Abstract
Background
Retinol-binding protein 4 (RBP4) may play an important role in the etiology of insulin resistance and metabolic syndrome. Few prospective data are available on the relationship between RBP4 and coronary heart disease (CHD). Further, previous studies did not distinguish among full-length and truncated forms of RBP4 that might have various biological activities.
Methods and Results
We measured plasma levels of full-length and several C-terminally truncated sub-fractions of RBP4 among 468 women who developed CHD and 472 matched controls in the Nurses’ Health Study cohort during 16 years of follow-up (1990–2006). We observed a temporal variation in the association of full-length RBP4 levels with CHD risk (P=0.04 for testing proportional hazard assumption). In the first 8 years of follow-up, after multivariate adjustment for covariates, the odds ratio (OR) of CHD risk comparing extreme quartiles of full-length RBP4 levels was 3.56 (95% CI: 1.21, 10.51; Ptrend=0.003), whereas this association was 0.77 (0.38, 1.56; Ptrend=0.44) in the follow-up period of 9–16 years. Results were similar for total RBP4 levels (summed levels of all RBP4 isoforms). Levels of the primary truncated isoform, RBP4-L, were not associated with CHD risk in any follow-up period; the ORs (95% CI) for extreme quartiles were 1.29 (0.50, 3.32) and 1.20 (0.64, 2.26) in the first and second 8 years of follow-up, respectively.
Conclusions
In this cohort of women, higher circulating full-length and total RBP4 levels were associated with increased risk of CHD in a time-dependent fashion. Additional data are warranted to confirm the current findings.
Keywords: acute myocardial infarction, retinol-binding protein 4, epidemiology, women, heart diseases
Retinol-binding protein 4 (RBP4) is a ~21 kDa protein secreted by adipocytes and hepatocytes. Strong animal experimental data have suggested that RBP4 is causally involved in the etiology of cardiometabolic diseases.1 First, RBP4 expression is inversely regulated by Glut4 expression. Further, a PPAR-γ agonist rosiglitazone lowered adipose RBP4 expression, normalized serum RBP4 levels, and reversed insulin resistance. Lastly, transgenic overexpression of RBP4, or injection of human RBP4 in normal mice, caused insulin resistance, while genetic deletion of RBP4 or lowering circulating RBP4 levels had the opposite effects.1 Although cross-sectional human studies generated somewhat inconsistent results between RBP4 levels and insulin resistance or diabetes,1–12 recent prospective studies have shown convincing evidence that supports the findings from animal experiments.13, 14
Despite the promising evidence for insulin resistance and diabetes, data regarding RBP4 levels in relation to cardiovascular disease (CVD) are rare. Nonetheless, positive associations were documented for circulating RBP4 or RBP4 expression levels with established CVD risk factors, including metabolic syndrome,6, 7, 15 overall/central obesity,15–18 dyslipidemia,3, 5, 6, 11, 15, 18, 19 inflammatory markers,20–22 and hypertension.18, 23 Moreover, RBP4 levels have been positively associated with carotid intima-media thickness23, 24 and prevalent CVD events.15, 25, 26 An intervention study conducted among children further demonstrated that fat mass loss following lifestyle intervention resulted in reduction in RBP4 levels, which was accompanied by decreased systemic inflammation and insulin resistance.20 Similarly, in a cross-sectional study an increase in RBP4 levels over 3 years also predicted worsening of insulin resistance and cardiometabolic risk.27 However, in the only prospective study of incident coronary heart disease (CHD), RBP4 levels were not associated with CHD risk.28 Heterogeneity in study design, age, race, and other participant characteristics, and limitations of the assays used,18, 29 across previous studies may explain the discordant observations. Another caveat to be considered is that circulating RBP4 molecules exist in full-length and several truncated forms.18, 30 Although the biology of different forms of RBP4 is unknown, limited evidence suggested that some forms might be more biologically active than others.18 Since the commonly used ELISA, Western blotting, and nephelometric assays do not distinguish different forms of RBP4, few studies have examined individual forms of RBP4.18 Furthermore, depending on the affinity of antibodies to various forms of RBP4, these prevalent assays may measure different forms with different affinity,29 introducing exogenous variability in the measurement of total RBP4 levels.
In the current prospective investigation, we specifically evaluated different forms of circulating RBP4 measured by a novel assay18, 31 in relation to the development of CHD among women in the Nurses’ Health Study (NHS).
METHODS
Study Populations
The NHS cohort consists of 121,700 female registered nurses aged 30 to 55 years who were enrolled in 1976. The response rate to study questionnaires was above 94% of potential person-time of follow-up. A total of 32,826 NHS participants provided blood samples in 1989–1990 via an overnight courier, and a frozen water bottle was included to keep the samples cool during transportation. The majority (97%) of these samples arrived within 26 hours since blood draw. Immediately upon arrival, whole blood samples were then centrifuged and aliquoted into cryotubes as plasma, buffy coat, and red blood cells, which were stored in the vapor phase of liquid nitrogen freezers at ≤−130°C until assessment of RBP4 levels. In a pilot study among 7 volunteers, we found an intra-class correlation coefficient (ICC) of 0.97 for total RBP4 levels in three split samples from the same participants. The split samples were processed under three conditions: immediately after collection, after 24 hours’ storage, and after 48 hours’ storage in a container cooled by a frozen water bottle. These data suggested that our sample collection conditions had minimal impact on RBP4 levels.
Nested Case-control Study Design
We prospectively identified and confirmed 468 incident cases of nonfatal myocardial infarction (MI) and fatal CHD from the date of blood draw through June 2006. We used risk-set sampling scheme to randomly select one control for each case from the rest of the population who remained free of CHD events when the case occurred. To control for confounding, we matched cases and controls for age at blood draw (±1 year), smoking status (never, past, and current), fasting status at blood draw (fasting for 10 hours or not), and date of blood draw. Cases and controls were all free of cancer and CVD at blood collection. In the current study, we had sufficient power (>80%) to detect odds ratios (OR) ≥1.6 comparing extreme quartiles.
The study protocol was approved by the institutional review board of the Brigham and Women’s Hospital and the Human Subjects Committee Review Board of the Harvard School of Public Health.
Assessment of Coronary Heart Disease
In baseline and all biennial follow-up questionnaires, we inquire about the occurrence of physician-diagnosed CHD events. We request medical records from participants who report having a nonfatal MI. Study physicians who are blinded to the exposure status of participants review all medical records and confirm or refute the self-reports of nonfatal MI using the World Health Organization criteria that require typical symptoms plus either diagnostic electrocardiographic findings or elevated cardiac enzyme levels.32 Deaths are identified by reports from next of kin, postal authorities, or by searching the National Death Index. In a validation study, we demonstrated that at least 98% of deaths among the NHS participants were identified using these approaches.33 Fatal CHD cases are identified if CHD is listed as the cause of death in autopsy reports, hospital records, or death certificates. Fatal CHD cases are then confirmed by a previous report of CHD and if there is no other more apparent or plausible cause of death. CHD cases are considered probable if study participants confirm diagnoses in telephone interviews or through mail but medical records are not obtained. We included both definite (83.5%) and probable (16.5%) nonfatal MI and fatal CHD cases in the current analysis. Unconfirmed CHD deaths were excluded.
Assessment of Plasma Levels of RBP4
Plasma samples from both cases and controls were processed for RBP4 assays at the same time. As 98% of blood samples were collected within 15 months (June 1989 to September 1990), the storage duration of these samples did not substantially differ among the participants. In addition, because blood collection time and subsequent CHD diagnosis date were entirely unrelated, case-control pairs identified in early follow-up periods had the same sample storage duration as those identified at later follow-up.
To minimize systematic measurement error, samples from each case-control pair were handled identically and analyzed in the same run by the same technicians in a random sequence under identical conditions for all assays. We used a novel quantitative mass spectrometry immunoassay (MSIA) to measure plasma levels of full-length and truncated forms of RBP4. This assay is based on the technique of Linear Time-of-Flight (TOF) that uses an internal reference standard (IRS) to internally normalize the MS signals.18, 31 Briefly, 50 μL of a 1/100 dilution of human plasma was mixed in a 1:1 ratio with 0.5% SDS (vol/vol). Mixed samples were incubated at room temperature for 20 minutes to liberate RBP4 from the endogenous transthyretin. Next, each sample was spiked with 50 μL of a 25 μg/μL solution of beta-lactoglobulin (β-Lac), which is used as the IRS. Samples were diluted to 1 mL with HEPES-buffered saline. RBP4 and β-Lac IRS were co-immuno-affinity purified from samples using MSIA-Tips tailored to specifically retrieve both these analytes. Mass spectra were generated by MALDI-TOF and analyzed with Zebra MS software (Intrinsic Bioprobes, Inc., Tempe, AZ).
This novel assay can distinguish full-length RBP4 from three truncated forms of RBP4, i.e., RBP4-L (the most abundant truncated form that lacks a leucine at the C-terminal end), RBP4-LL (lacks two C-terminal leucines), and RBP4-RNLL (the least abundant truncated form that lacks arginine, asparagine, and two leucines at the C-terminal end). To be comparable with most prior investigations that assayed total RBP4 levels only, we derived total RBP4 levels by adding the levels of full-length RBP4, RBP4-L, RBP4-LL, and RBP4–RNLL. Total RBP4 levels measured by MSIA correlate strongly with those determined by Western blotting (r = 0.91).18 Quality control samples were dispersed throughout each analytical run. Based on the measurements of these control samples, the average intra-assay coefficient of variation (CV) was 7.0% for full-length RBP4 and 10.5% for RBP4-L. Because RBP4-LL and RBP4-RNLL were not detectable in most quality control samples, CV data were not available for these two markers.
In the current investigation, we utilized existing data on an array of CVD risk markers, including total (TC), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol, fasting triacylglycerol (TG), high-sensitivity C-reactive protein (hsCRP), adiponectin, hemoglobin A1c (HbA1c), and creatinine levels, to explore the inter-relationship between RBP4 and these markers which were associated with RBP4 levels in prior investigations.15, 20, 21
Assessment of Covariates
In NHS questionnaires, we inquire about medical history, major lifestyle practices, and other risk factors for CHD, including body weight, cigarette smoking, physical activity, family history of MI, menopausal status, and post-menopausal hormone use. Information about history of hypertension, hypercholesterolemia, and diabetes was based on self-report. Body mass index (BMI) as weight in kilograms divided by the square of height in meters (kg/m2) was calculated to assess overall adiposity. Diet has been assessed using validated semiquantitative food frequency questionnaires every 2–4 years since 1980. We used covariates assessed using 1990 questionnaire in the analysis to control for confounding. We calculated and used cumulative averages of dietary variables through 1990 to represent long-term diet. We derived the estimated glomerular filtration rate (eGFR) using the following equation:
Statistical Methods
To explore the inter-relationship among individual RBP4 forms and the correlations between RBP4 levels and other CVD risk factors, we calculated Spearman partial correlation coefficients among controls and adjusted for age at blood draw, BMI, fasting status, smoking status, postmenopausal status, hormone use, physical activity, alcohol use, family history of heart disease, intakes of trans fat, polyunsaturated fat, and whole grains, use of aspirin, and eGFR.
We categorized the study population into quartiles according to the distribution of RBP4 levels among controls and used the lowest quartile as the reference group. Conditional logistic regression was used to estimate the OR of CHD by RBP4 quartiles. In nested case-control studies, ORs derived from conditional logistic regression models are unbiased estimates of hazard ratios or relative risks.35 In the multivariate analysis, we controlled for the aforementioned covariates and history of hypercholesterolemia, diabetes, or hypertension. P values for linear trend were calculated by entering an ordinal score based on the median value in each quartile of RBP4 levels into the multivariate models. Because the validity of estimates from conditional logistic regression analysis depends on the assumption of proportional hazard,35 we evaluated this assumption by testing the significance of interaction terms between RBP4 levels and length of follow-up. We used likelihood ratio tests to assess the significance of these interaction terms. In addition, we used restricted cubic spline regressions with 3 knots to model the dose-response relationship between RBP4 levels and risk of CHD.36 In this analysis, we excluded participants with the lowest and highest 5% of RBP4 levels to minimize potential impact of outliers. Tests for non-linearity were based on the likelihood ratio test, comparing the model with only the linear term to the model with the linear and the cubic spline terms.
All P values were two-sided. Ninety-five percent confidence intervals (95% CI) were calculated for ORs. Data were analyzed with the Statistical Analysis Systems software package, version 9.2 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Table 1 shows the characteristics of CHD cases and controls at blood collection in 1989–1990. Because of the matching design, cases and controls were similar for age and smoking status. Otherwise, as expected, cases had higher levels of CVD risk factors than controls. For example, cases had higher BMI, drank less alcohol, were more likely to have a history of hypertension, diabetes, and other chronic conditions, and were more likely to have a family history of MI than controls. The distribution of blood lipids, hsCRP, adiponectin, and HbA1c between cases and controls was also consistent with their higher risk status. In both cases and controls, full-length RBP4 was the most abundant form and accounted for >69.5% of total RBP4. RBP4-L accounted for the vast majority (95.4%) of total truncated forms of RBP4. For the two minor forms of truncated RBP4, only 20.4% of the participants had detectable RBP4-LL, and only 1.8% had detectable RBP4-RNLL. Although cases had higher levels of full-length and truncated forms of RBP4 than controls, the differences did not reach significance level. The cases and controls also had similar eGFR levels.
Table 1.
Baseline (1989–1990) characteristics of CHD patients and controls, the Nurses’ Health Study.
| Characteristics* | Cases (N=468) | Controls (N=472) | P value† |
|---|---|---|---|
| Age (year)‡ | 59.5±6.6 | 59.4±6.6 | 0.90 |
| Body mass index (kg/m2) | 26.5±5.2 | 25.3±4.3 | 0.0001 |
| Physical Activity (MET-hr/week) | 16.8±17.8 | 18.0±17.0 | 0.28 |
| Diet | |||
| Whole grain (g/d) | 16.8±12.4 | 16.3±11.0 | 0.56 |
| Trans fat intake (g/d) | 1.72±0.48 | 1.66±0.49 | 0.04 |
| Polyunsaturated fat intake (g/d) | 6.3±1.3 | 6.3±1.3 | 0.97 |
| Alcohol (g/d) | 5.5±8.8 | 6.8±9.7 | 0.04 |
| Smoking status (%)‡ | 0.85 | ||
| Current smoker | 25.2 | 23.9 | |
| Former smoker | 37.8 | 38.6 | |
| Never Smoked | 37.0 | 37.5 | |
| Medical history | |||
| Diabetes (%) | 13.7 | 5.1 | <0.0001 |
| Hypertension (%) | 48.5 | 26.3 | <0.0001 |
| Hypercholesterolemia (%) | 53.4 | 40.9 | 0.0001 |
| Parental MI before age 65 years (%) | 32.1 | 19.5 | <0.0001 |
| Fasting status (%)‡ | 70.7 | 70.6 | 0.95 |
| Menopausal status (%) | 0.54 | ||
| Pre-menopause | 11.3 | 13.1 | |
| Menopausal, current hormone users | 35.7 | 38.6 | |
| Menopausal, past hormone users | 18.2 | 16.5 | |
| Menopausal, never used hormone | 34.8 | 31.8 | |
| Use of aspirin (%) | 60.5 | 64.0 | 0.27 |
| RBP4 | |||
| Full-length RBP4 (μg/mL) | 65.7 (48.6, 92.4) | 62.5 (48.0, 86.8) | 0.12 |
| RBP4-L (μg/mL) | 26.0 (18.8, 36.6) | 25.0 (18.2, 36.2) | 0.24 |
| Total RBP4 (μg/mL) | 93.7 (70.3, 129.1) | 89.3 (67.5, 126.1) | 0.16 |
| CVD risk markers§ | |||
| HDL-C (mg/dL) | 51.2 (41.9, 62.6) | 57.9 (48.1, 67.1) | <0.0001 |
| LDL-C (mg/dL) | 141.8 (118.0, 166.4) | 137.0 (110.4, 161.0) | 0.01 |
| Total cholesterol (mg/dL) | 231.0 (207.0, 257.0) | 224.0 (200.0, 255.0) | 0.05 |
| Total to HDL-C ratio | 4.5 (3.6, 5.7) | 3.9 (3.2, 4.7) | <0.0001 |
| Fasting TG (mg/dL) | 121.0 (84.0, 177.0) | 105.0 (76.0, 147.0) | <0.0001 |
| hsCRP (mg/L) | 0.26 (0.11, 0.55) | 0.17 (0.08, 0.36) | <0.0001 |
| Adiponectin (μg/mL) | 7.4 (5.2, 10.2) | 8.6 (3.6, 21.7) | <0.0001 |
| Hemoglobin A1c (%) | 5.5 (5.3, 5.9) | 5.4 (5.2, 5.7) | <0.0001 |
| Glomerular filtration rate¶ | 82.5 (70.1, 96.6) | 76.2 (71.1, 83.8) | 0.79 |
Abbreviations: MET-hr, metabolic equivalent-hours; MI, myocardial infarction; RBP4, retinol binding protein-4; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triacylglycerol; hsCRP, high-sensitivity C-reactive protein; CVD, cardiovascular disease.
Plus-minus values are mean±standard deviation. Percentages are based on non-missing data. Levels of RBP-4 and other biomarkers are expressed as median (interquartile range) because of apparently skewed distributions.
P value estimates are based on Student’s t test for variables expressed as mean±standard deviation, Wilcoxon rank-sum test for variables expressed as medians, or Pearson χ2 test for variables expressed as percentages.
Matching factors.
Data of total, HDL-cholesterol, and total/HDL-C ratio were missing for 12 participants, and the number of participants with missing values was 22 for LDL-C, 60 for TG, 20 for CRP, 13 for creatinine, 12 for hemoglobin A1c, and 14 for adiponectin.
In controls, who represented the source population for cases, different RBP4 isoforms were significantly correlated with each other (Supplementary Table 1). Regarding the relationships between RBP4 levels and CVD risk markers, full-length RBP4 levels did not correlate with blood lipids, CRP, eGFR, adiponectin, or HbA1c after multivariate adjustment of BMI and other CVD risk factors (Table 2). In contrast, RBP4-L levels correlated positively with TC and fasting TG and inversely with eGFR, although the strength of correlations was modest. The same correlation pattern was found for total RBP4 levels. RBP4 levels were not correlated with BMI or waist circumference (data not shown).
Table 2.
Spearman partial correlation coefficients* among retinol binding protein-4 markers and other biomarkers in controls, the Nurses’ Health Study.
| Full-length RBP4 (μg/mL) | RBP4-L (μg/mL) | Total RBP4 (μg/mL) | |
|---|---|---|---|
| Full-length RBP4 (μg/mL; n=472) | 1.0 | 0.73† | 0.95† |
| RBP4-L (μg/mL; n=472) | - | 1.0 | 0.89† |
| Total RBP4 (μg/mL; n=472) | - | - | 1.0 |
| HDL-C (mg/dL; n=465) | −0.01 | −0.02 | −0.01 |
| LDL-C (mg/dL; n=461) | 0.03 | 0.06 | 0.05 |
| Total cholesterol (mg/dL; n=465) | 0.07 | 0.11‡ | 0.10‡ |
| Total to HDL-C ratio (n=465) | 0.06 | 0.09 | 0.07 |
| Fasting TG (mg/dL; n=441) | 0.09 | 0.14† | 0.12‡ |
| hsCRP (mg/L; n=461) | 0.01 | 0.06 | 0.03 |
| Glomerular filtration rate§ (n=472) | −0.06 | −0.20† | −0.13† |
| Adiponectin (μg/mL; n=463) | −0.02 | −0.04 | −0.01 |
| Hemoglobin A1c (%; n=465) | 0.02 | 0.04 | 0.03 |
Abbreviations: RBP4, retinol binding protein-4; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triacylglycerol; hsCRP, high-sensitivity C-reactive protein.
Spearman correlation coefficients were adjusted for age at blood draw (yr), body mass index (kg/m2), fasting status (yes, no), smoking status (current smoker, past smoker, non-smoker), menopausal status (yes, no), hormone use (current user, past user, and non-user), physical activity (MET-hrs/week), alcohol use (g/day), family history of heart disease (yes, no), intakes of trans fat, polyunsaturated fat, and whole grains (all in tertiles), and use of aspirin. Glomerular filtration rate was further adjusted except when calculating the correlation coefficient for this variable.
P<0.01
P<0.05
.
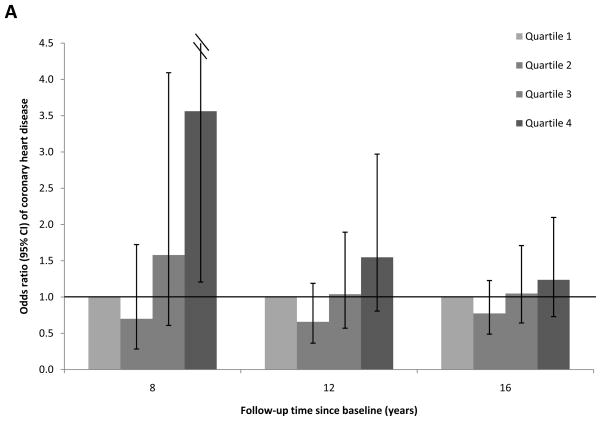
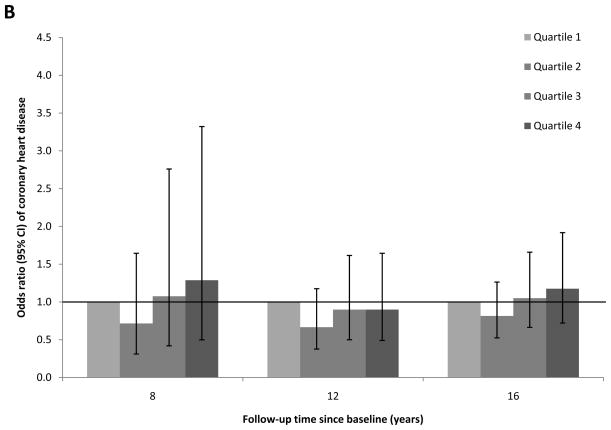
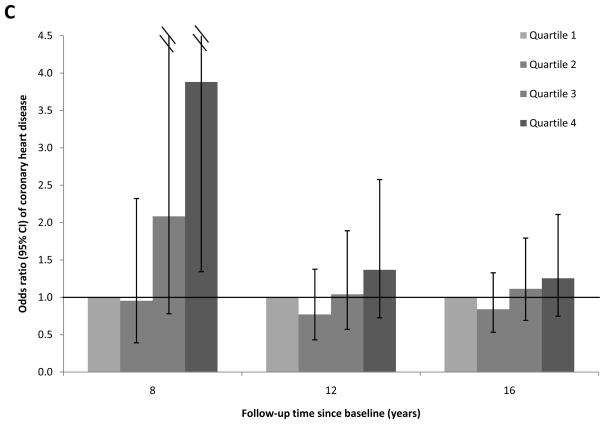
To evaluate the proportional hazard assumption, we first examined the temporality of associations between RBP4 levels and CHD risk over the total follow-up period of 16 years. We found evidence suggesting that the proportional hazard assumption was likely violated (Figure 1; Panels A-C): full-length RBP4 levels were associated with higher CHD risk during the first 8 years of follow-up, whereas this association was gradually attenuated during longer follow-up. A similar pattern was found for total RBP4 levels. In contrast, RBP4-L levels were not associated with CHD risk in any of these follow-up periods. When we formally tested the proportional hazard assumption, the P for interaction was 0.04 for follow-up period (first vs. second 8 years of follow-up) with full-length RBP4 quartiles. Because of this violation of the proportional hazard assumption, we focused on the associations for the first 8 years of follow-up (Table 3a), when the test for proportional hazard assumption was not significant (Pinteraction=0.31).
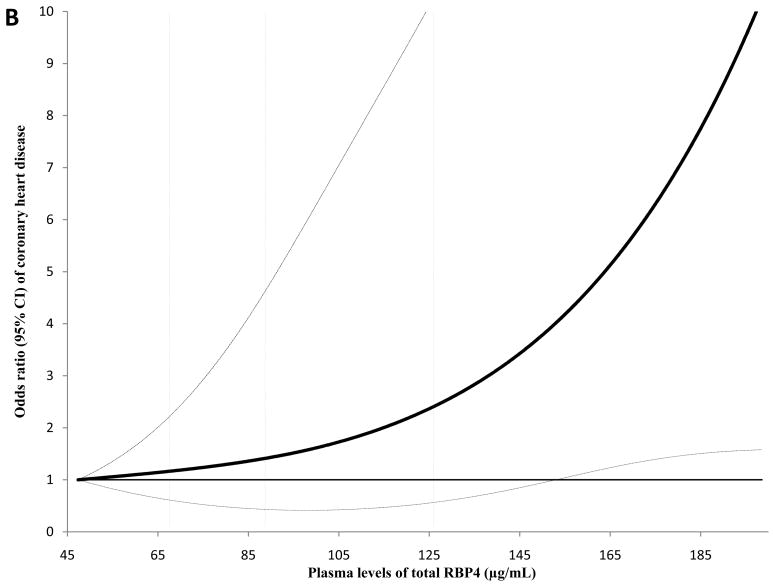
Figure 1.
Association between plasma RBP4 levels and risk of CHD by follow-up times, the Nurses’ Health Study. Odds ratios (95% CI) were adjusted for the same list of covariates listed in the footnote to model 2 in Table 3a. A, full-length RBP4; B, RBP4-L; C, total RBP4.
Table 3a.
Odds ratio (95% CI) of CHD associated with RBP4 levels during the first 8 years of follow-up, the Nurses’ Health Study
| Quartiles of RBP4 levels (μg/mL) | Ptrend | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 (lowest) | 2 | 3 | 4 (highest) | ||
| Full-length RBP4 | |||||
| Median (range) | 37.7 (22.1–47.9) | 55.5 (48.0–62.4) | 72.9 (62.5–85.9) | 106.3 (86.9–217.5) | |
| Case/control | 40/48 | 29/43 | 55/54 | 65/43 | |
| Model 1* | 1.0 | 0.96 (0.48, 1.93) | 1.72 (0.83, 3.56) | 2.92 (1.34, 6.38) | 0.002 |
| Model 2† | 1.0 | 0.70 (0.28, 1.72) | 1.58 (0.61, 4.09) | 3.56 (1.21, 10.51) | 0.003 |
| Model 3‡ | 1.0 | 0.62 (0.19, 2.01) | 1.76 (0.52, 5.98) | 4.45 (1.07, 18.51) | 0.007 |
| RBP4-L | |||||
| Median (range) | 14.3 (5.5–18.1) | 21.3 (18.2–24.9) | 30.4 (25.0–35.6) | 47.0 (36.2–148.0) | |
| Case/control | 43/49 | 35/49 | 58/41 | 53/49 | |
| Model 1* | 1.0 | 0.99 (0.52, 1.88) | 1.94 (0.96, 3.94) | 1.58 (0.79, 3.17) | 0.18 |
| Model 2† | 1.0 | 0.72 (0.31, 1.65) | 1.08 (0.42, 2.76) | 1.29 (0.50, 3.32) | 0.33 |
| Model 3‡ | 1.0 | 0.57 (0.18, 1.82) | 1.40 (0.38, 5.18) | 1.30 (0.33, 5.19) | 0.34 |
| Total RBP4 | |||||
| Median (range) | 54.9 (28.5–67.5) | 79.6 (67.6–88.7) | 107.1 (89.6–126.0) | 158.2 (126.1–350.2) | |
| Case/control | 37/51 | 34/46 | 55/45 | 63/46 | |
| Model 1* | 1.0 | 1.41 (0.70, 2.83) | 2.58 (1.21, 5.50) | 3.21 (1.47, 7.02) | 0.003 |
| Model 2† | 1.0 | 0.95 (0.39, 2.32) | 2.08 (0.78, 5.57) | 3.88 (1.34, 11.21) | 0.003 |
| Model 3‡ | 1.0 | 0.96 (0.30, 3.09) | 2.56 (0.71, 9.22) | 6.62 (1.55, 28.34) | 0.003 |
Model 1 was adjusted for the matching factors: age at blood draw, smoking status (never, past, current), fasting status (yes, no), and time of blood drawing.
Based on model 1, model 2 was further adjusted for body mass index (kg/m2), menopausal status (yes, no), hormone use (current user, past user, and non-user), physical activity (MET-hrs/week), alcohol use (g/day), parental history of MI before age 65 years (yes, no), use of aspirin (yes, no), glomerular filtration rate, intakes of whole grains, trans fat, and polyunsaturated fat (in tertiles), and history of hypercholesterolemia, diabetes, or hypertension (yes, no).
Based on model 2, model 3 was further adjusted for total cholesterol (mg/dL), triacylglycerol levels (mg/dL), high-sensitivity C-reactive protein (mg/L), hemoglobin A1c (%), and total adiponectin (μg/mL).
Characteristics of case-control pairs selected during 0–8 and 9–16 years of follow-up are shown in Supplementary Table 2. Cases occurring during the first 8 years of follow-up had significantly higher baseline levels of full-length and total RBP4 levels than controls. When examined as quartiles (Table 3a), women in the highest RBP4 quartile had an OR (95% CI) of 3.56 (1.21, 10.51) compared to those in the lowest quartile, after controlling for CHD risk factors that might act as confounders. Additional adjustment for blood lipids, hsCRP, adiponectin, and HbA1c further strengthened the associations (model 3). In a series of models, we adjusted for all covariates individually to demonstrate the confounding pattern (Supplementary Table 3). Adjustment of diet, use of aspirin, eGFR, physical activity, and history of hypertension and high cholesterol attenuated the association, whereas other covariates confounded the association in the opposite direction with various strengths. RBP4-L levels were not associated with CHD risk in any of these models. Associations for total RBP4 and full-length RBP4 were similar, although in each model the ORs were stronger for total RBP4 than for full-length RBP4. In contrast to the results in the first 8 years of follow-up, none of the RBP4 forms was associated with CHD risk in the second 8 years of follow-up (Table 3b).
Table 3b.
Odds ratio (95% CI) of CHD associated with RBP4 levels during 9–16 years of follow-up, the Nurses’ Health Study.
| Quartiles of RBP4 levels (μg/mL) | Ptrend | ||||
|---|---|---|---|---|---|
|
| |||||
| 1 (lowest) | 2 | 3 | 4 (highest) | ||
| Full-length RBP4 | |||||
| Median (range) | 38.4 (17.8–47.9) | 54.5 (48.0–62.4) | 71.8 (62.7–86.9) | 110.8 (87.0–239.6) | |
| Case/control | 74/70 | 69/75 | 68/64 | 68/75 | |
| Model 1* | 1.0 | 0.87 (0.53, 1.42) | 0.97 (0.57, 1.65) | 0.79 (0.44, 1.42) | 0.49 |
| Model 2† | 1.0 | 0.83 (0.47, 1.47) | 0.90 (0.48, 1.68) | 0.75 (0.38, 1.47) | 0.44 |
| Model 3‡ | 1.0 | 0.99 (0.55, 1.80) | 0.95 (0.49, 1.83) | 0.77 (0.38, 1.56) | 0.42 |
| RBP4-L | |||||
| Median (range) | 14.7 (5.7–18.1) | 21.5 (18.2–25.0) | 29.8 (25.0–36.1) | 50.4 (36.2–163.2) | |
| Case/control | 65/69 | 72/69 | 74/77 | 68/69 | |
| Model 1* | 1.0 | 1.07 (0.67, 1.72) | 1.04 (0.65, 1.68) | 0.99 (0.59, 1.68) | 0.91 |
| Model 2† | 1.0 | 1.00 (0.58, 1.72) | 1.06 (0.64, 1.85) | 1.20 (0.64, 2.26) | 0.53 |
| Model 3‡ | 1.0 | 1.06 (0.58, 1.91) | 1.14 (0.63, 2.07) | 1.24 (0.63, 2.46) | 0.52 |
| Total RBP4 | |||||
| Median (range) | 55.0 (28.6–67.5) | 77.7 (67.6–89.0) | 105.7 (89.7–126.1) | 168.0 (126.2–430.9) | |
| Case/control | 68/67 | 73/72 | 74/73 | 64/72 | |
| Model 1* | 1.0 | 1.00 (0.62, 1.61) | 0.97 (0.59, 1.61) | 0.80 (0.45, 1.43) | 0.41 |
| Model 2† | 1.0 | 0.86 (0.49, 1.50) | 0.91 (0.51, 1.65) | 0.71 (0.36, 1.41) | 0.37 |
| Model 3‡ | 1.0 | 0.92 (0.51, 1.67) | 0.88 (0.47, 1.66) | 0.74 (0.36, 1.50) | 0.39 |
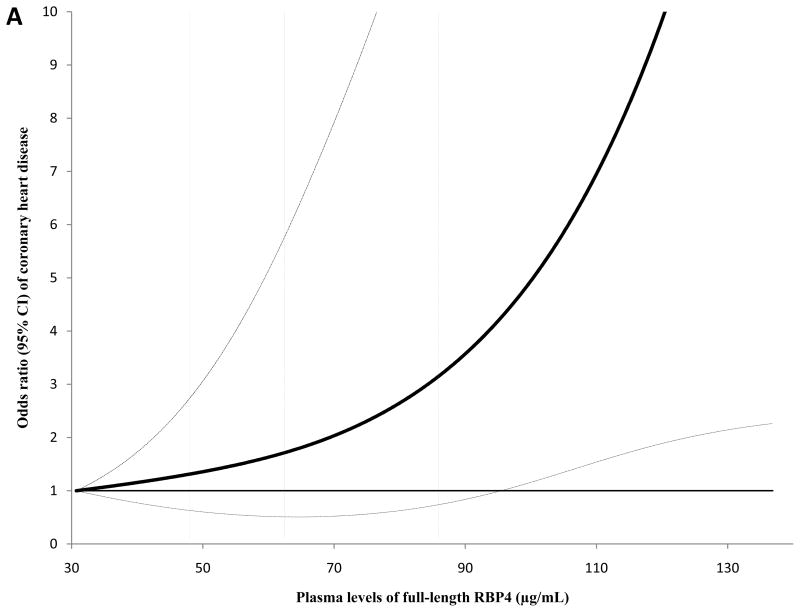
The dose-response relationship between full-length and total RBP4 levels and CHD risk during the first 8 years of follow-up is presented in Figure 2 (Panels A and B). We detected a significant linear association for both full-length RBP4 and total RBP4 levels with CHD; the P for linearity was 0.004 for full-length RBP4 and 0.01 for total RBP4, whereas the P for curvature was ≥0.61 for these two markers. We further estimated that the OR (95% CI) was 2.01 (1.33, 3.04) per standard deviation (SD) change of log-transformed full-length RBP4. This association was 1.65 (1.12, 2.42) for total RBP4 levels. When we included the extreme RBP4 values (top and bottom 5%) in this analysis, we observed similar dose-response relationships (Supplementary Figure; Panels A and B).
Figure 2.
Odds ratio of coronary heart disease (95% CI) by levels of full-length and total RBP4 in the first 8 years of follow-up. In this analysis, study participants with the lowest and highest 5% of RBP4 levels were excluded to minimize potential impact of outliers. Multivariate conditional logistic regression models were adjusted for the same set of covariates for model 2 in Table 3a. Solid lines are ORs and dashed lines are 95% CIs. The dotted vertical lines represent the cut-off points for making quartiles listed in Table 3a. A, full-length RBP4; B, total RBP4.
We performed several sensitivity analyses to examine the robustness of the associations during the first 8 years of follow-up. Among participants who were free of diabetes at baseline, full-length RBP4 levels were associated with higher CHD risk; the OR (95% CI) was 3.41 (1.09, 10.66; Ptrend=0.006) comparing extreme quartiles, and this association was 2.10 (0.74, 5.99; Ptrend=0.09) for RBP4-L levels and 3.84 (1.27, 11.57; Ptrend=0.004) for total RBP4. When we restricted the analysis to participants with eGFR levels ≥60 mL/min, we also observed similar associations: the OR (95% CI) was 3.25 (1.03, 10.29; Ptrend=0.02) for full-length RBP4, 1.21 (0.44, 3.33; Ptrend=0.51) for RBP4-L, and 3.89 (1.23, 12.33; Ptrend=0.02) for total RBP4. Lastly, when we repeated our analysis using definite CHD cases only, the associations did not change materially. The ORs (95% CIs) comparing extreme quartiles of full-length RBP4 levels were 3.04 (1.00, 9.20; Ptrend=0.01) in the first 8 years of follow-up and 0.82 (0.40, 1.70; Ptrend=0.69) in the second 8 years of follow-up.
DISCUSSION
In this prospective case-control study, full-length and total RBP4 levels were associated with CHD risk in a time-dependent manner in that these markers were strongly associated with a monotonically-increased risk of CHD during the first 8 years of follow-up, but associations were attenuated during longer follow-up. RBP4-L, the major truncated form of RBP4, was not associated with CHD risk in this population. These associations were largely independent of established cardiovascular risk factors, including lifestyle practices, diet, medical history, and CVD risk markers.
There have been few prospective investigations examining RBP4 levels and risk of incident CHD. In the only prior prospective investigation conducted among ~3,000 men and women living in the UK, total serum RBP4 levels measured using a particle-enhanced immunonephelometry assay were not associated with incident CHD after controlling for other CVD risk factors.28 This study was similar to ours in study design and participants’ age and race. Therefore, the inconsistency between these two studies may be due to other factors, such as the different methodology for measuring RBP4 levels.
Use of a novel mass spectrometry immunoassay to separate different isoforms of RBP4 is a strength of the current study. Although the biology of different isoforms of RBP4 is largely unknown, the current study suggests that full-length RBP4 may be the most biologically active isoform of RBP4 because this marker, but not the truncated isoforms, was strongly associated with CHD risk. On the other hand, levels of RBP4-L, but not full-length RBP4, were significantly correlated with fasting TG and TC levels in the current study, although the difference between the correlations was rather small. In this study, we cannot examine whether insulin resistance may explain the associations for full-length RBP4 because we do not have direct measurements of insulin resistance, such as the homeostasis model of assessment (HOMA) indices or euglycemic clamp data. Moreover, it is not surprising that full-length RBP4 levels were not associated with HbA1c as previous studies showed that serum RBP4 was much more tightly linked to insulin resistance than to glycemia per se.4 The two minor truncated forms of RBP4, i.e., RBP4-LL and RBP4-RNLL, were not detectable for most participants in our study, nor in a previous investigation,18 making it difficult to evaluate these forms in relation to disease outcomes in human studies. Nonetheless, more data are needed to elucidate the biology of individual isoforms of RBP4 with respect to CVD etiology. It is of particular interest to understand whether the expression levels of individual isoforms of RBP4 are different in adipose tissue and liver, and whether such a difference may explain the association between these isoforms and disease outcomes in humans.
In addition to the use of a novel assay, our investigation has several other strengths, including high follow-up rates, well-phenotyped clinical CHD, adjustment for a wide array of covariates, and rigorous quality control of laboratory procedures. Meanwhile, a few limitations of the current analysis are worth discussing. First, the study participants are all female nurses, and most are Caucasians. Therefore, it is unknown whether the results can be generalized to other races or populations with different characteristics. In addition, because of possible gender-differences in RBP4 metabolism,37 whether these results pertain to men is unclear. Second, although we controlled for many established CVD risk factors to reduce confounding, we cannot exclude the possibility of residual confounding. Of note, blood RBP4 levels increase among patients of renal insufficiency because of impaired renal clearance of RBP4.38, 39 Therefore, diseases that impair renal function, such as renal failure and diabetes, may confound the associations for RBP4, especially for the truncated isoforms.40 However, in the current analysis no participants were diagnosed with renal failure at baseline. When we excluded prevalent diabetes cases or restricted analyses to those with normal eGFR, we observed similar associations. Moreover, we controlled for eGFR and HbA1c levels in the analysis to minimize confounding by these conditions. Third, we measured only baseline RBP4 levels. A one-time measurement may not necessarily represent long-term average levels of RBP4, as RBP4 levels may change as a consequence of weight loss and possibly other factors.7, 41 This may potentially explain the observed attenuated association between RBP4 levels and CHD risk during extended follow-up (> 8 years). On the other hand, our pilot study for examining within-person stability of RBP4 levels demonstrated that the ICC was 0.80 for total RBP4 levels among 2–3 blood samples collected from 45 volunteers during a timeframe of 1–3 years, indicating that a single measurement of RBP4 level may reasonably represent average RBP4 levels over several years (unpublished data). Fourth, RBP4 molecules might possibly degrade to some extent after long-term storage, although this scenario was unlikely as the samples were stored at such a low temperature (≤−130°C). In addition, such degradation, if any, should influence all samples (both cases and controls) non-differentially, and, therefore, would likely attenuate true associations. Fifth, levels of transthyretin, a molecule that binds RBP4 and prevents its renal clearance,42 are not available. Therefore, we are unable to examine the role of transthyretin in the association between RBP4 and CHD. Previous studies have suggested positive associations between transthyretin and CVD risk factors,43 and transthyretin was modestly elevated in insulin-resistant people.5 More studies are warranted to explore the role of transthyretin in the associations of RBP4. Lastly, we cannot entirely exclude the possibility that the time-dependent associations are due to chance, more data are needed to elucidate the temporality of the association between RBP4 levels and CHD risk.
In conclusion, we found that full-length RBP4 levels were associated with a 3-fold increased risk of incident CHD in women, whereas truncated isoforms of RBP4 were not associated with CHD risk in this population. While we have found evidence that RBP4 may play a role in the development of CHD, and that different isoforms of RBP4 may not have the same biological effects, further studies are warranted to confirm these novel findings.
Supplementary Material
Clinical Perspective.
Evidence from animal experiments has clearly demonstrated a causal role of retinol-binding protein 4 (RBP4), a cytokine secreted by adipocytes and hepatocytes, in the etiology of insulin resistance. Although emerging data from human studies also suggest a link between this molecule and glucose intolerance, as well as many other cardiovascular risk factors, prospective studies examining RBP4 levels in relation to coronary heart disease (CHD) are few. With recent advances in the methodology of measuring RBP4 levels, investigators are now able to distinguish different forms of RBP4 that may have various biological activities and thus are likely associated with CHD risk differentially. Using data from the well-characterized female Nurses’ Health Study, we measured full-length and truncated forms of RBP4 and prospectively evaluated their associations with CHD risk in a nested case-control study. We found an interesting temporal pattern in the associations under investigation. After multivariate adjustment of established CHD risk factors, levels of the full-length RBP4 were significantly associated with elevated risk of CHD during the first 8 years of follow-up, whereas this association was gradually attenuated thereafter. The truncated forms of RBP4 were not associated with CHD in this investigation, and no temporal pattern was observed for these forms. These novel data support the notion that the full-length RBP4 is the most biologically-active form predicting future CHD events, although future studies are warranted to corroborate the temporal pattern of this association documented in the current study.
Acknowledgments
The authors are grateful to Dr. Mary Townsend for her contributions to the quality control and pilot studies of the RBP4 assay and her help with other laboratory analytical issues.
Funding Sources: This study was supported by research grants CA87969, HL34594, HL35464, CA55075, HL088521, and DK43051 from the National Institutes of Health. Dr. Sun is supported by a career development award K99HL098459 from the National Heart, Lung, and Blood Institute. Dr. Hu is a recipient of an American Heart Association Established Investigator Award.
Footnotes
Conflict of Interest Disclosures: Dr. Kiernan and Mr. Phillips are employees of Thermo Fisher Scientific Inc. and were paid to perform the laboratory assays of RBP4 levels. Dr. Kahn was the recipient of a research grant from Takeda Pharmaceutical Company. Dr. Kahn is also an inventor on a patent application related to RBP4.
References
- 1.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 2.Cho YM, Youn BS, Lee H, Lee N, Min SS, Kwak SH, Lee HK, Park KS. Plasma retinol-binding protein-4 concentrations are elevated in human subjects with impaired glucose tolerance and type 2 diabetes. Diabetes Care. 2006;29:2457–2461. doi: 10.2337/dc06-0360. [DOI] [PubMed] [Google Scholar]
- 3.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in nonobese subjects without type 2 diabetes. J Clin Endocrinol Metab. 2007;92:1886–1890. doi: 10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 4.Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, Kahn BB. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- 5.Kloting N, Graham TE, Berndt J, Kralisch S, Kovacs P, Wason CJ, Fasshauer M, Schon MR, Stumvoll M, Bluher M, Kahn BB. Serum retinol-binding protein is more highly expressed in visceral than in subcutaneous adipose tissue and is a marker of intra-abdominal fat mass. Cell Metab. 2007;6:79–87. doi: 10.1016/j.cmet.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Qi Q, Yu Z, Ye X, Zhao F, Huang P, Hu FB, Franco OH, Wang J, Li H, Liu Y, Lin X. Elevated retinol-binding protein 4 levels are associated with metabolic syndrome in chinese people. J Clin Endocrinol Metab. 2007;92:4827–4834. doi: 10.1210/jc.2007-1219. [DOI] [PubMed] [Google Scholar]
- 7.Tschoner A, Sturm W, Engl J, Kaser S, Laimer M, Laimer E, Weiss H, Patsch JR, Ebenbichler CF. Retinol-binding protein 4, visceral fat, and the metabolic syndrome: Effects of weight loss. Obesity (Silver Spring) 2008;16:2439–2444. doi: 10.1038/oby.2008.391. [DOI] [PubMed] [Google Scholar]
- 8.Perseghin G, Lattuada G, De Cobelli F, Esposito A, Belloni E, Canu T, Ragogna F, Scifo P, Del Maschio A, Luzi L. Serum retinol-binding protein-4, leptin, and adiponectin concentrations are related to ectopic fat accumulation. J Clin Endocrinol Metab. 2007;92:4883–4888. doi: 10.1210/jc.2007-0325. [DOI] [PubMed] [Google Scholar]
- 9.Takebayashi K, Suetsugu M, Wakabayashi S, Aso Y, Inukai T. Retinol binding protein-4 levels and clinical features of type 2 diabetes patients. J Clin Endocrinol Metab. 2007;92:2712–2719. doi: 10.1210/jc.2006-1249. [DOI] [PubMed] [Google Scholar]
- 10.Silha JV, Nyomba BL, Leslie WD, Murphy LJ. Ethnicity, insulin resistance, and inflammatory adipokines in women at high and low risk for vascular disease. Diabetes Care. 2007;30:286–291. doi: 10.2337/dc06-1073. [DOI] [PubMed] [Google Scholar]
- 11.Takashima N, Tomoike H, Iwai N. Retinol-binding protein 4 and insulin resistance. N Engl J Med. 2006;355:1392. doi: 10.1056/NEJMc061863. author reply 1394–1395. [DOI] [PubMed] [Google Scholar]
- 12.von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, Humpert PM. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- 13.van Hoek M, Dehghan A, Zillikens MC, Hofman A, Witteman JC, Sijbrands EJ. An rbp4 promoter polymorphism increases risk of type 2 diabetes. Diabetologia. 2008;51:1423–1428. doi: 10.1007/s00125-008-1042-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meisinger C, Ruckert IM, Rathmann W, Doring A, Thorand B, Huth C, Kowall B, Koenig W. Retinol-binding protein 4 is associated with prediabetes in adults from the general population: The cooperative health research in the region of augsburg (kora) f4 study. Diabetes Care. 2011;34:1648–1650. doi: 10.2337/dc11-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ingelsson E, Sundstrom J, Melhus H, Michaelsson K, Berne C, Vasan RS, Riserus U, Blomhoff R, Lind L, Arnlov J. Circulating retinol-binding protein 4, cardiovascular risk factors and prevalent cardiovascular disease in elderly. Atherosclerosis. 2009;206:239–244. doi: 10.1016/j.atherosclerosis.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 16.Tan BK, Chen J, Lehnert H, Kennedy R, Randeva HS. Raised serum, adipocyte, and adipose tissue retinol-binding protein 4 in overweight women with polycystic ovary syndrome: Effects of gonadal and adrenal steroids. J Clin Endocrinol Metab. 2007;92:2764–2772. doi: 10.1210/jc.2007-0091. [DOI] [PubMed] [Google Scholar]
- 17.Lee JW, Im JA, Lee HR, Shim JY, Youn BS, Lee DC. Visceral adiposity is associated with serum retinol binding protein-4 levels in healthy women. Obesity (Silver Spring) 2007;15:2225–2232. doi: 10.1038/oby.2007.264. [DOI] [PubMed] [Google Scholar]
- 18.Yang Q, Eskurza I, Kiernan UA, Phillips DA, Bluher M, Graham TE, Kahn BB. Quantitative measurement of full-length and c-terminal proteolyzed rbp4 in serum of normal and insulin-resistant humans using a novel mass spectrometry immunoassay. Endocrinology. 2012;153:1519–1527. doi: 10.1210/en.2011-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hutchison SK, Harrison C, Stepto N, Meyer C, Teede HJ. Retinol-binding protein 4 and insulin resistance in polycystic ovary syndrome. Diabetes Care. 2008;31:1427–1432. doi: 10.2337/dc07-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balagopal P, Graham TE, Kahn BB, Altomare A, Funanage V, George D. Reduction of elevated serum retinol binding protein in obese children by lifestyle intervention: Association with subclinical inflammation. J Clin Endocrinol Metab. 2007;92:1971–1974. doi: 10.1210/jc.2006-2712. [DOI] [PubMed] [Google Scholar]
- 21.Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ, 3rd, Rashidi AA, McGehee RE, Jr, Fried SK, Kern PA. Retinol binding protein 4 expression in humans: Relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barazzoni R, Zanetti M, Semolic A, Pirulli A, Cattin MR, Biolo G, Bosutti A, Panzetta G, Bernardi A, Guarnieri G. High plasma rbp4 is associated with systemic inflammation independently of low rbp4 adipose expression and is normalized by transplantation in non-obese, non-diabetic patients with chronic kidney disease. Clin Endocrinol (Oxf) 2011;75:56–63. doi: 10.1111/j.1365-2265.2011.03990.x. [DOI] [PubMed] [Google Scholar]
- 23.Solini A, Santini E, Madec S, Rossi C, Muscelli E. Retinol-binding protein-4 in women with untreated essential hypertension. Am J Hypertens. 2009;22:1001–1006. doi: 10.1038/ajh.2009.116. [DOI] [PubMed] [Google Scholar]
- 24.Bobbert T, Raila J, Schwarz F, Mai K, Henze A, Pfeiffer AF, Schweigert FJ, Spranger J. Relation between retinol, retinol-binding protein 4, transthyretin and carotid intima media thickness. Atherosclerosis. 2010;213:549–551. doi: 10.1016/j.atherosclerosis.2010.07.063. [DOI] [PubMed] [Google Scholar]
- 25.Ingelsson E, Lind L. Circulating retinol-binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. doi: 10.2337/dc08-1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cabre A, Lazaro I, Girona J, Manzanares J, Marimon F, Plana N, Heras M, Masana L. Retinol-binding protein 4 as a plasma biomarker of renal dysfunction and cardiovascular disease in type 2 diabetes. J Intern Med. 2007;262:496–503. doi: 10.1111/j.1365-2796.2007.01849.x. [DOI] [PubMed] [Google Scholar]
- 27.Goodman E, Graham TE, Dolan LM, Daniels SR, Goodman ER, Kahn BB. The relationship of retinol binding protein 4 to changes in insulin resistance and cardiometabolic risk in overweight black adolescents. J Pediatr. 2009;154:67–73. e61. doi: 10.1016/j.jpeds.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mallat Z, Simon T, Benessiano J, Clement K, Taleb S, Wareham NJ, Luben R, Khaw KT, Tedgui A, Boekholdt SM. Retinol-binding protein 4 and prediction of incident coronary events in healthy men and women. J Clin Endocrinol Metab. 2009;94:255–260. doi: 10.1210/jc.2008-0253. [DOI] [PubMed] [Google Scholar]
- 29.Graham TE, Wason CJ, Bluher M, Kahn BB. Shortcomings in methodology complicate measurements of serum retinol binding protein (rbp4) in insulin-resistant human subjects. Diabetologia. 2007;50:814–823. doi: 10.1007/s00125-006-0557-0. [DOI] [PubMed] [Google Scholar]
- 30.Kiernan UA, Tubbs KA, Nedelkov D, Niederkofler EE, Nelson RW. Comparative phenotypic analyses of human plasma and urinary retinol binding protein using mass spectrometric immunoassay. Biochem Biophys Res Commun. 2002;297:401–405. doi: 10.1016/s0006-291x(02)02212-x. [DOI] [PubMed] [Google Scholar]
- 31.Kiernan UA, Phillips DA, Trenchevska O, Nedelkov D. Quantitative mass spectrometry evaluation of human retinol binding protein 4 and related variants. PLoS One. 2011;6:e17282. doi: 10.1371/journal.pone.0017282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rose G, Blackburn H. Cardiovascular survey method. Geneva, Switzerland: World Health Organization; 1982. Monograph Series No. 56. [Google Scholar]
- 33.Stampfer MJ, Willett WC, Speizer FE, Dysert DC, Lipnick R, Rosner B, Hennekens CH. Test of the national death index. Am J Epidemiol. 1984;119:837–839. doi: 10.1093/oxfordjournals.aje.a113804. [DOI] [PubMed] [Google Scholar]
- 34.Lin J, Fung TT, Hu FB, Curhan GC. Association of dietary patterns with albuminuria and kidney function decline in older white women: A subgroup analysis from the nurses’ health study. Am J Kidney Dis. 2011;57:245–254. doi: 10.1053/j.ajkd.2010.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rothman KJ, Greenland S. Modern epidemiology. Philadelphia: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 36.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–561. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 37.Kotnik P, Fischer-Posovszky P, Wabitsch M. Rbp4: A controversial adipokine. Eur J Endocrinol. 2011;165:703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- 38.Henze A, Frey SK, Raila J, Tepel M, Scholze A, Pfeiffer AF, Weickert MO, Spranger J, Schweigert FJ. Evidence that kidney function but not type 2 diabetes determines retinol-binding protein 4 serum levels. Diabetes. 2008;57:3323–3326. doi: 10.2337/db08-0866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akbay E, Muslu N, Nayir E, Ozhan O, Kiykim A. Serum retinol binding protein 4 level is related with renal functions in type 2 diabetes. J Endocrinol Invest. 2010;33:725–729. doi: 10.1007/BF03346678. [DOI] [PubMed] [Google Scholar]
- 40.Frey SK, Nagl B, Henze A, Raila J, Schlosser B, Berg T, Tepel M, Zidek W, Weickert MO, Pfeiffer AF, Schweigert FJ. Isoforms of retinol binding protein 4 (rbp4) are increased in chronic diseases of the kidney but not of the liver. Lipids Health Dis. 2008;7:29. doi: 10.1186/1476-511X-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hammarstedt A, Pihlajamaki J, Graham TE, Kainulainen S, Kahn BB, Laakso M, Smith U. High circulating levels of rbp4 and mrna levels of ap2, pgc-1alpha and ucp-2 predict improvement in insulin sensitivity following pioglitazone treatment of drug-naive type 2 diabetic subjects. J Intern Med. 2008;263:440–449. doi: 10.1111/j.1365-2796.2007.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naylor HM, Newcomer ME. The structure of human retinol-binding protein (rbp) with its carrier protein transthyretin reveals an interaction with the carboxy terminus of rbp. Biochemistry. 1999;38:2647–2653. doi: 10.1021/bi982291i. [DOI] [PubMed] [Google Scholar]
- 43.Yoshida A, Matsutani Y, Fukuchi Y, Saito K, Naito M. Analysis of the factors contributing to serum retinol binding protein and transthyretin levels in japanese adults. J Atheroscler Thromb. 2006;13:209–215. doi: 10.5551/jat.13.209. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.