Abstract
Context
Cognitive rehabilitation has shown efficacy for improving cognition in patients with schizophrenia, but the underlying neurobiologic changes that occur during these treatments and support cognitive improvement are not well known.
Objective
To examine differential changes in brain morphology in early schizophrenia during cognitive rehabilitation versus supportive therapy.
Design
A 2-year, randomized-controlled trial with annual structural magnetic resonance imaging and cognitive assessments.
Setting
An outpatient research clinic at a university-based medical center providing comprehensive care services for patients with severe mental illness.
Patients
A total of 53 symptomatically stable, but cognitively disabled outpatients in the early course of schizophrenia or schizoaffective disorder.
Interventions
Cognitive enhancement therapy is an integrated approach to the remediation of cognitive impairments in schizophrenia that utilizes computer-assisted neurocognitive training and group-based social-cognitive exercises. Enriched supportive therapy is an illness management approach that provides psychoeducation and teaches applied coping strategies.
Main Outcome Measures
Broad areas of frontal and temporal gray matter change were analyzed using longitudinal voxel-based morphometry methods employing mixed-effects models, followed by volumetric analyses of regions demonstrating significant differential changes between treatment groups.
Results
Patients receiving cognitive enhancement therapy demonstrated significantly greater preservation of gray matter volume over the course of two years in the left hippocampus, parahippocampal gyrus, and fusiform gyrus, and significantly greater gray matter increases in the left amygdala (all corrected P < .040), compared with those receiving enriched supportive therapy. Less gray matter loss in the left parahippocampal and fusiform gyrus, and greater gray matter increases in the left amygdala were significantly related to improved cognition and mediated the beneficial cognitive effects of cognitive enhancement therapy.
Conclusion
Cognitive enhancement therapy may offer neurobiologic protective and enhancing effects in early schizophrenia that are associated with improved long-term cognitive outcomes.
Trial Registration
clinicaltrials.gov Identifier: NCT00167362
Schizophrenia is characterized by marked impairments in cognition,1,2 which place profound limitations on functional recovery.3,4,5 Evidence is increasingly accumulating that a variety of neurobiologic abnormalities contribute to cognitive impairment in the disorder. Progressive loss of gray matter,6 frontal hypofunction,7,8 and decreased white matter integrity,9,10 have been consistently observed in schizophrenia patients. Fronto-temporal dysfunction and gray matter loss in the prefrontal cortex, anterior cingulate, hippocampus, and superior temporal gyrus have all been linked to neurocognitive impairments in memory and executive function processes.11,12,13,14,15 Likewise, abnormalities in medial-temporal and medial-frontal brain networks, including the amygdala, fusiform gyrus, and orbitofrontal cortex have been implicated in social-cognitive impairments in perspective-taking, emotion perception, and foresightfulness.16,17 Given the growing appreciation of the central importance of cognitive impairments and their underlying neurobiologic mechanisms in schizophrenia, there is great interest in developing novel therapeutics that preserve or restore cognitive and brain function in the disorder.18
To date, the neurobiologically-based impairments in cognition observed in schizophrenia have had a limited response to pharmacotherapy,19,20 at the cost of continued disability.21 In contrast, psychosocial cognitive rehabilitation programs have emerged as effective methods for remediating the cognitive impairments in schizophrenia that limit functional recovery.22,23 A recent meta-analytic review of all randomized-controlled trials of cognitive remediation for individuals with schizophrenia found that on average, patients who participate in these programs experience nearly 4/10 of a standard deviation improvement in neurocognitive function, with modest improvements also seen in functioning and psychopathology.24 Those programs that provide more comprehensive integration with other psychosocial components beyond neurocognitive rehabilitation also showed greater effects on functioning. We have previously demonstrated that an integrated neurocognitive and social-cognitive rehabilitation program known as Cognitive Enhancement Therapy (CET25) can produce strong (Cohen’s d > 1.00) and lasting improvements in cognition and functioning among chronic patients who have had schizophrenia for many years.22,26 Further, very recently we provided evidence indicating that the cognitive and functional benefits of CET can be extended to individuals in the early course of the disorder, possibly capitalizing on a greater neurobiologic reserve in the first several years of the illness.27 After two years of treatment, young early course individuals receiving CET demonstrated substantial improvements in social and non-social cognition which ultimately translated into significant functional gains in employment, social functioning, major role adjustment and activities of daily living.28
Although the methods employed in CET and other cognitive rehabilitation programs are psychosocial in nature, improvements in cognition presumably produce associated neurobiological changes,29 thus the gains in neurocognitive and social-cognitive functioning in schizophrenia observed during cognitive rehabilitation could result in measurable changes in the brain. Furthermore, given that progressive neurobiologic deterioration has been observed in the disorder,6 cognitive rehabilitation might be best applied in the earliest phases of the illness to capitalize on a presumed neurobiologic and neuroplasticity reserve and protect against future neurobiological decline.29 Animal studies have repeatedly shown the ability of the brain to reorganize itself in response to environmental experiences,30 and previous work conducted with children with dyslexia supports the notion that cognitive training can induce a positive neurobiologic response.31 To date, only two published studies have examined the neurobiological effects of cognitive rehabilitation in schizophrenia. Wykes and colleagues32 studied the 3-month effects of cognitive rehabilitation in 12 patients with chronic schizophrenia using functional magnetic resonance imaging, and found significant increases in frontocortical activation among patients who received the treatment. However, potential associations of these functional changes with cognitive improvement were not examined. In addition, Wexler and colleagues found increased activation in the inferior frontal cortex after 10 weeks of verbal memory training in 8 patients with chronic schizophrenia, which was associated with verbal memory improvement.33 No studies have examined the long-term neurobiological effects of cognitive rehabilitation in early schizophrenia, or the association of these effects with cognitive changes that occur during early course treatment.
In this study we sought to characterize changes in brain morphology in a sample of early course patients previously reported on in a two-year randomized controlled trial of CET or an Enriched Supportive Therapy (EST) control,28 and examine the associations between brain structural and cognitive changes, in an effort to identify the potential neurobiological effects of cognitive rehabilitation in early schizophrenia. It was hypothesized that CET would exert a neuroprotective effect against gray matter loss in regions implicated in neurocognitive and social-cognitive impairment, and that these effects would be associated with better cognitive outcomes and mediate the previously demonstrated effects of CET on cognition.27
Method
Participants
Participants included 53 individuals in the early course of schizophrenia (n = 35) or schizoaffective disorder (n = 18) participating in a two-year randomized-controlled trial of CET. Patients were included in this trial if they were stabilized on antipsychotic medications and had a diagnosis of schizophrenia, schizoaffective, or schizophreniform disorder as assessed using the Structured Clinical Interview for DSM-IV,34 experienced their first psychotic symptom (including duration of untreated psychosis) within the past 8 years, had an IQ > 80, were not abusing substances for at least 2 months prior to study enrollment, and exhibited significant social and cognitive disability on the Cognitive Style and Social Cognition Eligibility Interview.22
Enrolled participants had an average age of 26.17 (SD = 6.51) years, two-thirds (n = 35) were male, and most were Caucasian (n = 36) or African American (n = 10). The majority (77%) of patients experienced their first psychotic symptom within the past 5 years, and on average, individuals had been ill for 3.22 (SD = 2.2) years since their first psychotic symptom. Most patients had completed some college education (n = 39), although the majority were not employed at study baseline (n = 38).
Treatments
Medications
All participants were maintained on antipsychotic medication for the treatment of schizophrenia or schizoaffective disorder as prescribed by a study psychiatrist. The majority of participants received atypical antipsychotic medications (n = 52), and were seen at least biweekly by a psychiatric clinical nurse specialist to evaluate efficacy, tolerability, and compliance. There were no significant differences in medication dose or clinician-estimated medication compliance between treatment groups at any point during the course of the study (see Table S1).
Cognitive Enhancement Therapy
Cognitive Enhancement Therapy (CET25) is an integrated, developmental approach to the remediation of social and non-social cognitive deficits in schizophrenia. The treatment consists of 60 hours of weekly computer-based neurocognitive training in attention, memory, and problem-solving using software developed by Ben-Yishay35 and Bracy,36 coupled with 45 weekly social-cognitive group sessions designed to address the key social-cognitive deficits that limit functional recovery from schizophrenia. Patients begin CET by first receiving a comprehensive neuropsychological assessment, and then meeting individually with a CET coach to develop a therapeutic alliance, review results of neuropsychological testing, and develop an initial treatment plan reflective of each patient’s goals for treatment. Neurocognitive training then proceeds in a hierarchical fashion, beginning with Ben-Yishay’s Orientation Remediation Module to improve aspects of attention and speed of processing, followed by addressing higher-order neurocognitive abilities in the domains of memory and executive functioning with Bracy’s PSSCogReHab software. To promote socialization and reinforce social-cognitive abilities that are the focus of the group curriculum, neurocognitive training is conducted in patient pairs with the assistance of a CET therapist/coach. After approximately 3 months of neurocognitive training in attention, 3 to 4 patient pairs come together to form a social-cognitive group. These groups provide patients with the necessary secondary socialization and experiential learning opportunities to develop a variety of social-cognitive abilities. Critical components of social cognition are addressed including perspective-taking, social gist abstraction, non-verbal communication, emotion management, and foresightfulness. The group curriculum includes both innovative cognitive exercises and psychoeducation that foster the development of social-cognitive abilities and effective social interaction. Generalization to “real world” settings is an explicit goal of CET, and is promoted through weekly homework assignments and individual coaching sessions tailored to the unique needs of the patient. CET integrates neurocognitive computer-based training with the social-cognitive group sessions to provide patients with a comprehensive approach to the remediation of cognitive deficits in schizophrenia. A complete description of the treatment can be found in the CET training manual.25
Enriched Supportive Therapy
Enriched Supportive Therapy (EST) is an individual psychotherapy approach that fosters illness management through psychoeducation and applied coping skills. The approach is based on components of the basic and intermediate phases of the demonstrably effective Personal Therapy.37 In EST, patients meet individually with a therapist to learn and practice a variety of stress reduction and illness management techniques designed to forestall relapse and enhance adjustment to the illness. The EST approach is designed to be sensitive to the patient’s stage of recovery, and divided into two phases, The first “basic” phase focuses on psychoeducation about schizophrenia, the role of stress in the disorder and symptom exacerbation, and introduces basic coping strategies to minimize and/or avoid stress in one’s life. The second “intermediate” phase of the treatment advances to a personalized approach toward the identification of early cues of distress and the application of healthy coping strategies to enhance adjustment. By tailoring the treatment to the patient’s stage of recovery, EST allows individuals to move through the phases of the treatment at their own pace. In the basic phase, patients meet weekly with a therapist, and in the intermediate phase, treatment is provided on a biweekly basis, although more frequent sessions were available if needed. EST components on illness and stress management were also made available to patients receiving CET through the social-cognitive group curriculum. No attempt was made to match the number of sessions or hours of treatment between CET and EST, and while individuals treated with CET did by design receive more hours of treatment, adherence, as defined by the percentage of scheduled sessions missed, was similar for both interventions (see Table S1).
Image Acquisition and Processing
Structural magnetic resonance images (MRI) were acquired from most patients using a 3-T Signa whole body scanner and head coil (GE Medical Systems, Milwaukee, WI), although a small proportion of patients (n = 7) received two-year scans on a 3-T Siemens whole body scanner and head coil (Siemens, Erhlangen, Germany). Structural MRI acquisition was identical between scanners and whole brain volume was acquired in 124 1.5mm-thick contiguous coronal slices with spoiled gradient recalled acquisition in steady state pulse sequence (TE = 5ms, TR = 25ms, acquisition matrix = 256 × 192, FOV = 24cm). After acquisition and initial quality control, images were normalized to standard MNI space and segmented into gray, white, and cerebrospinal fluid compartments using the unified segmentation algorithm based on a MNI template of adult brains in the Statistical Parametric Mapping software, version 5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London, UK).38 Segmented images were then smoothed using a 12mm Gaussian kernel, and radio frequency inhomogeneity artifacts were corrected during image post-processing using a bias correction algorithm built into the segmentation procedure. As this is the first study to examine the neuroanatomical effects of cognitive rehabilitation in schizophrenia, broad regions of interest (ROI) were specified a priori based on previous literature on the neurobiologic correlates of cognitive dysfunction in schizophrenia,11,17 and included amygdala, caudate, cingulate gyrus, dorsolateral prefrontal cortex, fusiform gyrus, hippocampus, parahippocampal gyrus, putamen, and superior temporal gyrus gray matter. Regions of interest were defined using the Wake Forest University PickAtlas toolbox for SPM5,39 with regional definitions outlined by Tzourio-Mazoyer and colleagues.40
Measures
Composite measures of general neurocognition and social cognition were included to assess the relationship between neurobiological and cognitive change during the two years of treatment. Individual measures used to construct these composites have been described in detail elsewhere.28 Briefly, a comprehensive neuropsychological testing battery was used to construct the general neurocognitive composite, which included immediate and delayed recall of stories A and B from the Revised Wechsler Memory Scale;41 List A total recall, as well as short-term and long-term free recall scores from California Verbal Learning Test;42 digit-span, vocabulary, picture arrangement, and digit symbol scores from the Revised Wechsler Adult Intelligence Scale;43 Trails B time to completion;44 categories achieved, perseverative and non-perseverative errors, and percentage of conceptual-level responses from the Wisconsin Card Sorting Test;45 total move score and ratio of initiation to execution time from the Tower of London;46 and cognitive-perceptual and repetition-motor neurological soft sign scores from the Neurological Evaluation Scale.47 These domains are reflective of those outlined by the NIMH-MATRICS committee as critical targets for cognitive enhancing treatments in schizophrenia.48 We found the internal consistency of this neurocognitive composite containing 18 variables from the aforementioned measures to be excellent (α = .88).
The social cognition composite included a purposively broad array of social-cognitive measures. These included the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT49), which has subsequently been recommended by the NIMH-MATRICS committee for the assessment of social cognition in schizophrenia,48 as well as two interview-based measures of social cognition, the Social Cognition Profile and the Cognitive Style and Social Cognition Eligibility Interview, which were developed in previous studies of CET.22 The MSCEIT is a 141-item, computer administered performance-based measure of emotion processing and management that has shown good psychometric properties in patients with schizophrenia.50,51 The Social Cognition Profile is a 50-item clinician-rated measure of social-cognitive behaviors gleaned from the literature on social cognition.52,53,54,55 The measure is based on Selman’s53 hierarchical stages of social-cognitive development and includes the domains of perceptive, supportive, tolerant, and self-confident behaviors indicative of adequate social cognition. The Cognitive Style and Social Cognition Eligibility Interview is a semi-structured interview designed, in part, to capture functional disability relevant to impaired social cognition and covers five broad domains, including lack of foresight, social gist extraction deficits, interpersonal ineffectiveness, vocational ineffectiveness, and difficulty adjusting to disability. Previous psychometric studies have indicated good inter-rater, test-retest, and internal reliability for both of these measures.22 We found the internal consistency of this social cognition composite, which consisted of 12 variables from the aforementioned measures, to be acceptable (α = .71).
Procedures
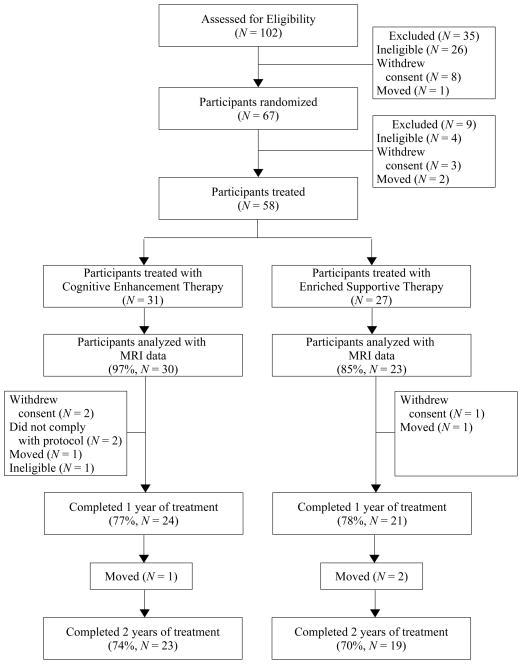
Upon recruitment, participants were randomly assigned by a project statistician to either CET or EST using computer-generated random numbers, and treated for two years in their respective treatment condition. Individuals were then assessed using structural MRI and the aforementioned neurocognitive and social-cognitive measures prior to the initiation of treatment, and annually for the two years of treatment. Initially 67 patients were randomized to a treatment condition, however only 58 received any treatment due to 9 patients moving, withdrawing, or being found ineligible upon further review prior to beginning psychosocial treatment (see Figure 1). Although 58 patients were treated and had cognitive and behavioral data available for analysis structural imaging data were only collected on 53 individuals, as 2 participants were too large to fit into the scanner, 1 had a metal object embedded in his thigh, 1 could not complete the scanning procedure due to anxiety, and 1 withdrew consent before undergoing imaging. While there were no significant differences between those who had imaging data and those who did not with regard to age, illness duration, sex, race, employment status, diagnosis, symptomatology, treatment assignment, or cognitive performance on the neurocognitive and social-cognitive composites; individuals who completed the MRI procedures were significantly more likely to have some college education, χ2(1, N = 58) = 8.14, p = .004. However, there were no significant differences between treatment conditions among individuals with MRI data with regard to demographics, attrition, or symptomatology at baseline (see Table S1). Of those with available imaging data, 8 had only an MRI at baseline, and 8 individuals had only two MRIs (6 with only baseline and year 1 scans, and 2 with only baseline and year 2 scans). Reanalysis excluding individuals with only baseline imaging data did not change the results (see Tables S2 and S3). This research was reviewed annually by the University of Pittsburgh Institutional Review Board, and all patients provided written informed consent prior to participation.
Figure 1.
Two-Year Participant Flow in a Randomized Trial of Cognitive Enhancement Therapy for Early Course Schizophrenia.
Data Analysis
Intent to treat analyses were conducted with all 53 patients who had structural MRI data for at least one study time point and received any exposure to their psychosocial treatment condition. Differential rates of gray matter change between CET and EST were first investigated using voxel-based morphometry employing linear mixed-effects models restricted to the anatomical ROIs outlined above. Significant treatment by time interactions showing differences in linear rates of change in gray matter density were the effects of interest in these models. When testing the significance of over 50,000 voxels in our ROI mask across both brain hemispheres, we used AlphaSim56 to conduct a Monte Carlo simulation based on our imaging parameters and ROIs to estimate a combined voxel-extent and alpha threshold that would maintain the corrected experiment-wise error rate at acceptable levels (p = .05). This approach is more powerful than alpha thresholds alone, as random field theory indicates that effects are less likely to be false positives when they cluster together.56 Taking this into account allows more information than just p-values to judge the veracity of an effect, and consequently, larger clusters of effects can be detected at greater alpha levels without sacrificing the overall experiment-wise error rate. The results of 10,000 different simulations indicated that a combined voxel-extent threshold of 220 voxels and an uncorrected alpha threshold of p = .005 was sufficient to keep the corrected experiment-wise rate at p < .05.
After identifying clusters of differential gray matter change between CET and EST patients over the two years of treatment using voxel-based morphometry, follow-up volumetric analyses were conducted by extracting gray matter volumes from SPM5 segmented images modulated by the Jacobian determinants of the images obtained during normalization to examine the differential effects of CET on gray matter volumes of specific anatomical regions. These volumetric data were then subjected to a series of linear mixed-effects trend models, employing an auto-regressive error structure most appropriate to longitudinal data, and allowing model intercepts and longitudinal trajectories to vary across subjects.57 All mixed-effects models, whether using voxel-based morphometry or extracted volumetric data, adjusted for potential demographic and medication confounds by including age, gender, IQ, illness duration, and medication dose as model covariates. In addition, although most (95%) data were collected on the same scanner, potential between-scanner differences were also controlled for by entering the scanner into the linear models as a covariate. Further, while only a small minority (2%) of structural scans demonstrated significant motion, sensitivity analyses were also conducted without these scans and revealed no significant differences in the results. All volumetric analyses also adjusted for intracranial volume, and p-values were corrected, when appropriate, for repeated inference testing of multiple volumetric regions within each cluster of results using Hochberg’s correction.58
Finally, mixed-effects growth curve models were employed to explore the associations between longitudinal changes in gray matter volume and cognition, after adjusting for age, gender, illness duration, medication dose, scanner, and intracranial volume. Significant associations between gray matter and cognitive change prompted the initiation of mediator analyses using Kraemer and colleagues’ mediator-analytic framework for clinical trials.59 In this framework, the mediating effect of changes in brain volume on the previously documented differential effects of CET on cognition28 is examined. If mediation is present, the indirect effect of CET on cognition through neurobiologic change will achieve statistical significance and reduce the direct effect of CET on cognition.60 The significance of the indirect effect of CET on cognition through neurobiologic change was calculated using estimates of the asymptotic distribution of indirect effects provided by MacKinnon and colleagues.61 No correction for multiple inference testing was employed in these exploratory analyses. Missing data for all analyses were handled using maximum-likelihood estimation.62,63,64
Results
Effects of Treatment on Two-Year Changes in Gray Matter Morphology
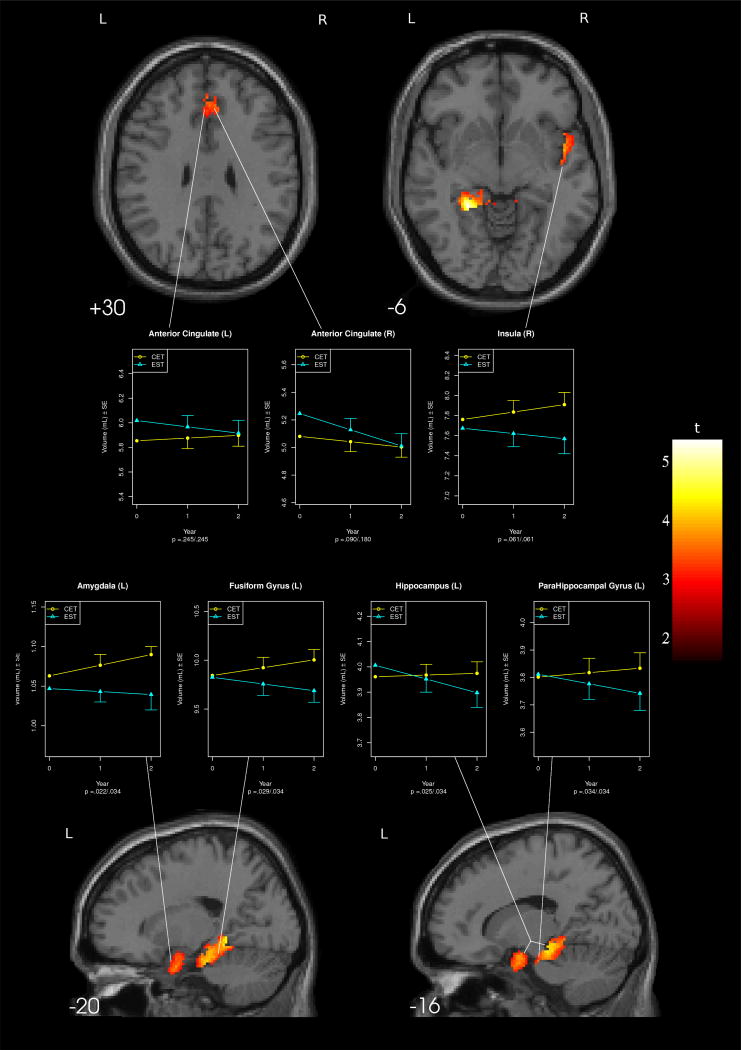
Voxel-based morphometric analyses using mixed-effects models showed three primary areas of differential gray matter change between patients receiving CET and those receiving EST over the course of two years of treatment (see Table 1 and Figure 2). Significant areas of differential effects included a cluster in the left medial temporal lobe, centering around the amygdala, parahippocampal gyrus, hippocampus, and fusiform gyrus; a cluster covering the bilateral anterior cingulate; and a cluster in right insula.
Table 1.
Voxel-Based Morphometric Analyses of Gray Matter Density Change in a Two-Year Trial of Cognitive Enhancement Therapy or Enriched Supportive Therapy (N = 53).
| MNI Coordinates (x, y, z) | Cluster Size | Location | BA | z | p | Direction |
|---|---|---|---|---|---|---|
| Main Effect of Time | ||||||
| −4, −66, −12 | 2925 | Bilateral cerebellum, left culmen | NA | 5.67 | < .001 | Loss |
| −2, −32, 34 | 553 | Left medial and posterior cingulate | 23/31 | 3.83 | < .001 | Loss |
|
| ||||||
| Treatment × Time Interaction | ||||||
| −26, −42, −6 | 803 | Left parahippocampal gyrus, left fusiform gyrus, left hippocampus | 35/36/37 | 4.90 | < .001 | EST loss > CET loss |
| −14, −2, −24 | 466 | Left parahippocampal gyrus, left amygdala | 28/34/38 | 3.49 | < .001 | EST loss > CET loss |
| 8, 40, 16 | 346 | Bilateral anterior cingulate | 9/10/32 | 3.46 | < .001 | EST loss > CET loss |
| 46, 0, −6 | 268 | Right insula | 22/38 | 3.56 | < .001 | EST loss > CET loss |
Note. BA = Brodmann’s Area, CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy
Figure 2.
Regions of Differential Gray Matter Change Among Participants Receiving Cognitive Enhancement Therapy Versus Enriched Supportive Therapy.
Follow-up tests of volumetric differences between treatment groups for specific regions identified in voxel-based morphometry analyses were consistent with a neuroprotective effect of CET against gray matter loss only for medial-temporal regions. As can be seen in Figure 2, EST patients demonstrated significantly greater gray matter loss over the course of two years in the left fusiform gyrus, hippocampus, and parahippocampal gyrus compared to CET patients. This trend was also apparent, although not statistically significant at the nominal alpha level in the right insula. In addition, CET patients demonstrated significantly greater gray matter increases in the left amygdala compared to patients receiving EST, who demonstrated no substantive change in left amygdala volume during the two years of study. Significant differential effects observed in the anterior cingulate using voxel-based morphometry were not maintained in volumetric analyses.
Relations Between Changes in Gray Matter Volume and Cognition
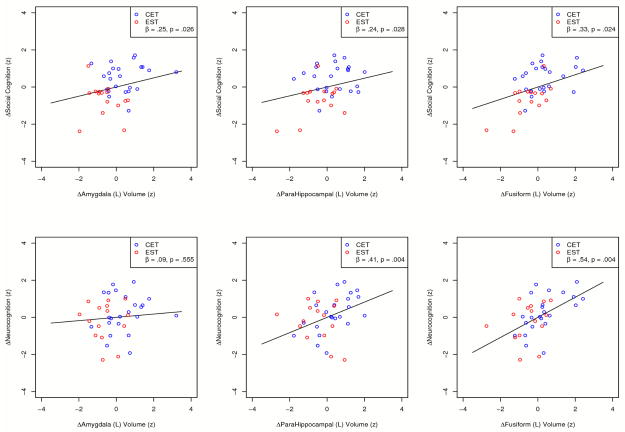
Having found that patients receiving CET demonstrated a decelerated loss of, and in some cases increase in gray matter volume over the course of two years of treatment compared to their EST counterparts, we proceeded to examine the relations between these differential rates of gray matter change and the beneficial cognitive effects of CET reported previously.27,28 Results from a series of mixed-effects growth models indicated that less loss of gray matter volume in the left parahippocampal and fusiform gyrus, and greater growth in left amygdala volume were all significantly related to greater two-year improvement in social cognition (see Figure 3). In addition, less loss of left parahippocampal and fusiform gyrus volume was also significantly related to more improvement in neurocognitive function. No significant relationships were observed between changes in anterior cingulate, left hippocampal, or right insula volume and change in cognition.
Figure 3.
Relations Between Neurobiologic and Cognitive Change During Two Years of Cognitive Enhancement Therapy or Enriched Supported Therapy.
Subsequent mediator analyses indicated that the neuroprotective effects of CET against gray matter loss in the left parahippocampal (z′ = 1.56, p = .039) and fusiform gyrus (z′ = 1.60, p = .033), as well as CET effects on left amygdala increases (z′ = 1.64, p = .029) all mediated the robust two-year effects of CET on social cognition previously reported from this trial.28 Further, CET effects protecting against gray matter loss in the left parahippocampal (z′ = 1.75, p = .026) and fusiform gyrus (z′ = 1.78, p = .022) also mediated the effects of CET on neurocognition.
Discussion
Cognitive rehabilitation approaches have emerged as effective methods for ameliorating cognitive impairment in schizophrenia.24 While the effects on cognition that these approaches produce have a presumed neurobiologic basis and when applied in early schizophrenia may exhibit a neuroprotective effect against loss of gray matter and brain function,29 no study has examined the long-term neurobiologic effects of cognitive rehabilitation in schizophrenia. We assessed brain morphology in a sample of early course schizophrenia patients treated for two years with Cognitive Enhancement Therapy (CET) or an active Enriched Supportive Therapy (EST) control. Results support our hypothesis that cognitive rehabilitation provides a neuroprotective effect against gray matter loss in key regions implicated in social and non-social cognitive impairment in schizophrenia. In particular, while patients receiving EST demonstrated loss of gray matter volume in the fusiform and parahippocampal gyrus, patients receiving CET demonstrated gray matter preservation in these areas, and even a significant differential increase in left amygdala gray matter volume. Consistent with previous reports on the effects of CET on cognitive and functional outcome,28 these neuroprotective effects were the greatest after the full two years of treatment, suggesting the benefits of long-term exposure to cognitive rehabilitation. Importantly, these differential effects of CET on gray matter change were significantly related to improved cognitive outcome, with patients who experienced less gray matter decline and greater gray matter increases also demonstrating significantly greater cognitive improvements over the two years of study. Further, these neurobiologic changes were found to be significant mediators of CET effects on cognition. These findings persisted after adjusting for a variety of potential demographic, illness and medication confounders, and suggest that CET can have direct benefits to the brains of patients with schizophrenia.
Despite the beneficial effects of CET on brain morphology demonstrated in this study, these findings need to be interpreted in the context of a number of important limitations. Although morphometric findings support a neuroprotective effect of CET against the gray matter loss seen during the early course of schizophrenia,6 and in the case of the amygdala, even increase in gray matter, in the absence of functional neuroimaging data the pathophysiological significance of these results for brain function is not clear. Overall structural changes in regional brain volumes were not large, but were reliably detectable, and may reflect functional changes. That we observed significant relations between increased gray matter and cognitive improvement, and that the effects of CET on gray matter change were significant mediators of CET effects on cognition, would suggest that brain functions sub-serving neurocognition and social cognition have been improved. Nonetheless, functional neuroimaging data are needed to better understand the effects of CET on brain function. An integration of morphometric and fMRI studies could be particularly informative in this regard.
It is also interesting to note that CET effects on brain regions commonly implicated in neurocognitive dysfunction in schizophrenia were quite modest. For example, no effects were seen in the dosrolateral prefrontal cortex, and only modest effects were observed in the anterior cingulate and hippocampus, which were not associated with neurocognitive change. Although gray matter change in the anterior cingulate and hippocampus might be more strongly related to individual neuropsychological tests, this pattern of findings parallels, to some degree, the cognitive effects observed in this trial of early course patients. In this population, we have observed much stronger effects on social cognition and noted a relative preservation of some general cognitive functions (particularly processing speed) among this sample.28 The absence of morphometric findings could reflect the better preserved neurocognitive capacity of early course patients.65 It is also possible that the effects of CET on brain regions implicated in neurocognitive impairment cannot be detected at a morphometric level, but that the primary effects of this approach on frontal brain regions is toward a normalization of functioning. To date, many studies have documented frontal hypofunction in schizophrenia,8 and if cognitive improvement occurs in the disorder it is also likely to be the result of improved brain function.32 As a consequence, while this study provides important information on the potential neuroanatomical effects of cognitive rehabilitation in early schizophrenia, future studies are clearly needed to continue to characterize the effects of CET on a variety of other neurobiologic parameters. It is important to remember, however, that significant relations were observed between changes in medial-temporal regions and neurocognition, as well as social cognition, suggesting the relevance of gray matter change in these regions to neurocognitive functioning. However, associations between gray matter and cognitive change were exploratory and not corrected for multiple inference testing, as such these results need to be interpreted with caution until confirmatory replications are available.
This study is also limited by the absence of an appropriately matched group of healthy individuals who could provide data on normative brain development in early adulthood. Although a large body of evidence has accumulated in schizophrenia research indicating a progressive loss of gray matter from the earliest phases of the disorder,6 healthy individuals also demonstrate some gray matter loss in early adulthood. However, loss appears to be greatest in the frontal cortex, not the subcortical regions demonstrating the most cognitive change in this study, which remain relatively stable or continue to grow after childhood.66
In summary, this investigation suggests that CET, a comprehensive cognitive rehabilitation approach, can protect against gray matter loss and may even support gray matter growth in medial-temporal areas of the brain in service of cognitive enhancement among early course schizophrenia patients. Although replication and further neurobiologic characterization is needed, these findings support the potential for cognitive rehabilitative approaches to positively affect the brain in schizophrenia. Further studies are needed to examine the durability of these effects on the brain, as Hogarty and colleagues26 and Wexler and Bell67 have both shown that cognitive rehabilitation can continue to confer benefits to schizophrenia patients even after the completion of treatment. Studies of neuronal mechanisms underlying brain change, such as possible effects of cognitive remediation on dopaminergic function,68 brain derived neurotrophic factor,69 as well as the genomic underpinnings of response to cognitive remediation are also needed.70
Supplementary Material
Table 2.
Changes in Gray Matter Volume During Two Years of Cognitive Enhancement Therapy or Enriched Supportive Therapy.
| Site/Cluster | CET (N = 30)
|
EST (N = 23)
|
Between-Group Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Year 1 | Year 2 | Baseline | Year 1 | Year 2 | ||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | t | p | pa | |
| Frontal | |||||||||
| Anterior Cingulate (L) | 5.85 (.83) | 5.88 (.82) | 5.90 (.88) | 6.02 (.99) | 5.97 (1.17) | 5.92 (.89) | 1.17 | .245 | .245 |
| Anterior Cingulate (R) | 5.08 (.72) | 5.04 (.71) | 5.00 (.74) | 5.25 (.85) | 5.13 (1.00) | 5.01 (.74) | 1.72 | .090 | .180 |
| Medial temporal | |||||||||
| Amygdala (L) | 1.06 (.13) | 1.08 (.11) | 1.09 (.12) | 1.05 (.13) | 1.04 (.15) | 1.04 (.12) | 2.35 | .022 | .034 |
| Fusiform Gyrus (L) | 9.84 (1.11) | 9.92 (1.00) | 10.00 (1.08) | 9.83 (1.36) | 9.76 (1.37) | 9.69 (1.18) | 2.23 | .029 | .034 |
| Hippocampus (L) | 3.96 (.35) | 3.97 (.33) | 3.97 (.35) | 4.01 (.46) | 3.95 (.50) | 3.90 (.46) | 2.28 | .025 | .034 |
| ParaHippocampal Gyrus (L) | 3.80 (.46) | 3.82 (.38) | 3.83 (.43) | 3.81 (.49) | 3.78 (.49) | 3.74 (.36) | 2.16 | .034 | .034 |
| Temporal | |||||||||
| Insula (R) | 7.76 (1.03) | 7.84 (.93) | 7.91 (.99) | 7.67 (1.10) | 7.62 (1.30) | 7.57 (.94) | 1.91 | .061 | - |
Note. All volumetric data are presented in cubic centimeters.
CET = Cognitive Enhancement Therapy, EST = Enriched Supportive Therapy
p-values are adjusted for multiple inference testing within each cluster of results using Hochberg’s correction
Acknowledgments
This work was supported by NIMH grants MH 60902 (MSK) and MH 79537 (SME). We thank Haranath Parepally, M.D., Diana Dworakowski, M.S., Mary Carter, Ph.D., Sara Fleet, M.S., and Michele Bauer for their help in various aspects of the study. We also thank our department chairman, David Kupfer, M.D. for extended support throughout the project, as well as the many patients who participated in this research and the dedication they showed to their recovery, which was a constant source of inspiration.
Footnotes
The authors report no conflicts of interest with this research.
References
- 1.Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: A quantitative review of the evidence. Neuropsychology. 1998;12(3):426–445. doi: 10.1037//0894-4105.12.3.426. [DOI] [PubMed] [Google Scholar]
- 2.Penn DL, Corrigan PW, Bentall RP, Racenstein J, Newman L. Social cognition in schizophrenia. Psychol Bull. 1997;121(1):114–132. doi: 10.1037/0033-2909.121.1.114. [DOI] [PubMed] [Google Scholar]
- 3.Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: Are we measuring the right stuff. Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- 4.Couture SM, Penn DL, Roberts DL. The Functional Significance of Social Cognition in Schizophrenia: A Review. Schizophr Bull. 2006;32(Suppl1):S44–63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MF, Kern RS, Heaton RK. Longitudinal studies of cognition and functional outcome in schizophrenia: implications for MATRICS. Schizophr Res. 2004;72(1):41–51. doi: 10.1016/j.schres.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 6.DeLisi LE. The Concept of Progressive Brain Change in Schizophrenia: Implications for Understanding Schizophrenia. Schizophr Bull. 2008;34(2):312–321. doi: 10.1093/schbul/sbm164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch Gen Psychiatry. 1986;43(2):114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- 8.Weinberger DR, Egan MF, Bertolino A, Callicott JH, Mattay VS, Lipska BK, Berman KF, Goldberg TE. Prefrontal neurons and the genetics of schizophrenia. Biol Psychiatry. 2001;50(11):825–844. doi: 10.1016/s0006-3223(01)01252-5. [DOI] [PubMed] [Google Scholar]
- 9.Lim KO, Hedehus M, Moseley M, de Crespigny A, Sullivan EV, Pfefferbaum A. Compromised white matter tract integrity in schizophrenia inferred from diffusion tensor imaging. Arch Gen Psychiatry. 1999;56(4):367–374. doi: 10.1001/archpsyc.56.4.367. [DOI] [PubMed] [Google Scholar]
- 10.Kubicki M, Park H, Westin CF, Nestor PG, Mulkern RV, Maier SE, Niznikiewicz M, Connor EE, Levitt JJ, Frumin M, et al. DTI and MTR abnormalities in schizophrenia: analysis of white matter integrity. Neuroimage. 2005;26(4):1109–1118. doi: 10.1016/j.neuroimage.2005.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70(2–3):117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Perlstein WM, Carter CS, Noll DC, Cohen JD. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. [DOI] [PubMed] [Google Scholar]
- 13.Sanfilipo M, Lafargue T, Rusinek H, Arena L, Loneragan C, Lautin A, Rotrosen J, Wolkin A. Cognitive performance in schizophrenia: relationship to regional brain volumes and psychiatric symptoms. Psychiatry Research: Neuroimaging. 2002;116(1–2):1–23. doi: 10.1016/s0925-4927(02)00046-x. [DOI] [PubMed] [Google Scholar]
- 14.Kerns JG, Cohen JD, MacDonald AW, Johnson MK, Stenger VA, Aizenstein H, Carter CS. Decreased conflict-and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am J Psychiatry. 2005;162(10):1833–1839. doi: 10.1176/appi.ajp.162.10.1833. [DOI] [PubMed] [Google Scholar]
- 15.Rüsch N, Spoletini I, Wilke M, Bria P, Di Paola M, Di Iulio F, Martinotti G, Caltagirone C, Spalletta G. Prefrontal--thalamic--cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93(1–3):79–89. doi: 10.1016/j.schres.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Eack SM, George MM, Prasad KMR, Keshavan MS. Neuroanatomical substrates of foresight in schizophrenia. Schizophr Res. 2008;103(1–3):62–70. doi: 10.1016/j.schres.2008.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pinkham AE, Penn DL, Perkins DO, Lieberman J. Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 2003;160(5):815–824. doi: 10.1176/appi.ajp.160.5.815. [DOI] [PubMed] [Google Scholar]
- 18.Carter CS, Barch DM. Cognitive Neuroscience-Based Approaches to Measuring and Improving Treatment Effects on Cognition in Schizophrenia: The CNTRICS Initiative. Schizophr Bull. 2007;33(5):1131–1137. doi: 10.1093/schbul/sbm081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keefe RSE, Bilder RM, Davis SM, Harvey PD, Palmer BW, Gold JM, Meltzer HY, Green MF, Capuano G, Stroup TS, et al. Neurocognitive Effects of Antipsychotic Medications in Patients With Chronic Schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64(6):633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 20.Sergi MJ, Green MF, Widmark C, Reist C, Erhart S, Braff DL, Kee KS, Marder SR, Mintz J. Cognition and Neurocognition: Effects of Risperidone, Olanzapine, and Haloperidol. Am J Psychiatry. 2007a;164(10):1585–1592. doi: 10.1176/appi.ajp.2007.06091515. [DOI] [PubMed] [Google Scholar]
- 21.Swartz MS, Perkins DO, Stroup TS, Davis SM, Capuano G, Rosenheck RA, Reimherr F, McGee MF, Keefe RSE, McEvoy JP, Hsiao JK, Lieberman JA. Effects of Antipsychotic Medications on Psychosocial Functioning in Patients With Chronic Schizophrenia: Findings From the NIMH CATIE Study. Am J Psychiatry. 2007;164(3):428–436. doi: 10.1176/ajp.2007.164.3.428. [DOI] [PubMed] [Google Scholar]
- 22.Hogarty GE, Flesher S, Ulrich R, Carter M, Greenwald D, Pogue-Geile M, et al. Cognitive enhancement therapy for schizophrenia. Effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry. 2004;61(9):866–876. doi: 10.1001/archpsyc.61.9.866. [DOI] [PubMed] [Google Scholar]
- 23.Bell M, Bryson G, Greig T, Corcoran C, Wexler BE. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Arch Gen Psychiatry. 2001;58(8):763–768. doi: 10.1001/archpsyc.58.8.763. [DOI] [PubMed] [Google Scholar]
- 24.McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A Meta-Analysis of Cognitive Remediation in Schizophrenia. Am J Psychiatry. 2007;164(12):1791–1802. doi: 10.1176/appi.ajp.2007.07060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogarty GE, Greenwald DP. Cognitive Enhancement Therapy: The Training Manual. University of Pittsburgh Medical Center; Authors: 2006. Available through www.CognitiveEnhancementTherapy.com. [Google Scholar]
- 26.Hogarty GE, Greenwald DP, Eack SM. Durability and mechanism of effects of Cognitive Enhancement Therapy. Psychiatr Serv. 2006;57(12):1751–1757. doi: 10.1176/ps.2006.57.12.1751. [DOI] [PubMed] [Google Scholar]
- 27.Eack SM, Hogarty GE, Greenwald DP, Hogarty SS, Keshavan MS. Cognitive Enhancement Therapy improves Emotional Intelligence in early course schizophrenia: Preliminary effects. Schizophr Res. 2007;89(1–3):308–311. doi: 10.1016/j.schres.2006.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eack SM, Greenwald DP, Hogarty SS, Cooley SJ, DiBarry AL, Montrose DM, Keshavan MS. Cognitive Enhancement Therapy for early course schizophrenia: Effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60(11):1468–1476. doi: 10.1176/appi.ps.60.11.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol. 1999;11(3):525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- 30.Buonomano DV, Merzenich MM. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21(1):149–186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 31.Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JDE. Neural deficits in children with dyslexia ameliorated by behavioral remediation: Evidence from functional MRI. Proc Natl Acad Sci. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wykes T, Brammer M, Mellers J, Bray P, Reeder C, Williams C, Corner J. Effects on the brain of a psychological treatment: cognitive remediation therapy Functional magnetic resonance imaging in schizophrenia. Br J Psychiatry. 2002;181(2):144–152. doi: 10.1017/s0007125000161872. [DOI] [PubMed] [Google Scholar]
- 33.Wexler BE, Anderson M, Fulbright RK, Gore JC. Preliminary evidence of improved verbal working memory performance and normalization of task-related frontal lobe activation in schizophrenia following cognitive exercises. Am J Psychiatry. 2000;157(10):1694–1697. doi: 10.1176/appi.ajp.157.10.1694. [DOI] [PubMed] [Google Scholar]
- 34.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 35.Ben-Yishay Y, Piasetsky EB, Rattok J. A systematic method for ameliorating disorders in basic attention. In: Meir MJ, Benton AL, Diller L, editors. Neuropsychological rehabilitation. New York: Guilford Press; 1985. pp. 165–181. [Google Scholar]
- 36.Bracy OL. PSSCogRehab [computer software] Indianapolis, IN: Psychological Software Services Inc; 1994. [Google Scholar]
- 37.Hogarty GE. Personal Therapy for schizophrenia and related disorders: A guide to individualized treatment. New York: Guilford; 2002. [Google Scholar]
- 38.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 39.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 40.Tzourio-Mazoyer N, Landeau B, Papthanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 41.Wechsler D. Manual for the Wechsler Memory Scale-Revised. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 42.Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test Manual. San Antonio, TX: Psychological Corp; 1987. [Google Scholar]
- 43.Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- 44.Reitan RM, Waltson D. The Halstead-Reitan Neuropsychological Test Battery. Tucson, AZ: Neuropsychology Press; 1985. [Google Scholar]
- 45.Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- 46.Culbertson WC, Zillmer EA. Tower of London-DX manual. 1996. Unpublished manuscript. [PubMed] [Google Scholar]
- 47.Buchanan RW, Heinrichs DW. The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res. 1989;27(3):335–350. doi: 10.1016/0165-1781(89)90148-0. [DOI] [PubMed] [Google Scholar]
- 48.Green MF, Nuechterlein KH, Gold JM, Barch DM, Cohen J, Essock S, et al. Approaching a consensus cognitive battery for clinical trials in schizophrenia: The NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry. 2004;56(5):301–307. doi: 10.1016/j.biopsych.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 49.Mayer JD, Salovey P, Caruso DR, Sitarenios G. Measuring emotional intelligence with the MSCEIT V2.0. Emotion. 2003;3(1):97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- 50.Nuechterlein KH, Green MF, Kern RS, Baade LE, Barch DM, Cohen JD, Essock S, Fenton WS, Frese FJ, III, Gold JM, et al. The MATRICS Consensus Cognitive Battery, Part 1: Test Selection, Reliability, and Validity. Am J Psychiatry. 2008;165(2):203–213. doi: 10.1176/appi.ajp.2007.07010042. [DOI] [PubMed] [Google Scholar]
- 51.Eack SM, Greeno CG, Pogue-Geile MF, Newhill CE, Hogarty GE, Keshavan MS. Assessing social-cognitive deficits in schizophrenia with the Mayer-Salovey-Caruso Emotional Intelligence Test. Schizophr Bull. doi: 10.1093/schbul/sbn091. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baldwin MW. Relational schemas and the processing of social information. Psychol Bull. 1992;112(3):461–484. [Google Scholar]
- 53.Selman RL, Schultz LH. Making a Friend in Youth. Chicago, IL: University of Chicago Press; 1990. [Google Scholar]
- 54.Wyer RS, Srull TK, editors. Handbook of social cognition. Vol. 1: Basic Processes. Hillside, NJ: Lawrence Earlbaum Association; 1994. [Google Scholar]
- 55.Brothers L. The social brain: A project for integrating primate behavior and neurophysiology in a new domain. Concepts in Neuroscience. 1990;1:27–51. [Google Scholar]
- 56.Ward DB. Simultaneous inference for fMRI data. Milwaukee, WI: Author; 2000. [Google Scholar]
- 57.Raudenbush DSW, Bryk DAS. Hierarchical Linear Models: Applications and data analysis methods. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 58.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 59.Kraemer HC, Wilson G, Fairburn CG, Agras W. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–884. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 60.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 61.MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39(3):384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society Series B (Methodological) 1977;39(1):1–38. [Google Scholar]
- 63.Gueorguieva R, Krystal JH. Move Over ANOVA Progress in Analyzing Repeated-Measures Data and Its Reflection in Papers Published in the Archives of General Psychiatry. Arch Gen Psychiatry. 2004;61(3):310–317. doi: 10.1001/archpsyc.61.3.310. [DOI] [PubMed] [Google Scholar]
- 64.Hamer RM, Simpson PM. Last observation carried forward versus mixed models in the analysis of psychiatric clinical trials. Am J Psychiatry. 2009;166(6):639–641. doi: 10.1176/appi.ajp.2009.09040458. [DOI] [PubMed] [Google Scholar]
- 65.Braw Y, Bloch Y, Mendelovich S, Ratzoni G, Gal G, Harari H, Tripto A, Levkovitz Y. Cognition in young schizophrenia outpatients: comparison of first-episode with multiepisode patients. Schizophr Bull. 2008;34(3):544–554. doi: 10.1093/schbul/sbm115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: a longitudinal MRI study. Nat Neurosci. 1999;2(10):861–862. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- 67.Wexler BE, Bell MD. Cognitive Remediation and Vocational Rehabilitation for Schizophrenia. Schizophr Bull. 2005;31(4):931–941. doi: 10.1093/schbul/sbi038. [DOI] [PubMed] [Google Scholar]
- 68.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, Klingberg T. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science. 2009;323(5915):800–802. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 69.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is Serum Brain-Derived Neurotrophic Factor a Biomarker for Cognitive Enhancement in Schizophrenia? Biol Psychiatry. 2009;66(6):549–553. doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bosia M, Bechi M, Marino E, Anselmetti S, Poletti S, Cocchi F, Smeraldi E, Cavallaro R. Influence of catechol-O-methyltransferase Val158Met polymorphism on neuropsychological and functional outcomes of classical rehabilitation and cognitive remediation in schizophrenia. Neurosci Lett. 2007;417(3):271–274. doi: 10.1016/j.neulet.2007.02.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.