211–224. doi: 10.1136/jnnp.11.3.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacko L. The lateral geniculate body in the New World monkeys. J Anat Soc India. 1954;3:62–74. [Google Scholar]
- Chacko L. The lateral geniculate body in gibbon (Hylobates hoolock) J Anat Soc India. 1955a;4:69–81. [Google Scholar]
- Chacko L. The lateral geniculate body of the chimpanzee. J Anat Soc India. 1955b;4:10–13. [Google Scholar]
- Chacko LW. A preliminary study of the distribution of cell size in the lateral geniculate body. J Anat. 1949;83:254–266. 253. [PubMed] [Google Scholar]
- Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. Anat Rec. 2005;287:1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- Dacey DM, Peterson BB, Robinson FR, Gamlin PD. Fireworks in the primate retina: in vitro photodynamics reveals diverse LGN-projecting ganglion cell types. Neuron. 2003;37:15–27. doi: 10.1016/s0896-6273(02)01143-1. [DOI] [PubMed] [Google Scholar]
- De Valois RL, Smith CJ, Kitai ST, Karoly AJ. Response of single cells in monkey lateral geniculate nucleus to monochromatic light. Science. 1958;127:238–239. doi: 10.1126/science.127.3292.238. [DOI] [PubMed] [Google Scholar]
- Deegan JF, Jacobs GH. Spectral sensitivity of gibbons: Implications for photopigments and color vision. Folia Primatol. 2001;72:26–29. doi: 10.1159/000049915. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Van Essen DC. Concurrent processing streams in monkey visual cortex. Trends Neurosci. 1988;11:219–226. doi: 10.1016/0166-2236(88)90130-0. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Hockfield S, Garren H, Van Essen DC. Antibody labeling of functional subdivisions in visual cortex: Cat-301 immunoreactivity in striate and extrastriate cortex of the macaque monkey. Vis Neurosci. 1990;5:67–81. doi: 10.1017/s0952523800000080. [DOI] [PubMed] [Google Scholar]
- DeYoe EA, Felleman DJ, Van Essen DC, McClendon E. Multiple processing streams in occipitotemporal visual cortex. Nature. 1994;371:151–154. doi: 10.1038/371151a0. [DOI] [PubMed] [Google Scholar]
- Dominy NJ, Lucas PW. Ecological importance of trichromatic vision to primates. Nature. 2001;410:363–366. doi: 10.1038/35066567. [DOI] [PubMed] [Google Scholar]
- Erwin E, Baker FH, Busen WF, Malpeli JG. Relationship between laminar topology and retinotopy in the rhesus lateral geniculate nucleus: Results from a functional atlas. J Comp Neurol. 1999;407:92–102. [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Receptive field properties of neurons in area V3 of macaque monkey extrastriate cortex. J Neurophysiol. 1987;57:889–920. doi: 10.1152/jn.1987.57.4.889. [DOI] [PubMed] [Google Scholar]
- Fries W. The projection from the lateral geniculate nucleus to the prestriate cortex of the macaque monkey. Proc R Soc Lond B Biol Sci. 1981;213:73–80. doi: 10.1098/rspb.1981.0054. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Saito N, Yamamoto M, Tanaka C. Immunocytochemical localization of the alpha-, beta I-, beta II- and gamma-subspecies of protein kinase C in the monkey visual pathway. Brain Res. 1994;658:155–162. doi: 10.1016/s0006-8993(09)90021-x. [DOI] [PubMed] [Google Scholar]
- Gallyas F. A principle for silver staining of tissue elements by physical development. Acta Morphol Acad Sci Hung. 1971;19:57–71. [PubMed] [Google Scholar]
- Goodchild AK, Martin PR. The distribution of calcium-binding proteins in the lateral geniculate nucleus and visual cortex of a New World monkey, the marmoset, Callithrix jacchus. Vis Neurosci. 1998;15:625–642. doi: 10.1017/s0952523898154044. [DOI] [PubMed] [Google Scholar]
- Hassler R. Comparative anatomy in day and night active primates. In: Hassler R, Stephan H, editors. Evolution of the Forebrain. Stuttgart: Thieme; 1966. pp. 419–434. [Google Scholar]
- Hendry SH, Yoshioka T. A neurochemically distinct third channel in the macaque dorsal lateral geniculate nucleus. Science. 1994;264:575–577. doi: 10.1126/science.8160015. [DOI] [PubMed] [Google Scholar]
- Hendry SH, Reid RC. The koniocellular pathway in primate vision. Annu Rev Neurosci. 2000;23:127–153. doi: 10.1146/annurev.neuro.23.1.127. [DOI] [PubMed] [Google Scholar]
- Hickey TL, Guillery RW. Variability of laminar patterns in the human lateral geniculate nucleus. J Comp Neurol. 1979;183:221–246. doi: 10.1002/cne.901830202. [DOI] [PubMed] [Google Scholar]
- Huberman AD, Chapman B. Evolution and development of eye-specific layers in the lateral geniculate nucleus. In: Kaas JH, editor. Evolution of Nervous Systems. Vol. 3. Oxford: Academic Press; 2007. pp. 319–328. Mammals. [Google Scholar]
- Jacobs GH, Deegan JF. Uniformity of colour vision in Old World monkeys. P Roy Soc Lond B Bio. 1999;266:2023–2028. doi: 10.1098/rspb.1999.0881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG, Hendry SH. Differential Calcium Binding Protein Immunoreactivity Distinguishes Classes of Relay Neurons in Monkey Thalamic Nuclei. Eur J Neurosci. 1989;1:222–246. doi: 10.1111/j.1460-9568.1989.tb00791.x. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Guillery RW, Allman JM. Some principles of organization in the dorsal lateral geniculate nucleus. Brain Behav Evol. 1972;6:253–299. doi: 10.1159/000123713. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Huerta MF, Weber JT, Harting JK. Patterns of retinal terminations and laminar organization of the lateral geniculate nucleus of primates. J Comp Neurol. 1978;182:517–553. doi: 10.1002/cne.901820308. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Huerta MF. The Subcortical Visual System of Primates. In: Stelis HD, Erwin J, editors. Comparative Primate Biology, Volume 4: Neurosciences. Vol. 4. New York: Liss; 1988. pp. 327–391. [Google Scholar]
- Kanagasuntheram R, Krishnamurti A, Wong WC. Observations on the lamination of the lateral geniculate body in some primates. Brain Res. 1969;14:623–631. doi: 10.1016/0006-8993(69)90203-0. [DOI] [PubMed] [Google Scholar]
- Kastner S, Schneider KA, Wunderlich K. Beyond a relay nuicleus: neuroimaging views on the human LGN. Progress in Brain Research. 2006;155:125–143. doi: 10.1016/S0079-6123(06)55008-3. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The Thalamus of Tarsius. J Anat. 1930;64:371–414. [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE, Penman GG. The projection of the petina in the lateral geniculate body. Proc R Soc Lond B. 1934;114:291. [Google Scholar]
- Le Gros Clark WE. The lateral geniculate body in the platyrrhine monkeys. J Anat. 1941a;76:131–140. [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE. The laminar organization and cell content of the lateral geniculate body in the monkey. J Anat. 1941b;75:419–433. [PMC free article] [PubMed] [Google Scholar]
- Le Gros Clark WE. The laminar pattern of the lateral geniculate nucleus considered in relation to colour vision. Doc Ophthalmol. 1949;3:57–64. doi: 10.1007/BF00162598. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Rodieck RW, Dreher B. Retinal ganglion cell classes in the Old World monkey: morphology and central projections. Science. 1981;213:1139–1142. doi: 10.1126/science.7268423. [DOI] [PubMed] [Google Scholar]
- Lucas PW, Darvell BW, Lee PK, Yuen TD, Choong MF. Colour cues for leaf food selection by long-tailed macaques (Macaca fascicularis) with a new suggestion for the evolution of trichromatic colour vision. Folia Primatol. 1998;69:139–152. doi: 10.1159/000021576. [DOI] [PubMed] [Google Scholar]
- Lueck CJ, Zeki S, Friston KJ, Deiber MP, Cope P, Cunningham VJ, Lammertsma AA, Kennard C, Frackowiak RS. The colour centre in the cerebral cortex of man. Nature. 1989;340:386–389. doi: 10.1038/340386a0. [DOI] [PubMed] [Google Scholar]
- Malpeli JG, Baker FH. The representation of the visual field in the lateral geniculate nucleus of Macaca mulatta. J Comp Neurol. 1975;161:569–594. doi: 10.1002/cne.901610407. [DOI] [PubMed] [Google Scholar]
- Malpeli JG, Lee D, Baker FH. Laminar and retinotopic organization of the macaque lateral geniculate nucleus: Magnocellular and parvocellular magnification functions. J Comp Neurol. 1996;375:363–377. doi: 10.1002/(SICI)1096-9861(19961118)375:3<363::AID-CNE2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Martin PR, White AJ, Goodchild AK, Wilder HD, Sefton AE. Evidence that blue-on cells are part of the third geniculocortical pathway in primates. Eur J Neurosci. 1997;9:1536–1541. doi: 10.1111/j.1460-9568.1997.tb01509.x. [DOI] [PubMed] [Google Scholar]
- McDonald CT, McGuinness ER, Allman JM. Laminar organization of acetylcholinesterase and cytochrome oxidase in the lateral geniculate nucleus of prosimians. Neuroscience. 1993;54:1091–1101. doi: 10.1016/0306-4522(93)90598-a. [DOI] [PubMed] [Google Scholar]
- McKeefry DJ, Zeki S. The position and topography of the human colour centre as revealed by functional magnetic resonance imaging. Brain. 1997;120(Pt 12):2229–2242. doi: 10.1093/brain/120.12.2229. [DOI] [PubMed] [Google Scholar]
- Merker B. Silver staining of cell bodies by means of physical development. J Neurosci Methods. 1983;9:235–241. doi: 10.1016/0165-0270(83)90086-9. [DOI] [PubMed] [Google Scholar]
- Mollon JD. “Tho' she kneel'd in that place where they grew…” The uses and origins of primate colour vision. J Exp Biol. 1989;146:21–38. doi: 10.1242/jeb.146.1.21. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Tigges J, Tigges M. Laminar organization of the lateral geniculate nucleus of the gorilla. Folia Primatol. 1998;69:377–380. doi: 10.1159/000021656. [DOI] [PubMed] [Google Scholar]
- Nassi JJ, Lyon DC, Callaway EM. The parvocellular LGN provides a robust disynaptic input to the visual motion area MT. Neuron. 2006;50:319–327. doi: 10.1016/j.neuron.2006.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien BJ, Abel PL, Olavarria JF. A morphological anomaly of the dorsal lateral geniculate nucleus in Macaca fascicularis. Cell Tissue Res. 1997;289:11–16. doi: 10.1007/s004410050847. [DOI] [PubMed] [Google Scholar]
- Polyak S. The Vertebrate Visual System. Chicago: Univ. of Chicago Press; 1957. [Google Scholar]
- Regan BC, Julliot C, Simmen B, Vienot F, Charles-Dominique P, Mollon JD. Fruits, foliage and the evolution of primate colour vision. Philos Trans R Soc Lond B Biol Sci. 2001;356:229–283. doi: 10.1098/rstb.2000.0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodiek RW. The Primate Retina. In: Stelis HD, Erwin J, editors. Comparative Primate Biology, Volume 4: Neurosciences. Vol. 4. New York: Liss; 1988. pp. 203–278. [Google Scholar]
- Rodman HR, Sorenson KM, Shim AJ, Hexter DP. Calbindin immunoreactivity in the geniculo-extrastriate system of the macaque: implications for heterogeneity in the koniocellular pathway and recovery from cortical damage. J Comp Neurol. 2001;431:168–181. doi: 10.1002/1096-9861(20010305)431:2<168::aid-cne1063>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Rohen J. Sehorgan. In: Hofer H, Schultz A, Starck D, editors. Primatologia: Handbuch der Primatenkunde. Base: Karger; 1962. pp. 1–210. [Google Scholar]
- Rosa MGP, Pettigrew JD, Cooper HM. Unusual pattern of retinogeniculate projections in the controversial primate Tarsius. Brain Behav Evol. 1996;48:121–129. doi: 10.1159/000113191. [DOI] [PubMed] [Google Scholar]
- Roy S, Jayakumar J, Martin PR, Dreher B, Saalmann YB, Hu D, Vidyasagar TR. Segregation of short-wavelength-sensitive (S) cone signals in the macaque dorsal lateral geniculate nucleus. Eur J Neurosci. 2009;30:1517–1526. doi: 10.1111/j.1460-9568.2009.06939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalmann YB, Kastner S. Cognitive and perceptualfunctions of the visual thalamus. Neuron. 2011;71:209–223. doi: 10.1016/j.neuron.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson KJ. Lamination of the dorsal lateral geniculate nucleus in carnivores of the weasel (Mustelidae), raccoon (Procyonidae) and fox (Canidae) families. J Comp Neurol. 1974;153:239–266. doi: 10.1002/cne.901530303. [DOI] [PubMed] [Google Scholar]
- Schiller PH, Malpeli JG. Functional specificity of lateral geniculate nucleus laminae of the rhesus monkey. J Neurophysiol. 1978;41:788–797. doi: 10.1152/jn.1978.41.3.788. [DOI] [PubMed] [Google Scholar]
- Schulz HD. Hohen Medizinischen Fakultaet. Frankfurt a. M., Germany: Johann Wolfgang Goethe-Universitaet Frankfurt a. M.; 1967. Metrische Untersuchungen an den Schichten des Corpus geniculatum laterale tag- und nachtaktiver Primaten. [Google Scholar]
- Sereno M, Allman JM. Cortical visual areas in mammals. In: Leventhal AG, editor. The Neural Basis of Visual Function. London: Macmillan; 1991. pp. 160–172. [Google Scholar]
- Sincich LC, Park KF, Wohlgemuth MJ, Horton JC. Bypassing V1: a direct geniculate input to area MT. Nat Neurosci. 2004;7:1123–1128. doi: 10.1038/nn1318. [DOI] [PubMed] [Google Scholar]
- Solnitzky O, Harman P. The lateral geniculate complex in the spider monkey, Ateles ater. Yale J Biol Med. 1943;15:615–639. [PMC free article] [PubMed] [Google Scholar]
- Stephan H, Frahm H, Baron G. New and revised data on volumes of brain structures in insectivores and primates. Folia Primatol. 1981;35:1–29. doi: 10.1159/000155963. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci. 1999;11:469–480. doi: 10.1046/j.1460-9568.1999.00461.x. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Ql HX, Kaas JH. Projections of the superior colliculus to subdivisions of the inferior pulvinar in New World and Old World monkeys. Vis Neurosci. 2000;17:529–549. doi: 10.1017/s0952523800174048. [DOI] [PubMed] [Google Scholar]
- Sumner P, Mollon JD. Catarrhine photopigments are optimized for detecting targets against a foliage background. J Exp Biol. 2000;203:1963–1986. doi: 10.1242/jeb.203.13.1963. [DOI] [PubMed] [Google Scholar]
- Tigges J. On colour vision in gibbon and orang-utan. Folia Primatol (Basel) 1963;1:188–198. [Google Scholar]
- Tigges J, Bos J, Tigges M. An autoradiographic investigation of the subcortical visual system in chimpanzee. J Comp Neurol. 1977;172:367–380. doi: 10.1002/cne.901720211. [DOI] [PubMed] [Google Scholar]
- Tigges J, Tigges M. Termination of retinofugal fibers and lamination pattern in the lateral geniculate nucleus of the gibbon. Folia Primatol. 1987;48:186–194. doi: 10.1159/000156294. [DOI] [PubMed] [Google Scholar]
- Tootell RB, Mendola JD, Hadjikhani NK, Ledden PJ, Liu AK, Reppas JB, Sereno MI, Dale AM. Functional analysis of V3A and related areas in human visual cortex. J Neurosci. 1997;17:7060–7078. doi: 10.1523/JNEUROSCI.17-18-07060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science. 1992;255:419–423. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]
- Yoshioka T, Hendry SH. Compartmental organization of layer IVA in human primary visual cortex. J Comp Neurol. 1995;359:213–220. doi: 10.1002/cne.903590203. [DOI] [PubMed] [Google Scholar]
- Yukie M, Iwai E. Direct projection from the dorsal lateral geniculate nucleus to the prestriate cortex in macaque monkeys. J Comp Neurol. 1981;201:81–97. doi: 10.1002/cne.902010107. [DOI] [PubMed] [Google Scholar]
- Zeki SM. Improbable areas in color vision. In: Chalupa LM, Werner JS, editors. The Visual Neurosciences. Vol. 2. Cambridge, MA: MIT Press; 2004. pp. 1029–1039. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.