Abstract
Background
Primary aldosteronism is the most common form of endocrine hypertension affecting approximately 8~10% of hypertensive subjects. Aldosterone production in PA occurs under low-renin conditions and the mechanisms that maintain the production of aldosterone in PA remain unknown.
Objective
This study was designed to compare the transcript profiles between aldosterone-producing adenoma (APA) and their adjacent adrenal gland (AAG) from the same adrenal.
Methods
Total RNA was extracted from 10 APA and 10 AAG; and subsequently analyzed by microarray and real-time quantitative RT-PCR (qPCR). The microarray data was paired for each APA-AAG, and analyzed by GeneSpring GX 11 with paired t-test and fold change calculations for each transcript. Changes identified by microarray analysis were confirmed by qPCR.
Results
Microarray analysis indicated that 14 genes had significantly up-regulated expression in APA compared to AAG. Among genes elevated were aldosterone synthase (CYP11B2) as well as novel transcription factors, calmodulin binding proteins, and other genes that have not been previously studied in APA. Selective analysis of 11 steroidogenic enzymes using microarray demonstrated that only CYP11B2 showed a significantly higher transcript level in APA compared to AAG (P<0.001). In contrast, AKR1C3 (17β-hydroxysteroid dehydrogenase type 5), CYP17 (17α-hydroxylase/17, 20 lyase), and CYB5 (cytochrome b5) showed significantly lower transcript level in APA (P<0.05).
Conclusion
The transcriptome analysis of APA compared with AAG showed several novel genes that are associated with APA phenotype. This gene list provides new candidates for the elucidation of the molecular mechanisms leading to PA.
Keywords: Transcriptome, aldosterone-producing adenoma, adjacent adrenal gland, biomarker
1. Introduction
Primary aldosteronism (PA) is the most common endocrine-related cause of hypertension and affects approximately 8~10% of hypertensive patients1, 2. Moreover, with an inappropriate production of aldosterone, PA is associated with more severe cardiovascular, renal and other complications compared to primary hypertension3, 4. In approximately 30~60% of PA patients, the cause is unilateral aldosterone-producing adenoma (APA) 1, 5–7.
Several studies have demonstrated that APA continue to express high levels of aldosterone synthase (CYP11B2), which maintains the capacity for aldosterone production8–12. Expression of this enzyme is mainly regulated by plasma potassium concentration and renin-angiotensin II system13–15. Aldosterone production in APA, however, occurs under low renin conditions16, 17, and the mechanisms that maintain high CYP11B2 expression and hypersecretion of aldosterone in APA remain unknown.
Previous studies have examined the gene expression pattern in APA compared to normal adrenal tissues8, 10–12, 18, however, direct comparison between APA and adjacent adrenal gland (AAG) tissue from the same patient has not been performed. Herein, we compared the gene expression profiles between APA and AAG tissues from 10 surgical specimens using high-density oligonucleotide microarray and real-time quantitative RT-PCR (qPCR). Our results define that some genes were expressed at significantly higher levels in APA than in AAG, which have not been identified as markers for APA in previous studies and might contribute to the pathophysiology of APA.
2. Material and Methods
2.1 Subjects and tissues
Human adrenal glands, which contained APA tissue, were obtained as part of standard pathological evaluation in collaboration with Tohoku University Hospital (Sendai, Japan) and University of Texas Southwestern Medical Center (Dallas, TX). Tumor and AAG tissues were macroscopically dissected and were then snap frozen and stored at −80°C. All samples were used under University Ethics Committee approval, and written informed consent was obtained from each patient. Thirteen adrenal glands were collected from patient with APA, two APA samples which showed less than 2-fold changes of CYP11B2 compared with the AAG tissue were excluded from our study, and one showed low quality of RNA was also excluded. Herein, we used 10 APA-AAG pairs for microarray analysis.
2.2 RNA isolation and cDNA generation
Total RNA was isolated from frozen tissue using RNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s instruction. The purity and quantity were assessed by a ND-1000 NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE).
For cDNA generation, 2 μg of total RNA was reverse transcribed using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturer recommendations and incubated at 25°C for 10 min, 37°C for 2 h, and 85°C for 5 seconds. The synthesized cDNA was subjected to 1:10 dilution for qPCR.
2.3 Microarray analysis
Total RNA isolated from APA and AAG tissues was treated with a first- and second-strand reverse transcription step, followed by a single in vitro transcription amplification that incorporated biotin-labeled nucleotides. Then the labeled RNA was hybridized to a bead chip containing more than 48,000 probes representing over 25,000 human genes (Illumina, San Diego, CA). The arrays were scanned at high resolution on the iScan system (Illumina). Results were analyzed using GeneSpring GX (version 11) software (Silicon Genetics, Redwood City, CA) to identify genome differences among APA and AAG.
2.4 Real-time quantitative RT-PCR (qPCR)
Primers and probes, for selected genes, were designed based on published sequences and gene amplification was performed using Taqman Gene Expression Assays (Applied Biosystems, Foster City, CA). The gene symbols and AB assay numbers are listed in Table 1. The primer and probe set for human CYP11B2 and CYP17 were designed using Primer Express 3.0 (Applied Biosystems, Foster City, CA) and purchased from IDT (Integrated DNA Technologies Inc., Coralville, IA) as described previously19.
Table 1.
Comparison of results from qPCR and microarray for APA-AAG pairs. The log2 fold changes was calculated for each APA-AAG pair and presented as mean ± S.E.M. P value was determined by paired t test for each transcript.
| Gene symbol | AB assay No. | qPCR(Log2 Fold change)
|
Microarray(Log2 Fold change)
|
||
|---|---|---|---|---|---|
| Mean±S.E.M | P | Mean±S.E.M | P | ||
| CYP11B2 | 6.01±0.88 | <0.001 | 3.28±0.41 | <0.001 | |
| PCP4 | Hs01113638_m1 | 3.38±0.69 | <0.001 | 2.80±0.67 | 0.004 |
| VPREB3 | Hs00353682_m1 | 3.30±0.40 | <0.001 | 2.39±0.32 | <0.001 |
| PRRX1 | Hs00246561_m1 | 1.69±0.25 | <0.001 | 1.39±0.22 | 0.001 |
| ALDH1A2 | Hs00180254_m1 | 3.09±0.60 | <0.001 | 1.45±0.45 | 0.010 |
| CYP17A1 | −0.97±0.41 | 0.042 | −0.73±0.23 | 0.023 | |
| CYB5 | Hs00157217_m1 | −1.77±0.53 | 0.009 | −1.76±0.31 | 0.001 |
| AKR1C3 | Hs00366261_m1 | −1.32±0.30 | 0.002 | −0.69±0.16 | 0.004 |
qPCR was performed using an ABI 7500 Fast Real-Time PCR System (Applied Biosystems), with 5 μl cDNA, 10 μl TaqMan Fast Universal PCR Master Mix (2Ҳ; Applied Biosystems), 900 nM of each primer, and 400 nM probe in a total volume of 20 μl per well according to the manufacturer’s instruction. Negative controls contained water instead of cDNA. Quantitative normalization of cDNA in each tissue-derived sample was performed using expression of 18S rRNA as an internal control. The generated Ct value of each gene was normalized by its respective Ct value of 18S rRNA (ΔCt). Each APA gene was then further normalized to the ΔCt value of its corresponding AAG (ΔΔCt). The final fold expression changes were calculated using the equation 2−ΔΔCt.
2.5 Statistical analysis
The fold change of qPCR data between APA and AAG pairs were converted to logarithms, and then analyzed using paired t-test with the SigmaStat 3.0 software package (Chicago, IL). The microarray data were analyzed using paired t-test with Benjamini Hochberg False discovery rate (FDR) correction using GeneSpring GX11 software. P value<0.05 was considered to be significant.
3. Results
3.1 Patient information
Before the removal of adrenal glands, all APA patients included in this study were diagnosed using adrenal vein sampling (AVS), which demonstrated lateralization of excess aldosterone production. All patients responded to adrenalectomy with normalization of aldosterone/renin ratio (ARR) and with decreased blood pressure and/or normalization of hypokalemia (Table 2). All adenoma cases were histopathologically diagnosed as an adrenocortical adenoma.
Table 2.
Clinical features of the APA patients. Relevant data were collected pre and post surgery, including SBP (systolic blood pressure), DBP (diastolic blood pressure), PRA (plasma renin activity), ARR (aldosterone/renin ratio), and ND (not detectable or not determined). Pre indicates the value obtained before surgery and post indicates the value obtained after the removal of the APA. Each case number corresponds to the number in the presented heatmap figures.
| Case No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 62 | 73 | 58 | 30 | 51 | 53 | 46 | 48 | 72 | 37 | |
| Sex | M | M | M | M | F | M | M | F | F | M | |
| SBP (mm Hg) | Pre | 154 | 149 | 167 | 164 | 168 | 183 | 157 | 146 | 158 | 120 |
| Post | 127 | 133 | 131 | 145 | 129 | 148 | 139 | 149 | 141 | 152 | |
|
|
|||||||||||
| DBP (mm Hg) | Pre | 92 | 89 | 101 | 103 | 97 | 105 | 92 | 95 | 83 | 90 |
| Post | 81 | 79 | 82 | 86 | 85 | 83 | 86 | 88 | 89 | 82 | |
|
|
|||||||||||
| Serum K+ (mEq/L) | Pre | 4.2 | 4.7 | 2.5 | 3.4 | 3.9 | 2.9 | 3.7 | 2.4 | 2.2 | 3.1 |
| Post | 4.8 | 4.7 | 3.9 | 4.1 | 4.5 | 3.8 | 3.4 | 5 | 3.9 | 3.8 | |
|
|
|||||||||||
| Plasma Aldosterone (ng/dL) | Pre | 20.9 | 15.8 | 35.3 | 15.4 | 15.8 | 42.7 | 28.3 | 30.8 | 32.7 | 26 |
| Post | ND | 8.7 | 6.2 | 8.1 | 7.9 | 10 | 6 | 17.4 | 11.3 | ND | |
|
|
|||||||||||
| PRA (ng/mL/h) | Pre | 0.3 | 0.3 | 0.2 | 0.3 | 0.1 | 0.4 | 0.6 | 0.1 | 0.5 | <0.6 |
| Post | ND | 0.4 | ND | 0.9 | 1.7 | 3.9 | 0.2 | 0.6 | 0.9 | ND | |
|
|
|||||||||||
| ARR (ng/dL: ng/mL/h) | Pre | 69.67 | 52.67 | 176.50 | 51.33 | 158.00 | 106.75 | 47.17 | 308.00 | 65.40 | >43 |
| Post | ND | 21.75 | ND | 9.00 | 4.65 | 2.56 | 30.00 | 29.00 | 12.56 | ND | |
|
|
|||||||||||
| Tumor Size (mm) | 9 | 8 | 14 | 12 | 15 | 18 | 19 | 17 | 18 | 19 | |
3.2 Differential gene expression in APA
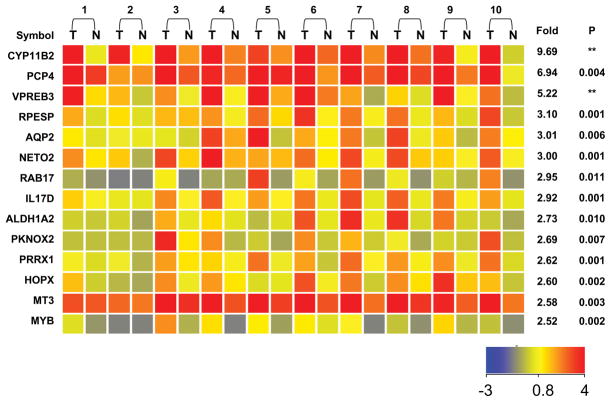
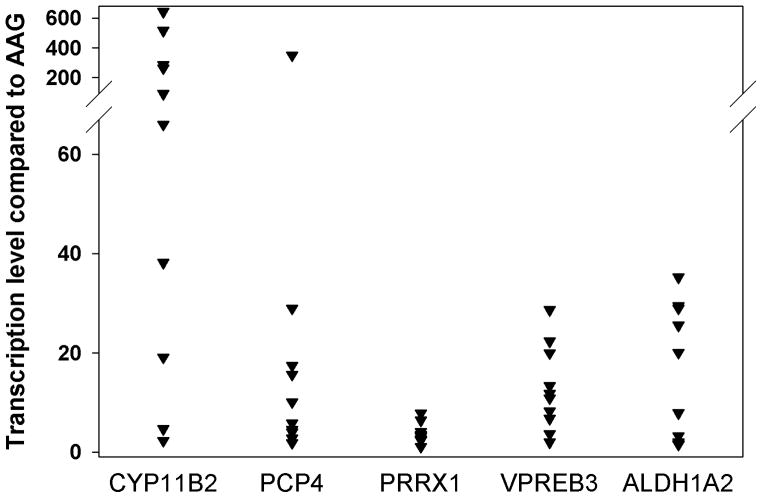
To evaluate global changes in gene expression associated with APA, RNA samples from eight adenomas were compared against the corresponding AAG tissue using microarray analysis. A gene list was compiled based on a minimum of a 2.5-fold increased level of expression that was found to be statistically significant (P<0.05). Fourteen genes were identified using these criteria and a heatmap representation of these genes is shown in Figure 1. Five genes in this list were chosen for qPCR validation, which confirmed the microarray data (Figure 2, Table 1). The major gene difference, which was observed in every APA-AAG sample pair, was CYP11B2 (Figure 2). Microarray data indicated a mean of 9.7-fold increased expression in APA (P<0.001), and the more quantitative method, qPCR, indicated a mean difference of 49-fold (Table 1). A comparison of microarray and qPCR was also performed for PCP4 (Purkinje cell protein 4), VPREB3 (pre-B lymphocyte 3), and PRRX1 (paired related homeobox 1). In each case qPCR generally agreed with microarray analysis (Table 1).
Figure 1.
Heatmap representation from microarray analysis showing transcripts with elevated expression in APA versus AAG tissue. The list was prepared based on genes that were significantly increased by greater than 2.5-fold (P<0.05) in APA compared to AAG. RNA from 10 APA-AAG pairs was used for microarray analysis on 20 arrays. The heatmap color indicates the expression value of each gene in the corresponding sample based on the log2 of signal strength (see color bar). The mean fold-induction of each APA-AAG pair with P value between all APA and AAG using paired t test are also provided (n=10). APA-AAG sample sets are paired by the indicated numbers. (** indicate a P value less than 0.001, T = APA; N = AAG)
Figure 2.
Quantification of up-regulated gene transcripts in APA-AAG pairs using qPCR. The vertical point scatterplot compares the expression of CYP11B2, PCP4, PRRX1, VPREB3, and ALDH1A2 between each APA-AAG pairs. Each plot represents the fold change within each sample pair, normalized to18S rRNA.
3.3 Steroidogenic enzymes
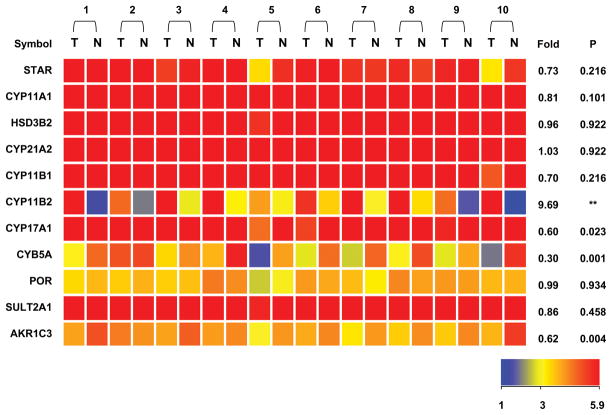
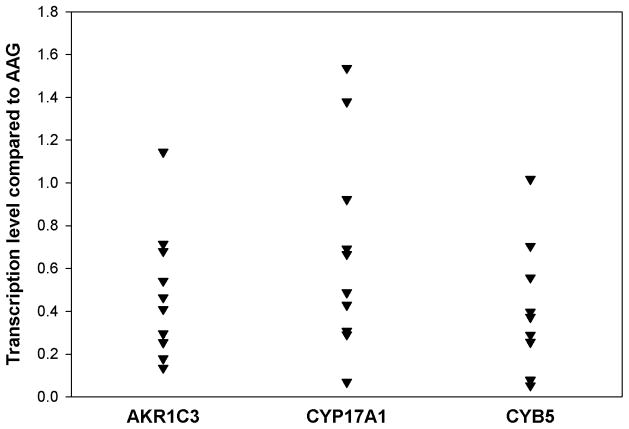
Eleven genes involved in the production of all classes of adrenal steroids were analyzed by microarray (Figure 3). In addition to a 9.7-fold increase in CYP11B2 expression, AKR1C3 (17β-hydroxysteroid dehydrogenase type 5), CYP17A1 (steroid 17α-hydroxylase/17,20 lyase), and CYB5 (Cytochrome b5) showed significantly lower expression in APA compared with AAG (P<0.05) (Figure 3). These genes also showed significant differences between APA and AAG when analyzed by qPCR (Figure 4, Table 2). Additionally, microarray analysis did not indicate any significant differences for the expression of cholesterol side-chain cleavage (CYP11A1), steroidogenic acute regulatory protein (StAR), 3β-hydroxysteroid dehydrogenase (HSD3B2), 21-hydroxylase (CYP21A2), 11β-hydroxylase (CYP11B1), cytochrome P450 oxidoreductase (POR) or steroid sulfotransferase type 2A1 (SULT2A1) (Figure 3).
Figure 3.
Comparison of steroidogenic enzyme transcripts in APA-AAG pairs using a heatmap derived from microarray analysis. RNA from 10 APA–AAG pairs was used for microarray analysis on 20 arrays. The heatmap color indicates the expression value of each gene in the corresponding sample based on the log2 of signal strength (see color bar). The mean fold-induction of each APA-AAG pair is also provided with P value between APA and AAG using paired t test. (n=10) Patient pairs are grouped by the indicated numbers. (** indicate a P value less than 0.001, T = APA; N = AAG)
Figure 4.
Quantification of the selected steroidogenic enzyme gene transcripts in APA-AAG pairs using qPCR. The vertical point scatterplot compares the expression of CYP17, CYB5, and AKR1C3 between APA and AAG. Each plot represents the fold change within each sample pair normalized to18S rRNA.
4. Discussion
In this study, we investigated the molecular mechanisms leading to APA by comparing the whole transcriptome of APA with their AAG tissues. Using microarray analysis, 14 transcripts were significantly elevated in APA, and qPCR assay confirmed the microarray results for the genes of interest. Most of the genes have not previously been studied in adrenal carcinoma or adenoma tissues, and several are good candidates as regulators of aldosterone secretion 8, 10–12, 20–22. One such example is PCP4, which is capable of binding calmodulin and regulating the dissociation of calcium from calmodulin 23, 24. The calcium-calmodulin cascade plays an important role in the expression of CYP11B211, 13, 25, and therefore PCP4 could participate in regulating aldosterone production. Another example is the gene that encoding PRRX1, a member of the paired family of homeobox proteins which is localized in the nucleus and functions as a transcription co-activator26, 27.
One unique feature of the current study is its use of paired APA-AAG samples from the same patient to study gene expression differences. This comparison led to the identification of several genes not previously noted in APA array studies, in which the control adrenal tissues were from autopsy or patients who underwent adrenalectomy along with a renalectomy due to renal carcinoma. Compared with these adrenal tissues, AAG showed morphological remodeling, but without transcriptional phenotype difference28. Moreover, APA production of aldosterone causes these patients to have low plasma renin activity and angiotensin II level (Ang II) 1, 5. Thus the adjacent adrenal tissue in this study is exposed to relatively lower Ang II levels, which was not controlled in previous studies8, 10. So the transcriptome comparison with paired APA-AAG could have less variation because these tissues are exposed to the same hormonal milieu. Interestingly, a qPCR analysis showed CYP11B2 expression between our AAG and normal adrenal gland (obtained from autopsy or renal cancer surgery) was not statistically different; and compared with the normal adrenal glands, the AAG showed smaller variation in CYP11B2 expression (data not shown). This is likely because most subjects, even those without primary aldosteronism are on a relatively high sodium diet and a relatively inactive glomerulosa. This concept is supported by the use of recently developed CYP11B2 antibodies that show normal adrenal glands exhibit few cells in the glomerulosa with detectable expression29.
Several studies suggested that APA have altered expression of several genes encoding various steroidogenic enzymes when compared to normal adrenal tissue. This observation has led many to speculate that the abnormal production of aldosterone and “hybrid” steroids such as 18-oxocortsiol by APA tissue may result from the disordered expression of multiple steroidogenic enzymes8, 11. As expected, our microarray data demonstrated that CYP11B2 was expressed at much higher levels in APA compared with AAG. In contrast, most transcripts encoding other steroidogenic enzymes were not different in APA, including StAR, which controls the acute rate-limiting first step in steroidogenesis30, 31. Nevertheless, we found decreased expression of the transcripts normally associated with adrenal androgen production including AKR1C3, CYP17A1, and CYB5 in APA. These results were consistent with the selectively increased aldosterone production from APA.
In the current study microarray analysis suggested that the fold difference for CYP11B2 expression was smaller than we and others have previously observed when APA tissue was compared to adrenal glands from autopsy and renal cancer patients8, 10, 32, 33. However, our qPCR analysis of the CYP11B2 transcript agrees with previous reports of a larger than 10-fold difference between APA and normal adrenal tissue8. This may be related to some cross-reactivity of the Illumina microarray CYP11B2 oligonucleotide with the highly similar 11β hydroxylase (CYP11B1) transcripts.
CYP11B2 is overexpressed in APA, which has been widely reported and also confirmed by our study1, 10, 12. In vitro and in vivo studies have shown that CYP11B2 expression is mainly regulated by Ang II, whereas, PA patients have low plasma renin activity, leaving the mechanism of autonomous CYP11B2 expression in APA unknown. Ye et al. showed that the expression of certain G-protein coupled receptors (GPCRs) were greatly up-regulated in APA when compared with normal adrenal glands10. We found a small but significant increase in the expression of several genes encoding GPCRs in APA using our pairwise comparison, but the overall fold increase was less than 2.5 (data not shown). These results further suggest that some GPCRs may be involved in the molecular mechanisms of APA, at least in some tumors. As noted previously, the ectopic expression of GPCRs may occur in only a small subset of APA10. Other studies suggest that the expression of transcription factors such as nerve growth factor induced clone B (NGFIB), steroidogenic factor-1 (SF-1), and dosage-sensitive sex reversal, adrenal hypoplasia congenita region on the X chromosome gene 1 (DAX-1)8, or signaling molecules such as calcium/calmodulin-dependent protein kinase I (CAMK I ) might increase CYP11B2 expression in APA12; however, these genes were not found to be differentially up-regulated in our study using pair-wise comparison.
Microarray analysis probes tens of thousands of genes simultaneously; therefore, it has become a useful tool to identify genes involved in tumorigenesis 18, 33–36. Several lines of evidence support the validity of our microarray analysis. First, our microarray results are in general agreement with the data obtained from qPCR. Second, a high CYP11B2 expression and low expression of AKR1C3, CYP17, and CYB5 are found in APA tissue, which is consistent with previous studies8, 11, 32. Our results also indicate a series of novel genes, which are highly expressed along with CYP11B2 in APA. These transcripts sort with CYP11B2 as markers for APA versus AAG and could contribute to Ang II independent CYP11B2 expression, aldosterone production and growth in APA. Elucidating the action of these genes in the adrenal cortex and APA might identify important mechanisms of APA growth and function, which might then lead to new strategies to diagnose and treat PA.
Acknowledgments
This work was supported by National Institutes of Health Grants DK-43140 and DK-069950 (to W.E.R.), a Synergy Award from the Cardiovascular Discovery Institute, Medical College of Georgia (to W.E.R.), a Clinical Scientist Award in Translational Research from the Burroughs-Wellcome Fund (#1005954 to R.J.A.), and the Houston J. and Florence A. Doswell Center for the Development of New Approaches for the Treatment of Hypertension at UT Southwestern. We would also like to acknowledge the editorial assistance of Dr. Mary Bassett.
Footnotes
6. Conflict of interest
None.
References
- 1.Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, Ganzaroli C, Giacchetti G, Letizia C, Maccario M, Mallamaci F, Mannelli M, Mattarello MJ, Moretti A, Palumbo G, Parenti G, Porteri E, Semplicini A, Rizzoni D, Rossi E, Boscaro M, Pessina AC, Mantero F. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–2300. doi: 10.1016/j.jacc.2006.07.059. [DOI] [PubMed] [Google Scholar]
- 2.Gordon RD. Primary aldosteronism--actual epidemics or false alarm? Arq Bras Endocrinol Metabol. 2004;48:666–673. doi: 10.1590/s0004-27302004000500012. [DOI] [PubMed] [Google Scholar]
- 3.Heymes C, Garnier A, Fuchs S, Bendall JK, Nehme J, Ambroisine ML, Robidel E, Swynghedauw B, Milliez P, Delcayre C. Aldosterone-synthase overexpression in heart: a tool to explore aldosterone’s effects. Mol Cell Endocrinol. 2004;217:213–219. doi: 10.1016/j.mce.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Rossi GP, Bernini G, Desideri G, Fabris B, Ferri C, Giacchetti G, Letizia C, Maccario M, Mannelli M, Matterello MJ, Montemurro D, Palumbo G, Rizzoni D, Rossi E, Pessina AC, Mantero F. Renal damage in primary aldosteronism: results of the PAPY Study. Hypertension. 2006;48:232–238. doi: 10.1161/01.HYP.0000230444.01215.6a. [DOI] [PubMed] [Google Scholar]
- 5.Stowasser M. Update in primary aldosteronism. J Clin Endocrinol Metab. 2009;94:3623–3630. doi: 10.1210/jc.2009-1399. [DOI] [PubMed] [Google Scholar]
- 6.Plouin PF, Amar L, Chatellier G. Trends in the prevalence of primary aldosteronism, aldosterone-producing adenomas, and surgically correctable aldosterone-dependent hypertension. Nephrol Dial Transplant. 2004;19:774–777. doi: 10.1093/ndt/gfh112. [DOI] [PubMed] [Google Scholar]
- 7.Mattsson C, Young WF., Jr Primary aldosteronism: diagnostic and treatment strategies. Nat Clin Pract Nephrol. 2006;2:198–208. doi: 10.1038/ncpneph0151. quiz, 191 p following 230. [DOI] [PubMed] [Google Scholar]
- 8.Bassett MH, Mayhew B, Rehman K, White PC, Mantero F, Arnaldi G, Stewart PM, Bujalska I, Rainey WE. Expression profiles for steroidogenic enzymes in adrenocortical disease. J Clin Endocrinol Metab. 2005;90:5446–5455. doi: 10.1210/jc.2005-0836. [DOI] [PubMed] [Google Scholar]
- 9.Connell JM, Fraser R, MacKenzie SM, Friel EC, Ingram MC, Holloway CD, Davies E. The impact of polymorphisms in the gene encoding aldosterone synthase (CYP11B2) on steroid synthesis and blood pressure regulation. Mol Cell Endocrinol. 2004;217:243–247. doi: 10.1016/j.mce.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 10.Ye P, Mariniello B, Mantero F, Shibata H, Rainey WE. G-protein-coupled receptors in aldosterone-producing adenomas: a potential cause of hyperaldosteronism. J Endocrinol. 2007;195:39–48. doi: 10.1677/JOE-07-0037. [DOI] [PubMed] [Google Scholar]
- 11.Lenzini L, Seccia TM, Aldighieri E, Belloni AS, Bernante P, Giuliani L, Nussdorfer GG, Pessina AC, Rossi GP. Heterogeneity of aldosterone-producing adenomas revealed by a whole transcriptome analysis. Hypertension. 2007;50:1106–1113. doi: 10.1161/HYPERTENSIONAHA.107.100438. [DOI] [PubMed] [Google Scholar]
- 12.Saner-Amigh K, Mayhew BA, Mantero F, Schiavi F, White PC, Rao CV, Rainey WE. Elevated expression of luteinizing hormone receptor in aldosterone-producing adenomas. J Clin Endocrinol Metab. 2006;91:1136–1142. doi: 10.1210/jc.2005-1298. [DOI] [PubMed] [Google Scholar]
- 13.Bassett MH, White PC, Rainey WE. The regulation of aldosterone synthase expression. Mol Cell Endocrinol. 2004;217:67–74. doi: 10.1016/j.mce.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Holland OB, Carr B, Brasier AR. Aldosterone synthase gene regulation by angiotensin. Endocr Res. 1995;21:455–462. doi: 10.3109/07435809509030462. [DOI] [PubMed] [Google Scholar]
- 15.Szekeres M, Turu G, Orient A, Szalai B, Supeki K, Cserzo M, Varnai P, Hunyady L. Mechanisms of angiotensin II-mediated regulation of aldosterone synthase expression in H295R human adrenocortical and rat adrenal glomerulosa cells. Mol Cell Endocrinol. 2009;302:244–253. doi: 10.1016/j.mce.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 16.Kempers MJ, Lenders JW, van Outheusden L, van der Wilt GJ, Schultze Kool LJ, Hermus AR, Deinum J. Systematic review: diagnostic procedures to differentiate unilateral from bilateral adrenal abnormality in primary aldosteronism. Ann Intern Med. 2009;151:329–337. doi: 10.7326/0003-4819-151-5-200909010-00007. [DOI] [PubMed] [Google Scholar]
- 17.Giacchetti G, Mulatero P, Mantero F, Veglio F, Boscaro M, Fallo F. Primary aldosteronism, a major form of low renin hypertension: from screening to diagnosis. Trends Endocrinol Metab. 2008;19:104–108. doi: 10.1016/j.tem.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Slater EP, Diehl SM, Langer P, Samans B, Ramaswamy A, Zielke A, Bartsch DK. Analysis by cDNA microarrays of gene expression patterns of human adrenocortical tumors. Eur J Endocrinol. 2006;154:587–598. doi: 10.1530/eje.1.02116. [DOI] [PubMed] [Google Scholar]
- 19.Pezzi V, Mathis JM, Rainey WE, Carr BR. Profiling transcript levels for steroidogenic enzymes in fetal tissues. J Steroid Biochem Mol Biol. 2003;87:181–189. doi: 10.1016/j.jsbmb.2003.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Soon PS, Gill AJ, Benn DE, Clarkson A, Robinson BG, McDonald KL, Sidhu SB. Microarray gene expression and immunohistochemistry analyses of adrenocortical tumors identify IGF2 and Ki-67 as useful in differentiating carcinomas from adenomas. Endocr Relat Cancer. 2009;16:573–583. doi: 10.1677/ERC-08-0237. [DOI] [PubMed] [Google Scholar]
- 21.Suh I, Guerrero MA, Kebebew E. Gene-expression profiling of adrenocortical carcinoma. Expert Rev Mol Diagn. 2009;9:343–351. doi: 10.1586/erm.09.13. [DOI] [PubMed] [Google Scholar]
- 22.Tacon LJ, Soon PS, Gill AJ, Chou AS, Clarkson A, Botling J, Stalberg PL, Skogseid BM, Robinson BG, Sidhu SB, Clifton-Bligh RJ. The glucocorticoid receptor is overexpressed in malignant adrenocortical tumors. J Clin Endocrinol Metab. 2009;94:4591–4599. doi: 10.1210/jc.2009-0546. [DOI] [PubMed] [Google Scholar]
- 23.Johanson RA, Sarau HM, Foley JJ, Slemmon JR. Calmodulin-binding peptide PEP-19 modulates activation of calmodulin kinase II In situ. J Neurosci. 2000;20:2860–2866. doi: 10.1523/JNEUROSCI.20-08-02860.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleerekoper QK, Putkey JA. PEP-19, an intrinsically disordered regulator of calmodulin signaling. J Biol Chem. 2009;284:7455–7464. doi: 10.1074/jbc.M808067200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spat A, Hunyady L. Control of aldosterone secretion: a model for convergence in cellular signaling pathways. Physiol Rev. 2004;84:489–539. doi: 10.1152/physrev.00030.2003. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez Perera O, Marrero A, Rodriguez Perez JC. What are the Hox genes? Its importance in vascular and renal disease. Nefrologia. 2006;26:195–205. [PubMed] [Google Scholar]
- 27.Noda M, Ohno S, Nakajin S. Mono-(2-ethylhexyl) phthalate (MEHP) induces nuclear receptor 4A subfamily in NCI-H295R cells: a possible mechanism of aromatase suppression by MEHP. Mol Cell Endocrinol. 2007;274:8–18. doi: 10.1016/j.mce.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Boulkroun S, Samson-Couterie B, Dzib JF, Lefebvre H, Louiset E, Amar L, Plouin PF, Lalli E, Jeunemaitre X, Benecke A, Meatchi T, Zennaro MC. Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism. Hypertension. 56:885–892. doi: 10.1161/HYPERTENSIONAHA.110.158543. [DOI] [PubMed] [Google Scholar]
- 29.Nishimoto K, Nakagawa K, Li D, Kosaka T, Oya M, Mikami S, Shibata H, Itoh H, Mitani F, Yamazaki T, Ogishima T, Suematsu M, Mukai K. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 95:2296–2305. doi: 10.1210/jc.2009-2010. [DOI] [PubMed] [Google Scholar]
- 30.Arakane F, Sugawara T, Nishino H, Liu Z, Holt JA, Pain D, Stocco DM, Miller WL, Strauss JF., 3rd Steroidogenic acute regulatory protein (StAR) retains activity in the absence of its mitochondrial import sequence: implications for the mechanism of StAR action. Proc Natl Acad Sci U S A. 1996;93:13731–13736. doi: 10.1073/pnas.93.24.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rone MB, Fan J, Papadopoulos V. Cholesterol transport in steroid biosynthesis: role of protein-protein interactions and implications in disease states. Biochim Biophys Acta. 2009;1791:646–658. doi: 10.1016/j.bbalip.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assie G, Auzan C, Gasc JM, Baviera E, Balaton A, Elalouf JM, Jeunemaitre X, Plouin PF, Corvol P, Clauser E. Steroidogenesis in aldosterone-producing adenoma revisited by transcriptome analysis. J Clin Endocrinol Metab. 2005;90:6638–6649. doi: 10.1210/jc.2005-1309. [DOI] [PubMed] [Google Scholar]
- 33.Lombardi CP, Raffaelli M, Pani G, Maffione A, Princi P, Traini E, Galeotti T, Rossi ED, Fadda G, Bellantone R. Gene expression profiling of adrenal cortical tumors by cDNA macroarray analysis. Results of a preliminary study. Biomed Pharmacother. 2006;60:186–190. doi: 10.1016/j.biopha.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Quackenbush J. Microarray analysis and tumor classification. In. N Engl J Med. 2006:2463–2472. doi: 10.1056/NEJMra042342. [DOI] [PubMed] [Google Scholar]
- 35.Giordano TJ, Kuick R, Else T, Gauger PG, Vinco M, Bauersfeld J, Sanders D, Thomas DG, Doherty G, Hammer G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676. doi: 10.1158/1078-0432.CCR-08-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laurell C, Velazquez-Fernandez D, Lindsten K, Juhlin C, Enberg U, Geli J, Hoog A, Kjellman M, Lundeberg J, Hamberger B, Larsson C, Nilsson P, Backdahl M. Transcriptional profiling enables molecular classification of adrenocortical tumours. Eur J Endocrinol. 2009;161:141–152. doi: 10.1530/EJE-09-0068. [DOI] [PubMed] [Google Scholar]