Abstract
The global emergence and spread of multidrug resistant (MDR) and extensively drug-resistant (XDR) tuberculosis (TB) has led to the reexamination of surgical therapy as a possible adjunctive therapy for the treatment of drug-resistant TB. We present a case of a 26-year-old HIV-seronegative patient with pulmonary XDR-TB refractory to medical therapy. Surgical resection of the patient’s solitary cavitary lesion was performed as adjunctive treatment and a successful outcome with a combination of surgery and medical therapy was achieved. We review the history of surgical therapy for TB and the literature published on the role of surgical therapy in the treatment of MDR- and XDR-TB. A total of 26 case series and cohort studies were reviewed demonstrating surgical resection is beneficial in the treatment of drug-resistant TB; however, the results may not be applicable to all settings as all studies were observational, tended to select “healthier” TB patients, and all surgeries were performed at specialized thoracic surgery centers. Additional well-designed studies are needed to determine the efficacy of surgery in the treatment of drug-resistant TB.
Introduction
Thoracic surgery was a common treatment for pulmonary tuberculosis (TB) in the pre-chemotherapy era after the discovery of Mycobacterium tuberculosis in 1882 by Robert Koch.1,2 Early surgical therapies consisted of a variety of collapse therapies including thoracoplasty, ball plombage, and induced pneumothorax.3 The first report of pulmonary resection was in 1891 and during the early twentieth century, surgery played a prominent role in TB management.2,4 However, after the advent of effective anti-TB medications in the mid-twentieth century, the use of surgical resection became limited in most countries. The global emergence of drug-resistant TB including multidrug resistant (MDR) and extensively drug-resistant (XDR) disease5 has led to the re-examination of surgery as an adjunctive treatment for highly drug-resistant TB. We present a case of XDR-TB treated with a combination of medical therapy and surgical resection and review the literature on the role of surgery in the treatment of MDR- and XDR-TB.
Case Report
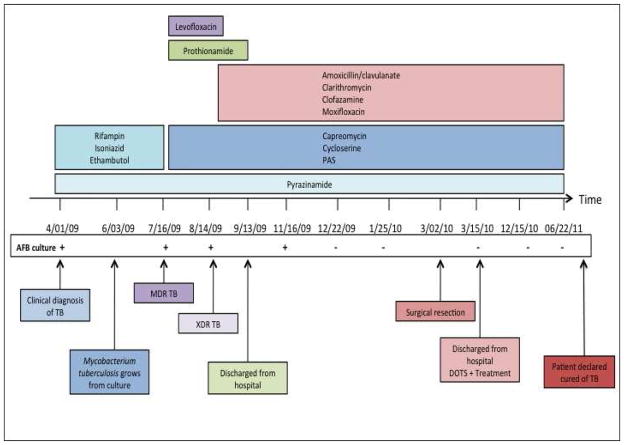
A 26-year-old HIV-seronegative male from the country of Georgia presented to the Georgian National Center for Tuberculosis and Lung Diseases (NCTBLD) in Tbilisi on April 1, 2009, with 2 weeks of fever, productive cough, and a 10-pound weight loss. The patient had been diagnosed with “community-acquired pneumonia” in December 2008 and received an empiric course of 7 days of an unknown antibiotic. The patient reported alcohol intake of 1–4 drinks weekly and smoking one half pack of cigarettes per day. He had received BCG vaccination as an infant. He had no other significant past medical history and was not taking any medications at the time of presentation. The patient was employed as a security guard at the NCTBLD for the three years prior to onset of symptoms. He reported not wearing a mask or respirator while patrolling the inpatient tuberculosis wards. The patient reported there was a new infection control policy that included wearing appropriate masks on patient floors; however, he stated he did not wear one because there were limited masks available and most hospital personnel were not wearing masks [at that time]. A chest radiograph revealed a left lower lobe infiltrate (Figure 1a). Sputum examination with Ziehl-Neelsen staining was positive for 3+ acid fast bacilli (AFB). Based on the above results, the patient was clinically diagnosed with pulmonary TB. He was admitted to the hospital, placed in respiratory isolation, and started on first-line anti-TB therapy with rifampin, isoniazid, pyrazinamide, ethambutol; he also received vitamin B6 (pyridoxine).
Figure 1. Chest radiography.
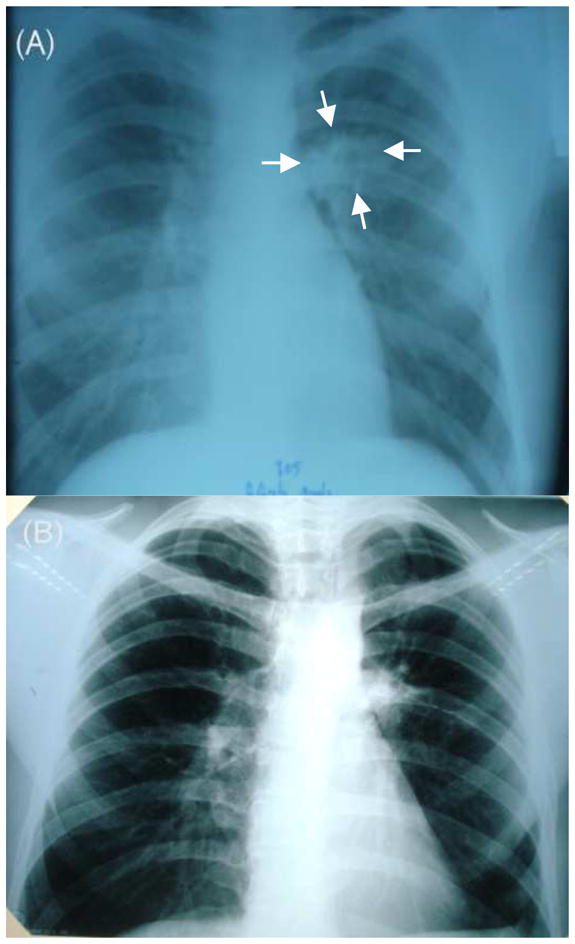
(A) Preoperative image showing left lower lobe infiltrate (arrows). (B) One month postoperative image, showing clear lung fields.
The initial sputum culture, performed on Löwenstein-Jensen medium, grew M. tuberculosis (MTB). The patient initially noted some symptomatic improvement after one month of anti-TB treatment but did not have complete resolution of symptoms. Drug susceptibility testing (DST) to first-line anti-TB drugs, utilizing the agar proportion method6 was performed by the Republic of Georgia National TB Reference Laboratory; it demonstrated resistance to all first-line drugs including rifampin, isoniazid, pyrazinamide, ethambutol, and streptomycin. The patient’s medications were subsequently changed to a second-line regimen that included capreomycin, levofloxacin, cycloserine, PAS, prothionamide, and pyrazinamide. Approximately one month later in August 2009, second-line DST results were available and identified further resistance to ethionamide, kanamycin, capreomycin, ofloxacin, and susceptibility to PAS. Based on the resistance pattern (resistance to isoniazid, rifampin, ofloxacin, capreomycin and kanamycin) which was further confirmed by subsequent positive cultures and DST, the patient was diagnosed with XDR-TB. Anti-TB medications were again modified and the individualized regimen included pyrazinamide, prothionamide, capreomycin, cycloserine, PAS, amoxicillin/clavulanate, clarithromycin, clofazamine, and moxifloxacin. Pyrazinamide, capreomycin, prothionamide, and moxifloxacin were continued to be used by the clinicians treating the patient even though the DST indicated resistance, given the limited treatment options with drugs which demonstrated in vitro susceptibility with the hope that some of these drugs might offer some benefit. Prothionamide was later stopped due to gastrointestinal toxicity. Sputum specimens collected from the patient in July and August 2009 remained culture positive for M. tuberculosis. In September 2009, he was discharged home to receive outpatient therapy delivered by direct observation.
After four months on the tailored treatment regimen, the patient had sputum culture conversion to negative in December 2009. The patient remained culture negative on follow up examinations; however, there was no radiological improvement of the left lower lobe infiltrate on chest radiograph. A CT scan of the lung was done and showed a localized 3 × 5 cm left lower lobe cavity with no evidence of bilateral disease (Figure 2). Given the lack of radiologic improvement and the high degree of drug resistance, the physicians caring for this patient felt that it was unlikely that this patient with XDR-TB would be cured with chemotherapy alone and referred the patient for evaluation for adjunctive surgical therapy. The NCTBLD drug resistance committee recommended surgical resection, and the patient was deemed a good operative candidate given his young adult age and no co-morbid illnesses, a preoperative body- mass index (BMI) of 20.8 kg/m2, and FEV1 value of 4.63 liters (99% of expected value).
Figure 2. Preoperative CT scan of the lung.
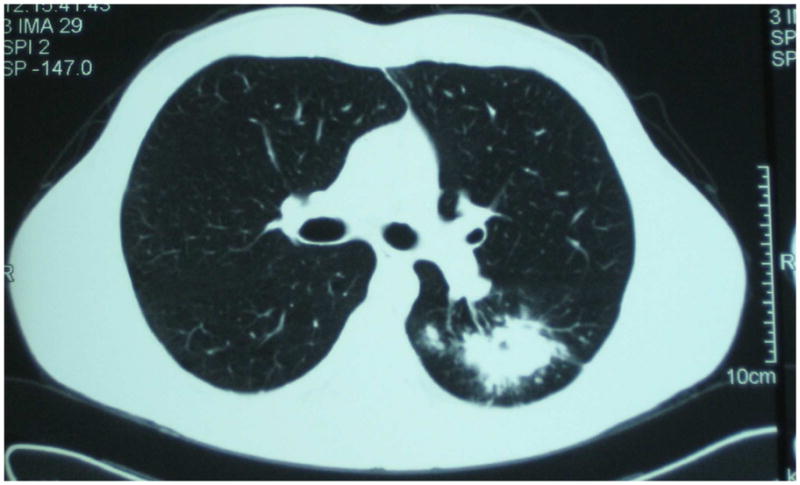
Cross Sectional view showing 3 × 5 cm left lung cavitary lesion.
In March 2010, surgical resection of the left lower lobe was performed. A left lateral posterior approach was used to remove the 6th segment of the left lower lobe. Gross pathology of the resected cavitary lesion showed caseous necrosis (Figure 3). The surgery time was approximately 90 minutes and there were no intraoperative complications. A chest tube placed during surgery was removed after three days. The patient was continued on the same anti-TB treatment regimen and had no postoperative complications. The surgical tissue specimen was AFB smear positive and culture positive (no DST performed) for MTB. The patient was discharged from the hospital two weeks after surgery. Chest radiography repeated one month after surgery showed no abnormalities (Figure 1B). The patient received anti-TB medication for 17 months post surgery (total of 23·5 months of XDR targeted TB medical treatment) and all follow up sputum cultures remained negative over the last 17 months of treatment. Given the completed course of treatment and negative follow up sputum cultures, the patient was declared cured of TB on July 15th, 2011.
Figure 3. Gross pathology of resected lung lesion.
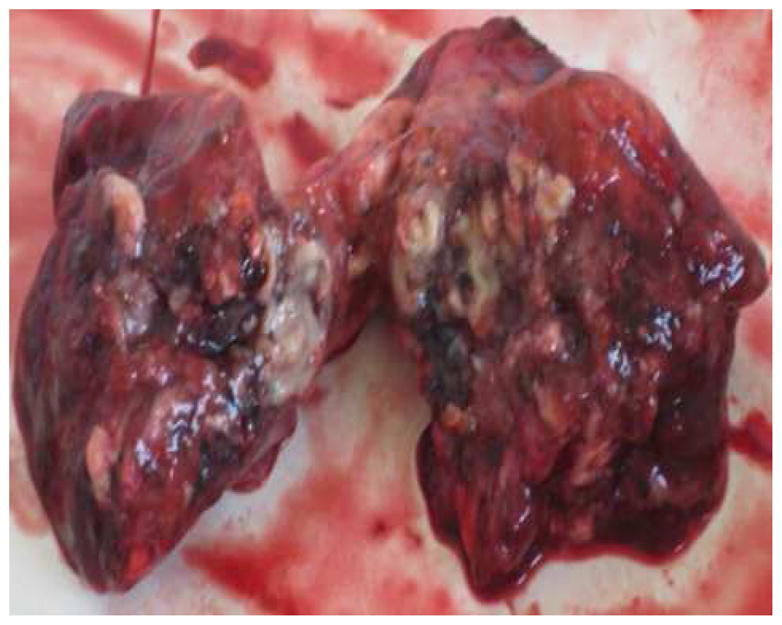
The inside of the cavity shows caseous necrosis.
Epidemiology and Treatment Outcomes of Drug-Resistant Tuberculosis
The WHO estimated that in 2009 there were 9·4 million incident cases (137 cases per 100,000 population) of TB worldwide with 1·7 million resulting deaths.7 Of particular concern, global surveillance data has indicated the highest level of drug-resistance ever recorded. The global emergence of drug-resistant TB including MDR-TB (defined as resistance to at least isoniazid and rifampicin) and XDR-TB (resistance to at least isoniazid, rifampicin, a fluoroquinolone and an injectable drug [kanamycin, amikacin, and/or capreomycin]) is an alarming issue in international TB control which presents enormous challenges not yet being sufficiently addressed.8,9 MDR- and XDR-TB are associated with significant increases in morbidity and mortality compared to drug susceptible disease,9. Additionally, XDR-TB outcomes have been found to be worse than MDR-TB.10 Each year an estimated half a million MDR-TB cases develop, of which only 7% are diagnosed and treated.11 Twenty-seven high-burden countries including Georgia account for 85% of all such cases, with the republics of the former Soviet Union having the highest rates of MDR- and XDR-TB in the world.7,12 Since a surveillance study of XDR-TB in 2006 described its global emergence there have been increasing reports with the highest rates seen in the WHO European Region.13,14 As of February 2011, 69 countries have reported at least one case of XDR-TB.15
The treatment of drug-resistant TB including MDR- and XDR-TB is extremely challenging. Unlike drug-susceptible treatment regimens which are evidence-based and developed based on results of randomized clinical trials,16 treatment of MDR- and XDR-TB has been based on anecdotal experience and expert opinion. Current regimens are lengthy, difficult to tolerate, not always available, and often ineffective. Less than 10% of persons with MDR-TB are receiving appropriate treatment according to international guidelines.11,17 For those receiving second-line therapy for MDR-TB, outcomes vary considerably across studies with a wide range of reported success (36–79%).18,19 A meta-analysis evaluating 33 cohort studies comprising over 8000 cases of MDR-TB, found an overall treatment success rate of 62% (95% CI 58–67%).20 XDR-TB has proved even more difficult to treat as effective medical regimens are further limited. Early reports have shown poor response to medical treatment (38–57% success rate) in HIV-seronegative persons and extremely high one-year mortality (81–98%) in persons with HIV.21–23 Given the poor response of drug-resistant TB to available medications, new drugs are urgently needed as well as additional interventions to help improve outcomes. While there are currently a number of anti-TB drugs in development, some which may be useful for drug-resistant TB, these will likely not be available for the next several to many years.24 Surgical resection may play an important role as adjunctive therapy for drug-resistant TB.2,
Historical Aspects of Surgery in the Treatment of Tuberculosis
Surgery is one of the oldest known treatments for tuberculosis. The earliest documented reports are from the beginning of the 19th century and describe the surgical drainage of tuberculous cavities.25 After this method proved ineffective and lost favor, attention shifted to surgical procedures which could reduce the volume of tuberculous affected lung, known collectively as collapse therapies. The objective of these procedures was to deprive the organism of oxygen, a rationale given credence by Koch’s discovery of MTB as an obligate aerobe.1 The first collapse therapy to gain widespread acceptance was induced pneumothorax. With a review of his experience in 1882, Carlo Forlanini helped to popularize the procedure in Europe from where it eventually spread to North America.26 A big boon to the practice of pneumothorax treatment was the discovery of x-rays in 1895, which allowed physicians to identify unilateral tuberculosis.
These patients were thought to be ideal candidates for pneumothorax treatment. Although no rigorous studies evaluating or proving the efficacy of this technique were performed, it was estimated over 100,000 pulmonary TB patients were treated by induced pneumothorax over the next quarter century.27 Thoracoplasty, the removal of ribs to collapse the chest wall, developed around the same time as pneumothorax treatment but was slower to win acceptance among surgeons. Samuel Freedlander, a thoracic surgeon in America, reported the outcome of 153 patients with pulmonary TB treated with thoracoplasty from 1932–34 and showed a benefit to those who received the procedure. After a minimum 2 year follow up, 21% of patients operated on experienced worsening disease or death as compared to 61% of patients who refused thoracoplasty.28 Similar observational reports showing favorable results with thoracoplasty were published and thoracoplasty became an accepted TB treatment.27,29 Other procedures that were utilized during this same period included phrenic nerve interruption, pneumoperitoneum, and extrapleural plombage (plastic balls placed in between the pleura and chest wall to collapse underlying lung). Generally, only patients with non-severe and unilateral disease were candidates for the above procedures. Additionally, many of the procedures were complicated by further spread of infection, bleeding, erosion of foreign material into lung, and rarely death.27 These limitations along with the discovery of streptomycin in 1943, which marked the beginning of effective chemotherapy for tuberculosis, led to the end of collapse therapies.
American Surgeons Lilienthal and Freedlander helped to pioneer the use of surgical resection in treating tuberculosis in the 1930s.30,31 Surgical therapy for TB became closely linked with the development of thoracic surgery as a specialty. As techniques were modified and improved, surgery became a commonly utilized adjunctive treatment along with developing antibiotic regimens. Throughout the 1950s, thousands of TB patients underwent surgical resection.3 In 1964, Francis reported on a prospective evaluation of over 1100 patients with unilateral cavitary disease who underwent surgical resection in the United Kingdom from 1953–54 and found greater than 80% of patients had no evidence of active TB and suffered no TB-related complications at five-year follow up.32 More published reports followed in the 1960s and early 1970s.33,34 However, as randomized controlled clinical trials demonstrated the effectiveness of new combination treatments for drug-susceptible TB,35 medical therapy became the standard and surgery was no longer indicated on a routine basis for TB treatment.
Despite the lack of clinical trial data on efficacy of adjunctive surgical therapy, some countries, such as Russia and other republics of the former Soviet Union, continued to perform a high number of surgical interventions, mainly surgical resection, since the 1980s.3,36 Some of these patients had “chronic” TB and had failed to respond to first-line drug therapy. A substantial proportion of these patients may have had drug-resistant TB but the laboratory capacity and infrastructure to document this often did not exist. A recent study from Russia found that the use of artificial pneumothorax as adjunctive treatment in patients with MDR-TB may lead to improved outcomes.37
The global emergence of MDR- and later XDR-TB has been paralleled by an increase in the number of reported surgical resections. These published reports on the use of surgical resection for treating pulmonary drug-resistant TB will be the focus of the remainder of this review.
Literature Review
In our review of the literature, we found 18 cases series and 8 cohort studies evaluating the outcomes of drug-resistant TB patients undergoing surgical resection. The case series studies evaluated drug-resistant TB patients who received medical treatment and had surgical resection performed while the cohort studies compared the outcomes of drug-resistant TB patients receiving medical treatment alone versus medical treatment plus surgical resection. No randomized controlled trials of the efficacy of adjunctive surgical therapy for the treatment of TB have been performed. Most reports were from Asia38–50, with only a few from each North America51–53, Europe54–59, and resource limited countries (Peru60, Egypt61, and South Africa62,63). Cases spanned from 1983–2008; MDR- and XDR-TB cases were both included with approximately 91% of cases reported as MDR. All surgeries were performed at either national TB center-affiliated hospitals or specialized thoracic surgery centers. A summary of all reports is shown in Tables 2 and 3.
Table 2.
Case studies of drug resistant pulmonary tuberculosis patients undergoing surgical resection along with medical treatment
| Author | Country | Years | Cohort size | Agea | MDR/ XDR | Preoperative treatment duration (months)b | Preoperative culture positive | Perioperative Complication/ Mortality rate | Post op Treatment Duration (months)b | Postoperative culture negative rate (%) | Favorable Outcome Rate |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Kang 201038 | Korea | 1996–2008 | 72 | 31 | 46/26 | 5 (2.5–13) | 81% | 15%/1.4% | - (18–24) | 78% | 90%c |
| Shiraishi 200939 | Japan | 2000–2007 | 56 | 46 | 56/0 | ≥ 3 | 32% | 16%/0 | 18 (8–84) | 100% | 95%d |
| Dravniece 200954 | Latvia | 1999–2005 | 17 | 42 | 0/17 | 12 (2–20) | 94% | 18%/0 | 14.5 (7–28) | 47% | 47%c |
| Park 200940 | Korea | 1998–2004 | 19 | 31 | 17/2 | 8.5 - | 53% | 0/0 | 12 - | 95% | 79%d |
| Orki 200956 | Turkey | 1997–2005 | 55 | 34 | 55/-e | 3.7 (2–8) | 11% | 29%/1.8% | 24 - | 95% | 95%d |
| Wang 200841 | China | 1995–2006 | 56 | 39 | 56/-e | 43.5 (3–240) | 100% | 25%/0 | 12 (6–18) | 91% | 75%d |
| Shiraishi 200842 | Japan | 2000–2006 | 5 | 44 | 0/5 | - | 100% | 0/0 | 19 - | 100% | 100%d |
| Mohsen 200761 | Egypt | 1995–2005 | 23 | 24 | 23/-e | - (3–6) | 35% | 35%/4.3% | - (18–24) | 100% | 91%d |
| Naidoo 200762 | South Africa | 1997–2005 | 27 | 34 | 27/-e | ≥ 3 - | 41% | 26%/ 0 | 18 - | 93% | 93%d |
| Kir 200657 | Turkey | 1993–2005 | 79 | 38 | 79/-e | 4 (2–8) | 15% | 39%/2.5% | - | 96% | 95%c |
| Kim 200643 | Korea | 1993–2004 | 79 | 29 | 61/18 | 14.5 (9–112) | 98% | 23%/1.2% | 18 (9–48) | 72% | 72%c |
| Somocurcio 200660 | Peru | 1999–2004 | 121 | 27 | 121/- | 15 (0.6–41) | 79% | 23%/5% | ≥12 - | 78% | 63%d |
| Takeda 200544 | Japan | 1998–2003 | 26 | 48 | 26/-e | 46 - | - | 23%/3.8% | - (18–24) | 92% | 89%d |
| Park 200245 | Korea | 1995–1999 | 49 | 35 | 49/- | ≥ 2 | 63% | 16%/0 | 18–24 - | 94% | 90%d |
| Chiang 200146 | Taiwan | 1990–1999 | 27 | 44 | 26/1 | 10 (2–33) | 52% | 11%/4% | 15 (8–24) | 92% | 89%d |
| Pomerantz 200151 | USA | 1983–2000 | 172 | 39 | 172/-e | ≥ 3 | 50% | 12%/3.3% | 24 - | 98% | >90%d |
| Vanleuven 199763 | South Africa | 1990–1995 | 62 | 34 | 62/-e | - | 39% | 23%/1.6% | 9 (0–26) | 89% | 80%d |
| Treasure 199552 | USA | 1986–1993 | 19 | 39 | 19/-e | - | 90% | 21%/0 | - | 89% | 89%d |
Median or Mean Age is shown
Median or mean length of preoperative and postoperative medical treatment and/or range is shown if reported. When not available minimum treatment duration is given
Favorable Outcome defined as cure, probable cure, or treatment completed as per Laserson et al72 or the WHO71
Favorable Outcome defined as cure, not further explained, or alive and negative sputum culture during follow up
Did not perform or no mention of second line drug susceptibility testing (DST) results
Table 3.
The effect of surgical resection on clinical outcomes from cohort studies comparing drug resistant tuberculosis patients undergoing combination surgical resection and medical treatment vs. medical treatment alone
| Author | Country | Years | Cohort Size ( % having surgery) | MDRa/ XDRb | Analysis | Overall treatment success | Effect of Surgical Resection on Clinical Outcomes | |
|---|---|---|---|---|---|---|---|---|
| Univariate HRc (95% CI) | Multivariate HRc (95% CI) | |||||||
| Jeon 200947 | Korea | 2001–2005 | 176 (9%) | XDR | Predictors of poor outcomed | 18% | 0.08 (.02–0.28) | 0.18 (0.04–0.78) |
| Kwon 200848 | Korea | 1995–2004 | 155 (23%) | MDR XDR |
Predictors of favorable outcomee | 66% | - | 11.35 (3.02, 42.74) |
| Keshavjee 200858 | Russia | 2000–2004 | 608 (9%) | MDR XDR |
Predictors of favorable outcomee | 66% | 1.24 (0.69–2.26) | - f |
| KIm 200849 | Korea | 2000–2002 | 1407 (4%) | MDR XDR |
Predictors of favorable outcomee | 45% | 2.72 (1.56, 4.73) | 3.87 (1.69, 8.88) |
| Kim 200750 | Korea | 1996–2005 | 211 (30%) | MDR XDR |
Predictors of poor outcomed | 63% | 1.36 (0.70, 2.65) | - f |
| Torun 200759 | Turkey | 1992–2004 | 252 (26%) | MDR | Predictors of favorable outcomee | 77% | 1.7 (0.8–3.5) | 1.5 (0.64, 3.46) |
| Leimane 200555 | Latvia | 2000 | 204 (9%) | MDR | Predictors of poor outcomed | 66% | 0.3 (0.1–1.2) | -f |
| Chan 200453 | USA | 1984–1998 | 205 (63%) | MDR | Predictors of initial favorable responseg | 75% | 4.63 (1.89, 11.37) | 4.23 (1.28, 13.93) |
Indications and Rationale for Surgery among Patients with Drug-Resistant Tuberculosis
Indications for surgical resection in tuberculosis are based on expert opinion and observational studies (Table 1). An early report of surgical resection for MDR-TB by Iseman et al., utilized selection criteria that with some minor modification are still currently used.64 They performed surgery for the following patients 1) such extensive drug resistance that there is a high likelihood of treatment failure or relapse 2) localized disease amenable to resection and 3) sufficient drug activity to reduce remaining mycobacterial burden enough to allow bronchial stump healing. The first group of patients included not only those with persistently positive sputum but also those with culture conversion to negative and a high chance of eventually failing medical treatment.
Table 1.
| A. Indications for Surgery in Drug-Resistant Tuberculosisa
| |
| |
|
| |
| B. Preoperative Work Up before Surgerya | Rationale |
|
| |
| - Chest Computerized tomography Scan65,66 | Evaluate extent of disease |
| - Pulmonary Function Testing65,66 | Guide Surgical Resection |
| - Ventilation Perfusion Scan66 | Ensure adequate pulmonary reserve to tolerate surgery |
| - Bronchoscopy | Rule out endobronchial tuberculosis, contralateral disease, and malignancy |
| - Echocardiogram | Rule out heart failure and pulmonary hypertension |
| - Nutritional Assessment65,66 | Ensure patient can tolerate and recover from surgery |
Most groups accepted patients with minimal scattered nodular or infiltrative bilateral disease for surgery, with the thought that the surgery in such cases was a “debulking” procedure and remaining lesions could be cleared with medications. The criteria recommending sufficient drug activity was not possible in many XDR-TB cases as they were often resistant to most drugs tested. In these patients, surgical resection may offer the only hope for cure given the limited available treatment options.42,54 Guides to the management of drug-resistant TB from Partners in Health65 and the WHO66 recommend similar indications for surgery as above in patients with MDR-TB,32,51,67 where as the CDC/IDSA/ATS tuberculosis treatment guidelines state only that surgical resection should be considered for MDR patients.16 Additionally, the WHO states adjuvant surgery should be considered in XDR-TB patients with localized disease.66 The importance of specialized surgical facilities and experienced thoracic surgeons has been stressed as a prerequisite for offering surgical resection to patients.16,66 Another generalized criterion employed by most is that patients have sufficient cardiopulmonary reserve to tolerate resection. While specific criteria were not usually given, some groups used a vital capacity < 50%, FEV1 < 800–2000ml, or cardiac insufficiency diagnosed by a cardiologist as exclusion criteria.45,60 Other indications for surgical resection not restricted to drug-resistant disease included the following complications of TB: bronchiectasis, empyema, hemoptysis, and aspergilloma.52,60,62,68
The purpose of surgery is to remove a large, focal burden of bacilli present in necrotic and nonviable lung tissue. The tuberculous cavity offers an ideal growth environment as its wall can limit drug penetration and its environment is thought to protect MTB from host defenses.69 Indeed, many of the studies reviewed found that patients who were preoperatively sputum culture negative often have positive cultures in resected lung tissue (27–100%).39,40,45 It is also postulated that cavities may be sites of drug resistance development. In a study of resected tuberculous lung tissue, bacillary growth was found to be most active in macrophages on the cavity surface, the site which harbored the majority of new drug resistance mutations. 69 The investigators found a lack of CD4+ and CD8+ T cells at the luminal surface which may explain active bacillary proliferation. It has also been speculated that after the removal of a major focus of tuberculosis the immune response to residual infection may be enhanced, similar to observed paradoxical reactions sometimes seen in tuberculosis medical treatment.64,70
Preoperative workup
In addition to routine laboratory work and AFB sputum smears and cultures, the preoperative workup reported across most studies was fairly standard and was targeted at localizing the tuberculosis lesion and evaluating cardiopulmonary function (Table 1). The preoperative workup generally included chest radiography followed by computed tomography. These imaging methods were most commonly employed to define the lesion location, identify bilateral disease, and help plan the surgical procedure and approach. Bronchoscopy was frequently used to rule out endobronchial tuberculosis, contralateral disease, and coexisting malignancy.39,41,56,57,61 Pulmonary function testing with spirometry, in some cases followed by a ventilation/perfusion scan, was done to ensure adequate pulmonary reserve. An echocardiogram was occasionally performed to rule out pulmonary hypertension and congestive heart failure.61 Some emphasized performing a comprehensive nutrition evaluation preoperatively and giving appropriate supplementation for malnourished patients. Additionally, one group hospitalized patients who were socioeconomically deprived to ensure they received optimized medical treatment and nutrition support prior to surgery.56
Surgical Procedure
The most common approach for surgical resection in most reports was through a muscle sparing posterolateral thoracotomy. In contrast, an anterior approach was occasionally used and one group recently started performing minimally invasive resection by video-assisted thoracoscopic surgery (VATS) for patients with (1) limited and localized disease, (2) no peribronchial reactive lymph node enlargement, and (3) without severe pleural adhesions. 38,39 An extrapleural dissection was utilized when possible in order to avoid contamination of the pleural space.41,42,56,61,63 The different types of resections performed included pneumonectomy, lobectomy, segmentectomy, wedge resection, and some combination of these procedures. The balance of removing all affected lung and desire to preserve pulmonary function was used to determine which type of resection was done. In patients with bilateral cavitary lesions, the largest lesion was usually resected or in a minority of cases a staged resection of bilateral lesions was done.43,57 The most controversial aspect of surgical resection seemed to be whether bronchial stump reinforcement was needed to help prevent bronchopleural fistula (BPF) formation. Limited peribronchial tissue dissection was also utilized by most groups in an effort to promote healing of the bronchial stump.61 Postoperatively, the pleural space was routinely drained with a chest tube. Occasionally if resection created a large residual space, a thoracoplasty or muscle tent was used to reduce the open space to help prevent further complications.39
Data on the Clinical Efficacy of Surgical Resection for Drug Resistant Tuberculosis
For the following studies, a favorable outcome was either defined as cure, probable cure, or treatment completed as defined by the WHO71 or an international expert consensus group72 or as alive with continued negative sputum culture follow up unless otherwise noted (Table 2, Table 3).
Cases Series
In 18 case series studies, a total of 964 pulmonary TB patients with drug-resistant disease (895 MDR/69 XDR) underwent surgical resection with cohort sizes ranging from 5–172 (Table 2).38–46,51,52,54,56,57,60–63 A few studies included surgical resection in drug susceptible TB cases and these patients were not included in this analysis.44,52,62 All persons were either HIV-seronegative or not tested for HIV. Lobectomy was the most commonly performed procedure (51.7%), followed by pneumonectomy (37.7%), bi-lobectomy or lobectomy plus wedge or segmental resection (6.3%), and segmental or wedge resection (4.3%). In four studies multiple operations were performed for the removal of bilateral cavities39,43,51,57 Across all studies except one there was a minimum of 2 months of preoperative treatment with anti-tuberculosis medications; however, the range was considerable (0.6–240 months). Preoperative positive sputum cultures rates varied between 11–100% (median 53%). The indication for surgery in over 95% of all drug-resistant TB cases across all studies was either medical failure or a clinicians estimation of a high likelihood for relapse with only a few studies including patients operated on for complications of drug-resistant TB such as empyema or hemoptysis.38,56,60 Postoperative medical treatment was generally continued for 12–24 months utilizing the same regimen used preoperatively. The medical regimens varied among the studies with most utilizing individualized regimens based on DST results for first line and when available second line drugs with a goal of including at least 3 effective drugs.38,39,42,54,56,57,61,62
Among the 18 cases series reports, rates of postoperative culture conversion from positive to negative (47–100%, median 92.5%) and favorable outcomes (47–100%, median 89.5%) with few exceptions compared favorably to previously reported outcomes in persons with drug-resistant TB20,73 who received medical treatment alone and in most cases was higher (Table 2). Comparable rates of favorable outcomes were seen both among persons undergoing early or late resection, and in those with positive or negative preoperative cultures. The lowest rates of success were reported from Latvia (47%) and Peru (63%).54,60 The lower success rates among the Latvian cohort might be explained by the fact that they included only XDR-TB patients and many had severe disease: resistant to a mean of 9.2 drugs and 29% with bilateral cavities.54 Even within this study a trend towards worse outcomes was seen with increasing drug resistance and presence of bilateral cavities. Similarly, the Peruvian cohort had very severe disease at baseline, as measured by low FEV1, high drug resistance, bilateral disease, and tuberculosis complications.60 Another study found the following factors to be significantly associated with treatment failure: low BMI, primary resistance, fluoroquinolone resistance, and the presence of bilateral cavities.43 In contrast, a study comparing outcomes in both MDR- and XDR-TB patients undergoing resection and receiving tailored drug regimens based on DST showed no significant difference in favorable outcome rates (93% vs. 85%, p=0.24).38 In this study, amoxicillin-clavulanate, clarithromycin, and/or rifabutin were included as part of the antibiotic regimen when three active drugs were not available based on DST. In regards to timing of surgical resection, Park et al, found a non-statistically significant trend towards improved outcomes when surgery was performed ≤ 6 months after starting medical therapy45; however, no other study showed a difference of outcomes based on timing of surgery. Two studies evaluated the effect of increasing drug resistance on outcomes, and only the Latvian study showed a trend towards more favorable outcomes with less drug resistance.43,54 In the case series from Kang et al, all 4 patients who had minimal invasive resection with VATS achieved favorable outcomes with no treatment failures. No studies indicated a difference in outcomes based on the type of resection performed.
Perioperative morbidity and mortality rates were between 0–39% (median 23%) and 0–5% (median 1.3%), respectively (Table 2). The most commonly encountered postoperative complications were prolonged air leak (4.3%), empyema +/− BPF (3.6%), BPF alone (3.4%), infection (3.1%), bleeding (2.5%), and respiratory insufficiency (1.4%). Rare complications (< 1%) included chylothorax, nerve lesions, space problems, and postpneumonectomy syndrome. Somocurcio et al, found that preoperative hemoptysis and low baseline pulmonary function (FEV1 <1000 ml or vital capacity <50%) were significant risk factors for postoperative complications.60 While Wang et al, showed that endobronchial disease and no bronchial reinforcement were significantly related to increased incidence of BPF, 41 others found a low incidence of BPF in patients both with and without bronchial reinforcement.38,39,45 Rates of reoperation for persistent or recurrent TB were low, with the exception of one study in which 11% required reoperation for persistent disease. 43
Cohort Studies
The cohort sizes for the 8 observational studies measuring the effect of surgery on outcomes ranged from 205–1407 patients with between 4–63% undergoing surgical resection (Table 3). One study included only XDR-TB patients, 4 both MDR- and XDR-TB, and 3 MDR-TB only. 47–50,53,55,58,59 All studies were retrospective and evaluated the effect of surgical resection on overall outcome. The overall favorable outcomes rate ranged from 18–75% (median 66%), with the poorest outcome (18%) seen among the XDR-TB only cohort. There was a favorable trend towards improved outcomes in patients undergoing surgical resection in 7 of 8 studies, including the XDR-TB cohort. Utilizing an aggressive surgical approach (63% surgery rate) in 205 MDR-TB patients, Chan showed a significant improvement in initial favorable response, defined by at least three negative monthly sputum cultures, in those patients undergoing surgery (aOR 4.23, 95% CI 1.28, 13.93).53 In evaluating a cohort of 155 MDR- and XDR-TB patients, Kwon et al. found surgical resection (aOR 11.35, 95% CI 3.02, 42.7448) along with BMI ≥ 18.5 and the use of ≥ 4 effective drugs were all significant predictors of favorable outcome in multivariate analysis. Another Korean cohort of 1407 MDR- and XDR-TB patients also demonstrated surgical resection (aOR 3.87–95% CI 1.69) as well as BMI≥ 18.5 were associated with improved treatment success rates in multivariate analysis.49 While overall favorable outcomes were extremely low, Jeon found in a multivariate analysis that surgical resection (OR 0.18–95% CI .04, .78) along with linezolid use were both associated with a lower risk of poor outcome in XDR-TB patients. 47 All other studies showing a trend towards favorable outcomes with surgery had a non-significant effect. The study showing worse outcomes with surgery was likely due the fact the most patients from this MDR- and XDR-TB cohort who underwent resection had XDR-TB, and XDR was the most significant predictor of treatment failure.50
While the above data is the best available comparing outcomes in MDR- and XDR-TB patients undergoing medical treatment alone versus medical treatment along with adjunctive surgery it does have many limitations. All the studies were retrospective and none were specifically designed to evaluate the role of surgery on outcomes. Two studies did not reveal their criteria for performing surgery.49,58 Additionally, none of the studies compared the characteristics of patients having surgery with those not having surgery, thus making it difficult to estimate how similar the two groups were and introducing possible selection bias.
Conclusions
Treatment of highly drug-resistant TB is extremely challenging and adjunctive surgical therapy has been used to try and enhance the relatively poor outcomes seen with medical therapy alone when treating MDR- and XDR-TB patients. The case history presented earlier illustrates the potential benefits of surgical resection as an adjunctive treatment for drug-resistant TB. Rising rates of drug-resistant TB and limited effective medical treatment necessitates the consideration of adjunctive treatments such as surgical resection in carefully selected patients. Surgical resection has long played a role in the treatment of tuberculosis and recent observational studies show high treatment success rates, a trend towards improved outcomes, and acceptable morbidity when used as adjunctive treatment for MDR- and XDR-TB. The quality of data on the efficacy of adjunctive surgical therapy is relatively poor. No randomized, controlled trials of surgical resection have been performed, and there are many biases in patient selection for surgery including a likely bias towards operating on “healthier” patients. However, even with these limitation it is reasonable to consider surgical resection early in the course of treatment for drug-resistant TB based on the available data, WHO recommendations, and expert opinion.
To further define and optimize the role of surgical resection in treating drug-resistant TB areas of uncertainty need to be addressed. Well designed trials, either case control studies or randomized, controlled trials if ethically feasible, comparing medical vs. combination medical and surgical treatment would help determine the benefit and efficacy of surgery on outcomes. HIV-infected patients need to be included in future studies as they are at an increased risk for poor outcomes with medical treatment.21 The previously reported studies do not include HIV-infected patients. Studies to assess risk factors for favorable outcomes, cost effectiveness, optimal timing of surgery and duration of medical therapy could enhance the beneficial role of surgery. Further studies in resource poor countries along with capacity building will help determine the role of surgery in these areas, which have some of the highest rates of drug-resistant TB and poorest reported outcomes. Determining the role of surgical experience and postoperative care in influencing outcomes would also help determine who and where is best to perform adjunctive surgical resection for drug-resistant TB. Answering the above questions will help us utilize surgery as an effective adjunctive treatment for drug-resistant TB.
Figure 4.
Clinical course, AFB culture results, and antibiotic regimens for our patient.
Acknowledgments
Financial Support:
R.R.K. supported in part by the NIH Fogarty International Center (D43TW007124 and D43TW007124-06S1) and the Atlanta Clinical and Translational Science Institute (NIH/NCRR UL1RR025008).
Footnotes
Authorship: All authors have reviewed and approved the manuscript. Additionally, all authors have contributed significantly to this work. No other writing assistance was provided in the preparation of this manuscript.
Search strategy and selection criteria
We searched PubMed using the terms “tuberculosis”, “Mycobacterium tuberculosis”, “multidrug resistant”, “extensively drug resistant”, and “surgery”. Additionally, references from relevant journal articles were used to identify further sources. Only English language papers were reviewed. The lack of drug susceptibility testing was used as an exclusion criterion. No date restrictions were used in these searches.
Conflicts of interest
We declare that we have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koch R. Classics in infectious diseases. The etiology of tuberculosis: Robert Koch. Berlin, Germany 1882. Rev Infect Dis. 1982;4:1270–4. [PubMed] [Google Scholar]
- 2.Sihoe AD, Shiraishi Y, Yew WW. The current role of thoracic surgery in tuberculosis management. Respirology. 2009;14:954–68. doi: 10.1111/j.1440-1843.2009.01609.x. [DOI] [PubMed] [Google Scholar]
- 3.Perelman MI, Strelzov VP. Surgery for pulmonary tuberculosis. World J Surg. 1997;21:457–67. doi: 10.1007/pl00012270. [DOI] [PubMed] [Google Scholar]
- 4.Tuffier T. De la resection du sommet du poumon. Semin Med. 1891;11:202. [Google Scholar]
- 5.WHO. WHO/HTM/TB/20103. Geneva: World Health Organization; 2010. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response. [Google Scholar]
- 6.WHO. Guidelines for surveillance of drug resistance in tuberculosis. 4. Geneva: World Health Organization; 2009. WHO/HTM/TB/2009422 2009. [Google Scholar]
- 7.WHO. WHO/HTM/TB/20107 2010. Geneva: World Health Organization; 2010. Global tuberculosis control: WHO report 2010. [Google Scholar]
- 8.Raviglione MC. Facing extensively drug-resistant tuberculosis-- a hope and a challenge. N Engl J Med. 2008;359:636–8. doi: 10.1056/NEJMe0804906. [DOI] [PubMed] [Google Scholar]
- 9.Gandhi NR, Nunn P, Dheda K, et al. Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet. 375:1830–43. doi: 10.1016/S0140-6736(10)60410-2. [DOI] [PubMed] [Google Scholar]
- 10.Migliori GB, Sotgiu G, D’Arcy Richardson M, et al. MDR-TB and XDR-TB: drug resistance and treatment outcomes. Eur Respir J. 2009;34:778–9. doi: 10.1183/09031936.00059409. [DOI] [PubMed] [Google Scholar]
- 11.Caws M, Ha DT. Scale-up of diagnostics for multidrug resistant tuberculosis. Lancet Infect Dis. 10:656–8. doi: 10.1016/S1473-3099(10)70188-2. [DOI] [PubMed] [Google Scholar]
- 12.Wright A, Zignol M, Van Deun A, et al. Epidemiology of antituberculosis drug resistance 2002–07: an updated analysis of the Global Project on Anti-Tuberculosis Drug Resistance Surveillance. Lancet. 2009;373:1861–73. doi: 10.1016/S0140-6736(09)60331-7. [DOI] [PubMed] [Google Scholar]
- 13.Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs-- worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55:301–5. [PubMed] [Google Scholar]
- 14.Sotgiu G, Ferrara G, Matteelli A, et al. Epidemiology and clinical management of XDR-TB: a systematic review by TBNET. Eur Respir J. 2009;33:871–81. doi: 10.1183/09031936.00168008. [DOI] [PubMed] [Google Scholar]
- 15.WHO. WHO/HTM/TB/20113 2011. Geneva: World Health Organization; 2010. Towards universal access to diagnosis and treatment of multidrug-resistant and extensively drug-resistant tuberculosis by 2015:WHO progress report 2011. [Google Scholar]
- 16.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167:603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 17.Organization WH. WHO/HTM/TB/2009426 2009. Geneva, Switzerland: WHO; 2009. Global tuberculosis control control: a short update to the 2009 report. [Google Scholar]
- 18.Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–28. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- 19.Olle-Goig JE, Sandy R. Outcomes of individualised treatment for multidrug-resistant tuberculosis before DOTS-plus. Int J Tuberc Lung Dis. 2005;9:765–70. [PubMed] [Google Scholar]
- 20.Orenstein EW, Basu S, Shah NS, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:153–61. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 21.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 181:80–6. doi: 10.1164/rccm.200907-0989OC. [DOI] [PubMed] [Google Scholar]
- 22.Eker B, Ortmann J, Migliori GB, et al. Multidrug- and extensively drug-resistant tuberculosis, Germany. Emerg Infect Dis. 2008;14:1700–6. doi: 10.3201/eid1411.080729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leimane V, Dravniece G, Riekstina V, et al. Treatment outcome of multidrug/extensively drugr-esistant tuberculosis in Latvia, 2000–2004. The European respiratory journal: official journal of the European Society for Clinical Respiratory Physiology. 2010;36:584–93. doi: 10.1183/09031936.00003710. [DOI] [PubMed] [Google Scholar]
- 24.Burman WJ. Rip Van Winkle wakes up: development of tuberculosis treatment in the 21st century. Clin Infect Dis. 50(Suppl 3):S165–72. doi: 10.1086/651487. [DOI] [PubMed] [Google Scholar]
- 25.Hastings J, Storks R. A case of tuberculous excavationof the left lung treated by perforation of the cavity through the walls of the chest. Lond Med Gaz. 1844:378. [Google Scholar]
- 26.Forlanini C. A contribuzione della terapia chirurgica della tisi; ablazione del polmone? pneumatorace artificiale? Gazz d osp. 1882:3, 537, 85, 601, 9, 17, 25, 41, 57, 65, 89, 705. [Google Scholar]
- 27.Daniel T. Captain of Death: The Story of Tuberculosis. Rochester, NY: University of Rochester Press; 1997. Collapse and Mutilation; pp. 195–202. [Google Scholar]
- 28.Meade R. A history of thoracic surgery. Springfield, IL: C.C. Thomas; 1961. [Google Scholar]
- 29.Carter BN. The Late Results of Thoracoplasty in the Treatment of Pulmonary Tuberculosis. Ann Surg. 1936;104:552–9. doi: 10.1097/00000658-193610440-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freedlander SO. Lobectomy in pulmonary tuberculosis: Report of Case. J Thorac Surg. 1935;5:132–42. [Google Scholar]
- 31.Lilienthal H. Pneumonectomy for sarcoma of lung in tuberculosis patient. J Thorac Surg. 1933;2:600–11. [Google Scholar]
- 32.Francis RS, Curwen MP. Major Surgery for Pulmonary Tuberculosis: Final Report. Tubercle. 1964;45(SUPPL):5–79. [PubMed] [Google Scholar]
- 33.Mital OP, Agarwala MC. Results of 150 resections for pulmonary tuberculosis. Indian J Chest Dis. 1970;12:83–90. [PubMed] [Google Scholar]
- 34.Snelling MR, Kam CM. Treatment of 164 patients with chronic pulmonary tuberculosis by pneumonectomy in a developing country. Tubercle. 1968;49:187–91. doi: 10.1016/0041-3879(68)90021-4. [DOI] [PubMed] [Google Scholar]
- 35.Saltini C. Chemotherapy and diagnosis of tuberculosis. Respir Med. 2006;100:2085–97. doi: 10.1016/j.rmed.2006.09.015. [DOI] [PubMed] [Google Scholar]
- 36.Perelman MI. Tuberculosis in Russia. Int J Tuberc Lung Dis. 2000;4:1097–103. [PubMed] [Google Scholar]
- 37.Motus IY, Skorniakov SN, Sokolov VA, et al. Reviving an old idea: can artificial pneumothorax play a role in the modern management of tuberculosis? The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2006;10:571–7. [PubMed] [Google Scholar]
- 38.Kang MW, Kim HK, Choi YS, et al. Surgical treatment for multidrug-resistant and extensive drug-resistant tuberculosis. Ann Thorac Surg. 89:1597–602. doi: 10.1016/j.athoracsur.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Shiraishi Y, Katsuragi N, Kita H, Tominaga Y, Kariatsumari K, Onda T. Aggressive surgical treatment of multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2009;138:1180–4. doi: 10.1016/j.jtcvs.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Park SK, Kim JH, Kang H, Cho JS, Smego RA., Jr Pulmonary resection combined with isoniazid-and rifampin-based drug therapy for patients with multidrug-resistant and extensively drug-resistant tuberculosis. Int J Infect Dis. 2009;13:170–5. doi: 10.1016/j.ijid.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 41.Wang H, Lin H, Jiang G. Pulmonary resection in the treatment of multidrug-resistant tuberculosis: a retrospective study of 56 cases. Ann Thorac Surg. 2008;86:1640–5. doi: 10.1016/j.athoracsur.2008.07.056. [DOI] [PubMed] [Google Scholar]
- 42.Shiraishi Y, Katsuragi N, Kita H, Toishi M, Onda T. Experience with pulmonary resection for extensively drug-resistant tuberculosis. Interact Cardiovasc Thorac Surg. 2008;7:1075–8. doi: 10.1510/icvts.2008.185124. [DOI] [PubMed] [Google Scholar]
- 43.Kim HJ, Kang CH, Kim YT, et al. Prognostic factors for surgical resection in patients with multidrug-resistant tuberculosis. Eur Respir J. 2006;28:576–80. doi: 10.1183/09031936.06.00023006. [DOI] [PubMed] [Google Scholar]
- 44.Takeda S, Maeda H, Hayakawa M, Sawabata N, Maekura R. Current surgical intervention for pulmonary tuberculosis. Ann Thorac Surg. 2005;79:959–63. doi: 10.1016/j.athoracsur.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 45.Park SK, Lee CM, Heu JP, Song SD. A retrospective study for the outcome of pulmonary resection in 49 patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2002;6:143–9. [PubMed] [Google Scholar]
- 46.Chiang CY, Yu MC, Bai KJ, Suo J, Lin TP, Lee YC. Pulmonary resection in the treatment of patients with pulmonary multidrug-resistant tuberculosis in Taiwan. Int J Tuberc Lung Dis. 2001;5:272–7. [PubMed] [Google Scholar]
- 47.Jeon DS, Kim DH, Kang HS, et al. Survival and predictors of outcomes in non-HIV-infected patients with extensively drug-resistant tuberculosis. Int J Tuberc Lung Dis. 2009;13:594–600. [PubMed] [Google Scholar]
- 48.Kwon YS, Kim YH, Suh GY, et al. Treatment outcomes for HIV-uninfected patients with multidrug-resistant and extensively drug-resistant tuberculosis. Clin Infect Dis. 2008;47:496–502. doi: 10.1086/590005. [DOI] [PubMed] [Google Scholar]
- 49.Kim DH, Kim HJ, Park SK, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med. 2008;178:1075–82. doi: 10.1164/rccm.200801-132OC. [DOI] [PubMed] [Google Scholar]
- 50.Kim HR, Hwang SS, Kim HJ, et al. Impact of extensive drug resistance on treatment outcomes in non-HIV-infected patients with multidrug-resistant tuberculosis. Clin Infect Dis. 2007;45:1290–5. doi: 10.1086/522537. [DOI] [PubMed] [Google Scholar]
- 51.Pomerantz BJ, Cleveland JC, Jr, Olson HK, Pomerantz M. Pulmonary resection for multi-drug resistant tuberculosis. J Thorac Cardiovasc Surg. 2001;121:448–53. doi: 10.1067/mtc.2001.112339. [DOI] [PubMed] [Google Scholar]
- 52.Treasure RL, Seaworth BJ. Current role of surgery in Mycobacterium tuberculosis. Ann Thorac Surg. 1995;59:1405–7. doi: 10.1016/0003-4975(95)00145-b. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 53.Chan ED, Laurel V, Strand MJ, et al. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2004;169:1103–9. doi: 10.1164/rccm.200308-1159OC. [DOI] [PubMed] [Google Scholar]
- 54.Dravniece G, Cain KP, Holtz TH, Riekstina V, Leimane V, Zaleskis R. Adjunctive resectional lung surgery for extensively drug-resistant tuberculosis. Eur Respir J. 2009;34:180–3. doi: 10.1183/09031936.00047208. [DOI] [PubMed] [Google Scholar]
- 55.Leimane V, Riekstina V, Holtz TH, et al. Clinical outcome of individualised treatment of multidrug-resistant tuberculosis in Latvia: a retrospective cohort study. Lancet. 2005;365:318–26. doi: 10.1016/S0140-6736(05)17786-1. [DOI] [PubMed] [Google Scholar]
- 56.Orki A, Kosar A, Demirhan R, Saygi A, Arman B. The value of surgical resection in patients with multidrug resistant tuberculosis. Thorac Cardiovasc Surg. 2009;57:222–5. doi: 10.1055/s-0029-1185458. [DOI] [PubMed] [Google Scholar]
- 57.Kir A, Inci I, Torun T, Atasalihi A, Tahaoglu K. Adjuvant resectional surgery improves cure rates in multidrug-resistant tuberculosis. J Thorac Cardiovasc Surg. 2006;131:693–6. doi: 10.1016/j.jtcvs.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 58.Keshavjee S, Gelmanova IY, Farmer PE, et al. Treatment of extensively drug-resistant tuberculosis in Tomsk, Russia: a retrospective cohort study. Lancet. 2008;372:1403–9. doi: 10.1016/S0140-6736(08)61204-0. [DOI] [PubMed] [Google Scholar]
- 59.Torun T, Tahaoglu K, Ozmen I, et al. The role of surgery and fluoroquinolones in the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2007;11:979–85. [PubMed] [Google Scholar]
- 60.Somocurcio JG, Sotomayor A, Shin S, et al. Surgery for patients with drug-resistant tuberculosis: report of 121 cases receiving community-based treatment in Lima, Peru. Thorax. 2007;62:416–21. doi: 10.1136/thx.2005.051961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mohsen T, Zeid AA, Haj-Yahia S. Lobectomy or pneumonectomy for multidrug-resistant pulmonary tuberculosis can be performed with acceptable morbidity and mortality: a seven-year review of a single institution’s experience. J Thorac Cardiovasc Surg. 2007;134:194–8. doi: 10.1016/j.jtcvs.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 62.Naidoo R. Active pulmonary tuberculosis: experience with resection in 106 cases. Asian Cardiovasc Thorac Ann. 2007;15:134–8. doi: 10.1177/021849230701500211. [DOI] [PubMed] [Google Scholar]
- 63.van Leuven M, De Groot M, Shean KP, von Oppell UO, Willcox PA. Pulmonary resection as an adjunct in the treatment of multiple drug-resistant tuberculosis. Ann Thorac Surg. 1997;63:1368–72. discussion 72–3. [PubMed] [Google Scholar]
- 64.Iseman MD, Madsen L, Goble M, Pomerantz M. Surgical intervention in the treatment of pulmonary disease caused by drug-resistant Mycobacterium tuberculosis. Am Rev Respir Dis. 1990;141:623–5. doi: 10.1164/ajrccm/141.3.623. [DOI] [PubMed] [Google Scholar]
- 65.Health Pi. Adjuvant Therapies and Strategies. The PIH Guide to the Medical Management of Multidrug-Resistant Tuberculosis United States Partners in Health. 2003:29–32. [Google Scholar]
- 66.Organization WH. WHO/HTM/TB/2008402 2008. Geneva, Switzerland: WHO; 2008. Guidelines for the programmatic management of drug-resistant tuberculosis. [Google Scholar]
- 67.Sung SW, Kang CH, Kim YT, Han SK, Shim YS, Kim JH. Surgery increased the chance of cure in multi-drug resistant pulmonary tuberculosis. Eur J Cardiothorac Surg. 1999;16:187–93. doi: 10.1016/s1010-7940(99)00158-x. [DOI] [PubMed] [Google Scholar]
- 68.Olcmen A, Gunluoglu MZ, Demir A, Akin H, Kara HV, Dincer SI. Role and outcome of surgery for pulmonary tuberculosis. Asian Cardiovasc Thorac Ann. 2006;14:363–6. doi: 10.1177/021849230601400503. [DOI] [PubMed] [Google Scholar]
- 69.Kaplan G, Post FA, Moreira AL, et al. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099–108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Orlovic D, Smego RA. Paradoxical tuberculosis reactions in HIV-infected patients. Int J Tuberc Lung Dis. 2001;5:370–5. [PubMed] [Google Scholar]
- 71.WHO. WHO/TB/99260 1999. Geneva: WHO; 1999. Protocol for the implementation of individualized treatment regimens for multi-drug resistant tuberculosis in resource-poor settings. [Google Scholar]
- 72.Laserson KF, Thorpe LE, Leimane V, et al. Speaking the same language: treatment outcome definitions for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2005;9:640–5. [PubMed] [Google Scholar]
- 73.Jacobson KR, Tierney DB, Jeon CY, Mitnick CD, Murray MB. Treatment outcomes among patients with extensively drug-resistant tuberculosis: systematic review and meta-analysis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2010;51:6–14. doi: 10.1086/653115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Organization WH. WHO/HTM/TB/2006361 2006. Geneva, Switzerland: WHO; 2006. Guidelines for the programmatic management of drug-resistant tuberculosis. [Google Scholar]