Abstract
Dose-limiting peripheral neuropathy (PN) is frequently reported with the use of thalidomide and bortezomib, novel proteasome inhibitors. While these two agents have significant activity in multiple myeloma (MM), the combination and the associated PN have not been fully examined in untreated patients. The objective of this study was to report the baseline prevalence and occurrence of PN in newly diagnosed MM patients treated with bortezomib and thalidomide. Twenty-seven patients (11 men and 16 women) with previously untreated MM were prospectively monitored for PN. Total neuropathy score reduced (TNSr) was calculated at baseline and after every two cycles of bortezomib treatment. The median cumulative dose of bortezomib was 35.6 mg/m2 (median 8 cycles) and of thalidomide was 16.8 g. Only three subjects showed mild PN at baseline (whole group median TNSr 0). At the end of treatment, PN developed in 26 patients (median TNSr 8). PN was of mild to moderate severity (TNSr grade 1 = 11, grade 2 = 10, grade 3 = 5, and grade 4 = 0). Nerve conduction studies showed axonal physiology in all except three subjects in whom demyelinating physiology was noted. The median TNSr was 17 in the demyelinating group and 9 in the axonal group. There was no significant correlation of TNSr with cumulative bortezomib or thalidomide dose. At follow-up, 80% of patients had become asymptomatic after discontinuation of the chemotherapy. We conclude that bortezomib and thalidomide combination chemotherapy induces a reversible length-dependent sensory>motor, predominantly axonal, large-fiber>small-fiber polyneuropathy. In a subset, a more severe demyelinating polyneuropathy may develop.
Keywords: ortezomib, CIPN, thalidomide, total neuropathy score, toxic neuropathy
Introduction
Bortezomib (Velcade®) represents a novel class of anticancer agents that have the ability to affect a combination of cellular regulatory mechanisms primarily through proteasome inhibition, a pathway important in regulation of cell cycle, neoplastic growth, and metastasis (Kane et al., 2003; Jagannath, 2007; Mateos and San Miguel, 2007). Bortezomib has demonstrated clinical efficacy in newly diagnosed and in recurrent multiple myeloma (MM). Peripheral neuropathy (PN) is a known toxicity of bortezomib. New onset or worsening of existing PN has been noted throughout the cycles of treatment in 25–55% of patients (Richardson et al., 2003; Bang et al., 2006; Harousseau et al., 2006; Mateos et al., 2006; Badros et al., 2007; Kane et al., 2007; Kropff et al., 2007; Min et al., 2007; Rosinol et al., 2007). In these studies, 37–81% of patients had signs or symptoms of PN at baseline evaluation (Richardson et al., 2006; Badros et al., 2007).Although the incidence of more severe PN [National Cancer Institute-Common Toxicity Criteria (NCI-CTC) grade ≥3 sensory PN] is low, up to one-third of the patients require dose reduction or discontinuation of bortezomib due to PN (Bang et al., 2006; Richardson et al., 2006; Badros et al., 2007). In the majority of patients, dose reduction results in improvement of the PN symptoms (Bang et al., 2006; Richardson et al., 2006; Badros et al., 2007).
Thalidomide (alpha-[n-phthalimido] glutarimide) is an U.S. Food and Drug Administration-approved drug for the treatment of MM (Barlogie et al., 2001). The mechanism of action of thalidomide is poorly understood but thought to involve both immunomodulation and antiangiogenesis. Like bortezomib (Diggle, 2001; Richardson et al., 2002), thalidomide is known to cause length-dependent predominantly sensory axonal neuropathy that affects large and small fibers with an incidence ranging from 1 to 70% (Sheskin, 1980; Wulff et al., 1985). Bortezomib and thalidomide as single agents show activity in less than 50% of patients with newly diagnosed MM (Richardson et al., 2006). This prospective study was designed to characterize the baseline prevalence of underlying PN and the development and progression of chemotherapy-induced peripheral neuropathy (CIPN) in newly diagnosed MM patients treated with the combination of bortezomib and thalidomide.
Materials and Methods
Untreated Salmon-Durie stage II/III MM subjects with an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 were included. All adult subjects had a measurable paraprotein in either serum or urine [monoclonal immunoglobulin (Ig) spike on serum electrophoresis IgG >3.5 g/dl or IgA >2.0 g/dl; kappa or lambda light chain excretion >1 g/day on 24-h urine protein electrophoresis] and plasmacytosis.
Bortezomib was administered intravenously at an initial dose of 1.3 mg/m2 on days 1, 4, 8, and 11 every 21 days. Thalidomide was administered orally at bedtime starting with day 1 of the first cycle of bortezomib at an initial dose of 50 mg and escalated by 50 mg each week to a maximum tolerated dose of 150 mg daily. Patients were treated for a minimum of three and maximum of eight cycles. All patients underwent a directed neurological evaluation including history and examination at baseline and after every two cycles (Cornblath et al., 1999). Nerve conduction without electromyography was performed at baseline, every 2 months, and at study termination using standard techniques. Nerve conduction study consisted of sequential evaluation of sural and peroneal nerves using a standardized protocol. Nerve conduction study of median and ulnar sensory and motor nerves was performed if abnormalities were noted in lower limb nerves. The total neuropathy score reduced (TNSr), a composite score ranging from 0 to 32 points that includes symptoms, signs, and nerve conduction values, was calculated at baseline and every 2 months (Cavaletti et al., 2006; 2007). The presence of neuropathy was defined as TNSr ≥2.
Patients were planned to be treated for a minimum of four cycles to obtain at least a 50% reduction in the tumor burden. Patients proceeding to transplantation came off study at the time eligibility for transplantation. For patients not undergoing transplantation, therapy was continued for a maximum of eight-cycles. In a subset of patients, bortezomib was continued beyond the eight cycles at a lower ‘maintenance dose’ at the discretion of the treating oncologist. Skin biopsy for measurement of intra-epidermal fiber density was done at baseline for all subjects and at study termination in 14 subjects using standard techniques (McArthur et al., 1998).
The presence and severity of CIPN were established during each visit and correlations made to the cumulative dosages of bortezomib and thalidomide. PN was characterized as to the fiber type, physiology (axonal or demyelinating), length-dependent vs. length-independent relationship to cumulative dose, and number of cycles of treatment.
A dose-related neurotoxic effect of bortezomib and thalidomide was determined through regression analysis with the change in TNSr as the dependent variable. Bortezomib and thalidomide doses were used as independent variables. Statistical analysis for comparison of changes in electrophysiological and skin density measures was done using Wilcoxon rank sum and Mann-Whitney U tests, with the level of significance set at 0.05. This study was approved by the Johns Hopkins Medicine institutional review board. All subjects provided written informed consent.
Results
A total of 27 MM subjects (11 men and 16 women; median age 58 years; range 37–83 years) were included. Ten had IgG lambda paraproteins, 8 IgG kappa, 7 IgA kappa, and 2 kappa light chains (Table 1).
Table 1.
Patient demographics, TNS, and chemotherapy dosages.*
| Patient number | Age/sex | Diagnosis | TNSr (B) | TNSr (end) | Bortezomib (mg/m2) | Thalidomide (g) |
|---|---|---|---|---|---|---|
| 1 | 67/F | IgGlambda | 0 | 10 | 31.8 | 24 |
| 2 | 51/F | IgA kappa | 0 | 18 | 28.4 | 16.8 |
| 3 | 61/M | IgG kappa | 1 | 12 | 42 | 16.8 |
| 4 | 51/F | IgGlambda | 0 | 14 | 38 | 24 |
| 5 | 50/F | IgGlambda | 0 | 10 | 26 | 5.6 |
| 6 | 37/F | IgGlambda | 0 | 6 | 38.2 | 22.1 |
| 7 | 65/F | IgA kappa | 0 | 14 | 22.3 | 5.8 |
| 8 | 58/M | IgA kappa | 0 | 7 | 42 | 16.8 |
| 9 | 58/F | IgGlambda | 0 | 7 | 38.3 | 24 |
| 10 | 83/M | IgG kappa | 5 | 15 | 39.2 | 20 |
| 11 | 48/M | IgGlambda | 0 | 8 | 31.6 | 17.9 |
| 12 | 55/F | IgG kappa | 2 | 12 | 42 | 20 |
| 13 | 48/M | Kappa light chain | 3 | 8 | 35.6 | 4.5 |
| 14 | 56/M | IgA kappa | 0 | 19 | 33.2 | 15.7 |
| 15 | 61/F | IgG kappa | 0 | 7 | 42 | 20 |
| 16 | 78/M | IgG kappa | 5 | 24 | 34 | 10 |
| 17 | 55/M | IgG kappa | 0 | 15 | 39.2 | 21 |
| 18 | 57/F | IgA kappa | 0 | 8 | 40.4 | 16.7 |
| 19 | 68/F | IgGlambda | 0 | 7 | 36.8 | 19.9 |
| 20 | 60/M | IgG kappa | 0 | 17 | 27.8 | 15.7 |
| 21 | 54/F | IgA kappa | 0 | 3 | 21.6 | 3.7 |
| 22 | 58/M | IgGlambda | 0 | 7 | 37 | 18 |
| 23 | 71/M | IgG kappa | 0 | 19 | 11 | 5 |
| 24 | 64/F | IgGlambda | 0 | 13 | 35.6 | 11.6 |
| 25 | 62/F | IgA kappa | 0 | 5 | 21.8 | 10.5 |
| 26 | 48/F | Kappa light chain | 0 | 1 | 31.9 | 17.5 |
| 27 | 67/F | IgGlambda | 0 | 3 | 42 | 21 |
| Mean | 59 | 1 | 11 | 33.7 | 15.7 | |
| Median | 58 | 0 | 8 | 35.6 | 16.8 |
B, baseline; end, end of treatment; TNSr, total neuropathy score reduced; TNS, total neuropathy score; Ig, immunoglobulin.
Thalidomide and bortezomib dosages are cumulative at the time of final TNS measurement.
At baseline, three had PN (TNSr ≥2) (Table 1). All were NCI-CTC grade 1 neuropathy, and only one was symptomatic with mild numbness or weakness. The median TNSr for all patients at baseline was 0 (range 0–5). At baseline, five (19%) had abnormal intra-epidermal nerve fiber density (McArthur et al., 1998). Two patients who did not have PN defined by TNSr had abnormal intra-epidermal fiber density at baseline.
Twenty-one subjects completed eight cycles of bortezomib and thalidomide therapy. In seven subjects, bortezomib and thalidomide (one each after cycles 3, 5, 6, and 7 and two after four cycles) were discontinued before eight cycles due to disease progression. The median cumulative doses of bortezomib and thalidomide were 36 mg/m2 (range 11–42 mg/m2) and 16.8 g (range 3.7–24 g), respectively. The average administered doses of bortezomib were 1.1 mg/m2 on days 1, 4, 8, and 11 every 21 days and 110 mg of thalidomide daily.
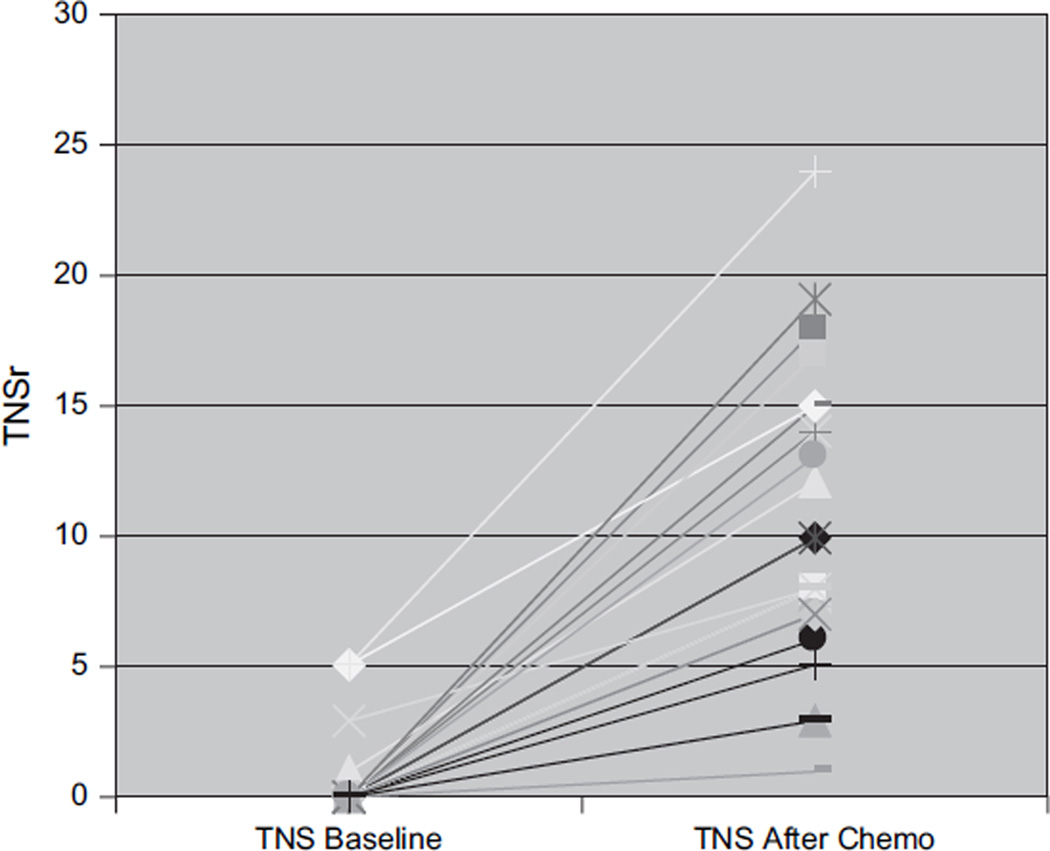
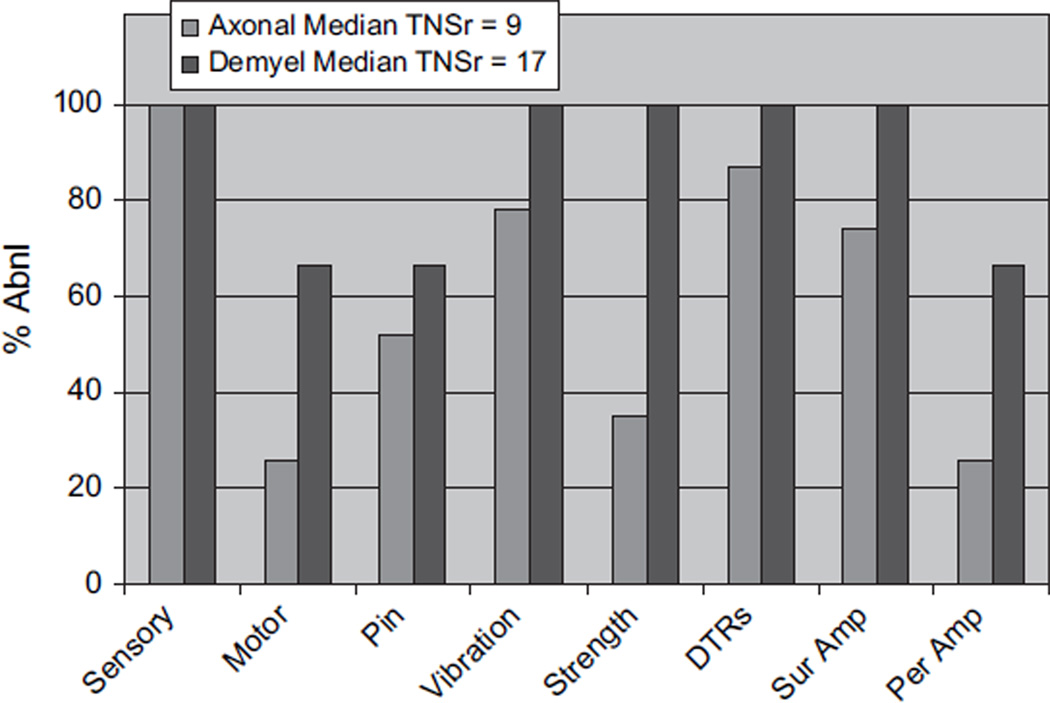
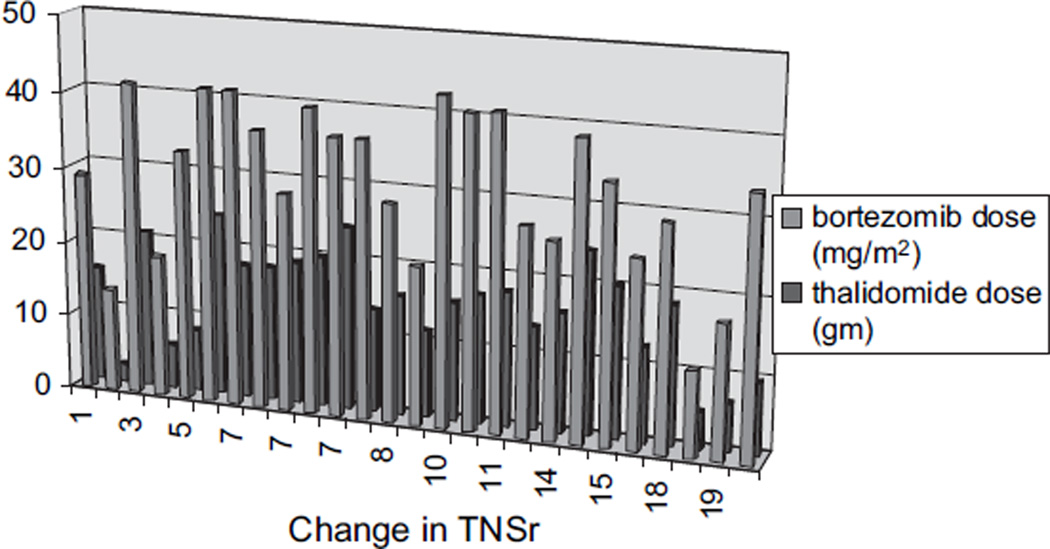
CIPN developed in 26 of 27 patients by the time of their last evaluation (median TNSr 10 and range 1–24) (Table 1; Fig. 1). With the PN being graded on the basis of the TNSr (TNSr 2–8 = grade 1; TNSr 9–16 = grade 2; TNSr 17–24 = grade 3; and TNSr 25–32 = grade 4), 11 were grade 1, 10 grade 2, 5 grade 3, and none grade 4 (Fig. 1). All 26 developed paresthesias/dysesthesias in a length-dependent fashion and 3 also complained of perioral or scalp tingling and numbness. Nine were on neuropathic pain medications, and eight required narcotic analgesics. Most were significantly symptomatic by cycles 4–6, requiring neuropathic pain medication and/or reduction of the dose of bortezomib (9) and thalidomide (8). In all subjects in whom the dose was reduced or treatment discontinued, neuropathic pain improved. Eight complained of weakness, and four had autonomic symptoms of orthostasis and/or diarrhea. Examination showed length-dependent loss to pin (14 subjects) and vibration (21), distal weakness (11), and absent ankle reflexes (23). NC showed reduced or absent sural amplitudes (20) and reduced peroneal motor amplitudes (8). In 23 patients, the nerve conduction findings at the end of the treatment period were ‘axonal’ with reduced amplitudes and preserved distal motor latencies and conduction velocities. In the other three, the physiology was demyelinating with reduced conduction velocities, partial motor conduction block, and prolonged F-wave latencies (Table 2). Table 3 details the baseline and end of treatment data for sural and peroneal nerves. Comparison of baseline to end of treatment revealed significant changes in sural amplitude (p < 0.001), peroneal amplitude (p < 0.05), and peroneal conduction velocity (p < 0.001) but not in sural conduction velocity (p = 0.069). Comparison of the demyelinating with the axonal group (Fig. 2) revealed that the neuropathy was more severe (median TNSr 17 vs. 9) and associated with areflexia and greater motor involvement both clinically and physiologically in the demyelinating group. Skin biopsy was performed in 14 at follow-up, with the intraepidermal nerve fiber density (IENFD) decreasing at the distal leg site from 10.6 ± 6.0 (median 9.7) to 9.1 ± 4.6 fibers/mm (median 8.4) (p = 0.2). The IENFD at the proximal thigh site was 18.2 ± 8.0 at baseline and 18.3 ± 7.2 at follow-up. Significant PN requiring neuropathic medications and/or dose reduction developed in all patients by the fifth or sixth cycle of therapy. There was no significant association between change in TNSr with cumulative bortezomib (p = 0.21) or thalidomide dose (p = 0.45). Subjects receiving higher cumulative doses of both bortezomib and thalidomide did not have higher TNSr values (Fig. 3). Presence of pre-existing PN did not appear to predict worsening of PN, although the number of patients with pre-existing neuropathy was too small to provide a statistical comparative analysis (p = 0.98).
Figure 1.
Baseline TNSr and end of treatment TNSr in the 27 subjects depicting the worsening of TNSr after chemotherapy with bortezomib and thalidomide. TNSr, total neuropathy score reduced.
Table 2.
Nerve conduction findings in three patients with demyelinating neuropathy.
| DL (ms) |
CMAP D/P (mV) |
CV (m/s) |
F wave (ms) |
|
|---|---|---|---|---|
| Patient number 7: peroneal | ||||
| Baseline | 3.1 | 5.5/4.5 | 52 | 42.8 |
| 5 cycles | 4.5 | 2.6/0.8 | 26 | NR |
| 1-year follow-up | 4 | 2.4/2.1 | 44 | 48.8 |
| Patient number16: median | ||||
| Baseline | 4.5 | 7.1 | 50 | 31.3 |
| 4 cycles | 4.9 | 3.2 | 44 | NR |
| 8 cycles | 4.4 | 1. 8 | 37 | NR |
| Patient number 20: peroneal | ||||
| Baseline | 3.5 | 4.2 | 40 | 56.7 |
| 6-cycle follow-up | 4.6 | 2.5 | 31 | 75.9 |
CV, conduction velocity; DL, distal latency; D/P, distal/proximal; NR, no response.
Table 3.
Nerve conduction findings in all patients with toxic neuropathy.
| Patient number |
Baseline data | After 8 cycles or last follow-up | ||||||
|---|---|---|---|---|---|---|---|---|
| Sural SNAP (μV) |
Peroneal CMAP (mV) |
Sural CV (m/s) |
Peroneal CV (m/s) |
Sural SNAP (μV) |
Peroneal CMAP (mV) |
Sural CV |
Peroneal CV |
|
| 1 | 9.4 | 4.9 | 41 | 47 | 6.5 | 3.9 | 37 | 43 |
| 2 | 21.5 | 4.9 | 42 | 48 | 0 | 1. 2 | 41 | |
| 3 | 7.8 | 3.4 | 34 | 44 | 3.2 | 3 | 36 | 39 |
| 4 | 21 | 5 | 42 | 52 | 2.5 | 3.9 | 31 | 42 |
| 5 | 26 | 2.3 | 45 | 53 | 8 | 2 | 40 | 40 |
| 6 | 15.2 | 7.4 | 65 | 53 | 17.6 | 6.4 | 54 | 41 |
| 7 | 14.7 | 5.5 | 46 | 52 | 3.2 | 2.6 | 34 | 27 |
| 8 | 21.9 | 5 | 51 | 49 | 11 | 3.9 | 40 | 42 |
| 9 | 33.1 | 3.6 | 48 | 51 | 11. 5 | 1. 8 | 39 | 54 |
| 10 | 6.7 | 1.7 | 42 | 43 | 0 | 1. 3 | 38 | |
| 11 | 22.7 | 3.2 | 40 | 55 | 17 | 5.8 | 43 | 40 |
| 12 | 31.8 | 1. 6 | 46 | 42 | 11.7 | 1 | 42 | 48 |
| 13 | 11. 5 | 3.4 | 40 | 53 | 6.6 | 3.7 | 40 | 42 |
| 14 | 11. 3 | 4.7 | 55 | 40 | 0 | 0.6 | 34 | |
| 15 | 24 | 6.4 | 60 | 50 | 6.2 | 3.9 | 60 | 51 |
| 16 | 8.9 | 1. 9 | 37 | 41 | 0 | 0 | ||
| 17 | 17 | 4.9 | 49 | 51 | 3 | 4.4 | 45 | 44 |
| 18 | 16.5 | 6.6 | 41 | 51 | 4.1 | 6.6 | 42 | 44 |
| 19 | 11 | 5.6 | 41 | 46 | 3.6 | 3.1 | 37 | 43 |
| 20 | 12.9 | 4.2 | 41 | 40 | 2.30 | 2.5 | 34 | 31 |
| 21 | 31.6 | 6.7 | 41 | 44 | 23.9 | 6.4 | 40 | 42 |
| 22 | 11.1 | 7.8 | 40 | 41 | 2.3 | 5.5 | 44 | 39 |
| 23 | 12.9 | 3.2 | 49 | 55 | 0 | 0.8 | 40 | |
| 24 | 15.2 | 3.3 | 45 | 43 | 0 | 0.6 | 40 | |
| 25 | 23.9 | 5.6 | 42 | 47 | 18.4 | 5 | 44 | 42 |
| 26 | 19.3 | 2.1 | 44 | 48 | 13.2 | 3.3 | 42 | 45 |
| 27 | 19.4 | 2.9 | 41 | 48 | 14.8 | 3.9 | 50 | 49 |
| Mean | 17.7 | 4.4 | 44.7 | 47.7 | 7.1 | 3.2 | 41.6 | 41.5 |
| Median | 16.5 | 4.7 | 42.0 | 48.0 | 4.1 | 3.3 | 40.0 | 42.0 |
| Normal values for sensory nerve conduction studies | ||||||||
| Amplitude >9 μV | ||||||||
| Conduction velocity – lower limb >39 m/s, upper limb >49 m/s | ||||||||
| Normal values for motor nerve conduction studies. | ||||||||
| Nerve | DL (ms) | CV (m/s) | Amplitude (mV) | F (ms) |
|---|---|---|---|---|
| Peroneal | <5.6 | >39 | >2 | <56 |
| Tibial | <6.1 | >39 | >2 | <56 |
| Median | <4.3 | >49 | >4 | <32 |
| Ulnar | <3.5 | >49 | >4 | <33 |
CV, conduction velocity; SNAP, sensory nerve action potential.
Figure 2.
TNSr and its subcomponents in the two groups, those developing axonal with those developing demyelinating neuropathy. Subjects developing demyelinating neuropathy had higher TNSr and more changes in strength, vibration, and neurophysiological change. TNSr, total neuropathy score reduced.
Figure 3.
Change in TNSr is plotted against the cumulative doses of bortezomib and thalidomide. Subjects receiving higher cumulative doses of both bortezomib and thalidomide did not have higher TNSr values. TNSr, total neuropathy score reduced.
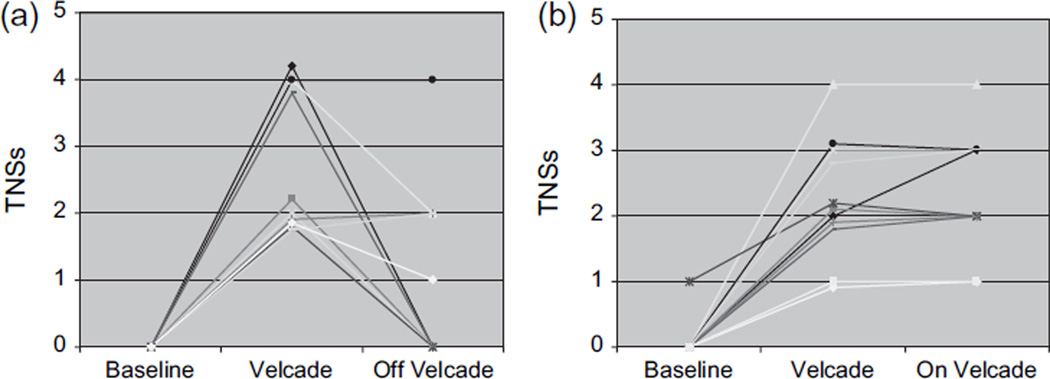
Follow-up information after completion of the eight study cycles of bortezomib and thalidomide study was available for 23 patients (2 died and 2 were lost to follow-up). Ten patients discontinued the two drugs completely, while the other 13 continued on lower maintenance doses of the two drugs after completion of the research protocol. Of the 10 who were off the drugs, 5 became asymptomatic, 1 improved (less symptoms), and 3 were unchanged at a median time frame of 2 months of follow-up (range 1–5 months) (Fig. 4a). Thirteen continued to receive a maintenance therapy, with the two drugs in varying dosages after the research study. In this group, 11 continued to have the same degree of symptoms and 2 became slightly worse (Fig. 4b).
Figure 4.
Reversibility of toxic neuropathy in the two groups: (a) those who completely stopped bortezomib and thalidomide; the median TNSs for 10 patients who stopped the drug was 1 at follow-up and (b) those who continued on a lower dose; median TNSs was 3 for those who continued with bortezomib. TNS, total neuropathy score; TNSs, TNS symptom score.
Discussion
In our study, bortezomib and thalidomide combination therapy induced a length-dependent sensory>. motor, axonal, large<small-fiber polyneuropathy that was cumulative with each additional cycle. In three subjects, demyelinating PN developed with more severe symptoms, and even though the neuropathy was more severe in these subjects, the symptoms and physiology improved after discontinuation of therapy in these patients. In other patients, the neuropathic symptoms appeared to stabilize or improve with discontinuation of the drug and addition of neuropathic medications.
Baseline neuropathy
Unlike previous studies that reported that 37– 81% of patients had PN at baseline in MM patients (Richardson et al., 2006; Badros et al., 2007), our study revealed baseline neuropathy in only 11% of patients. The primary reason for this is that our patients were all previously untreated with any potentially neurotoxic drugs. All had a recent diagnosis of MM rather than refractory MM with longer duration of illness as in prior studies. In addition, our criteria for defining neuropathy were more stringent than those previously described. In our study, the presence of symptoms alone was not sufficient to diagnose neuropathy. In other studies, e.g., a patient with tingling in hands or difficulty buttoning was called neuropathy even though this may potentially be secondary to entrapment neuropathy or radiculopathy among other potential causes. A recent study looking at 31 previously untreated MM patients reported the incidence of baseline neuropathy similar to ours with 3 (12.9%) patients reporting paresthesias and having nerve conduction study findings of axonal sensory motor neuropathy (Plasmati et al., 2007).
Development of neuropathy
We report a much higher percentage of patients developing a neuropathy (96%: 42% grade 1, 38% grade 2, and 19% grade 3) compared with 25–55% reported previously (Richardson et al., 2003; Bang et al., 2006; Harousseau et al., 2006; Mateos et al., 2006; Badros et al., 2007; Kane et al., 2007; Kropff et al., 2007; Min et al., 2007; Rosinol et al., 2007). The percentages of subjects with more severe neuropathy and percentage of subjects reducing dose or discontinuing bortezomib in our study were similar to previous studies. The first reason for the differences observed in the higher incidence of CIPN is the rigorous measures used to assess neuropathy in this study. Most other studies have relied on the NCI-CTC for assessing CIPN as done by the treating physician. We used a comprehensive and independent physician-directed approach using multiple modalities – symptoms, signs, and physiology – to assess neuropathy. A second reason may be the fact that we have assessed previously untreated MM patients with relatively low frequency of baseline neuropathy (see below). In the presence of existing neuropathy, development of additional symptoms could conceivably not be noted by the patient or new signs not so easily detected. Third, a synergistic effect of the bortezomib and thalidomide is possible though we are not able to address this because all subjects in this study received both drugs. The PN associated with thalidomide typically occurs in the setting of chronic administration and at higher doses than were used in our study (Chaudhry et al., 2002; Plasmati et al., 2007). The dose of thalidomide in this study was 15 g compared with at least 20 g typically seen when thalidomide is used as monotherapy (Chaudhry et al., 2002; Cavaletti et al., 2004; Plasmati et al., 2007). It is possible that the two together may have produced additive neuropathy. Bang et al. (2006) compared bortezomib and bortezomib with thalidomide in a retrospective review of 95 patients and were able to show that the combined group had higher incidence of neurotoxicity compared with bortezomib alone (74 vs. 32%). Last, the prospective and combined approach of patients being seen by both an oncologist and a neurologist provides a unique opportunity for more careful evaluation of neuropathy.
Physiology
Although as previously reported, most patients develop an axonal neuropathy (Richardson et al., 2006), we report that a subset of patients also develop a demyelinating polyneuropathy, which is more severe, albeit reversible. Sensory amplitudes were reduced in 74% of the patients as reported in previous reports, but unlike previous reports, we also report that 30% of patients have reduction of motor amplitudes.
Neuropathy vs. neuronopathy
As reported previously, we also found that most patients develop a length-dependent stocking-glove pattern of symptoms and signs. Not only were the symptoms and signs distally prominent, the nerve conduction studies showed a length-dependent gradient affection of sensory nerve action potential amplitudes. Given the symptom onset in the lower limbs with distal sensory loss, the absent ankle reflexes, and the additional involvement of compound muscle action potential amplitudes, we feel that bortezomib and thalidomide induce a ‘dying-back’ degeneration of sensory and motor nerves that in turn is indicative of a toxic distal axonopathy (Spencer and Schaumburg, 1978; Schaumburg and Spencer, 1979). Sensory and motor neuronopathy cannot, however, be excluded because selective involvement of largest neurons, which have the longest processes, may give the erroneous impression that a dying-back axonopathy is occurring when the primary site of injury may actually be at the neuronal level (Xu et al., 1989). In addition, 11% of the patients developed perioral or scalp paresthesias, suggesting a possible sensory ganglionopathy in this group.
Dose-dependent effect
Even though we clearly demonstrate a cumulative toxic effect, with worsening neuropathy with each additional cycle, peaking at about the sixth cycle, we were unable to document a correlation between cumulative dose and TNSr. One reason for this is the fact that most patients ended up reducing the dose by the time they reached their eighth and final cycle. Richardson et al. (2006) have shown a dose relationship with PN occurring in 21% of patients receiving bortezomib at 1 mg/m2 and in 37% of patients receiving 1.3 mg/m2 per cycle. The PN in this cohort increased with each additional cycle of treatment, plateauing at cycle 5 (approximately 30 mg/m2).
Reversibility
Like previous studies, dose reduction or discontinuation of bortezomib resulted in improvement in the majority of the patients (Bang et al., 2006; Richardson et al., 2006; Badros et al., 2007). This study is also unique in defining follow-up of neuropathy after the drugs have been discontinued. We were able to show that 80% of patients who discontinued taking thalidomide and bortezomib became asymptomatic. All these patients were asymptomatic prior to receiving chemotherapy, symptomatic on chemotherapy, and again became asymptomatic after stopping the two drugs. This aspect is also supportive of the fact that most of the toxicities can be attributed to bortezomib because most thalidomide-induced neuropathies are not readily reversible. Because we were only able to follow the symptoms after discontinuation of the medication, we are unable to comment on the recovery of signs or nerve conduction attributes. In those patients who continued the medications, their symptoms remained unchanged or only mildly worsened, suggesting that it is possible to find a lower maintenance dose that is beneficial for the underlying disease but does not cause a disabling or functionally limiting neuropathy.
Skin biopsy
We used punch skin biopsies to assess subjects for small-fiber nerve involvement at baseline as well as for progression after four to eight cycles of chemotherapy. The biopsies were well tolerated and were not associated with any adverse events. Five subjects had mild reductions in IENFD at the distal leg at baseline, and there was a mild, albeit not significant, decrease in distal leg IENFD among the 14 subjects who had follow-up biopsies. This degree of change was less pronounced than the changes seen in large-fiber measures such as nerve conduction velocity and TNSr and suggests that bortezomib- and thalidomide-induced PN is predominantly large fiber.
Monitoring of neuropathy
Our study showed the value of monitoring for PN in patients receiving drugs potentially toxic to the peripheral nerves. Total neuropathy score (TNS) is a validated measure useful in following patients with CIPN (Chaudhry et al., 1994; 1996; Cornblath et al., 1999). Other measures used to monitor toxic neuropathy such as common toxicity criteria, ECOG scales, Ajani criteria, and WHO, do not include directed examination and nerve conduction studies; hence the symptoms assessed may not be measuring dysfunction of the peripheral nerves or polyneuropathy. In addition, the range of the other measures is limited as opposed to TNS, which is better able to detect presence, severity, and longitudinal changes in CIPN (Cavaletti et al., 2007).
References
- Badros A, Goloubeva O, Dalal JS, Can I, Thompson J, Rapoport AP, Heyman M, Akpek G, Fenton RG. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110:1042–1049. doi: 10.1002/cncr.22921. [DOI] [PubMed] [Google Scholar]
- Bang SM, Lee JH, Yoon SS, Park S, Min CK, Kim CC, Suh C, Sohn SK, Min YH, Lee JJ, Kim K, Seong CM, Yoon HJ, Cho KS, Jo DY, Lee KH, Lee NR, Kim CS. A multicenter retrospective analysis of adverse events in Korean patients using bortezomib for multiple myeloma. Int J Hematol. 2006;83:309–313. doi: 10.1532/IJH97.A30512. [DOI] [PubMed] [Google Scholar]
- Barlogie B, Tricot G, Anaissie E. Thalidomide in the management of multiple myeloma. Semin Oncol. 2001;28:577–582. doi: 10.1016/s0093-7754(01)90027-2. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Beronio A, Reni L, Ghiglione E, Schenone A, Briani C, Zara G, Cocito D, Isoardo G, Ciaramitaro P, Plasmati R, Pastorelli F, Frigo M, Piatti M, Carpo M. Thalidomide sensory neurotoxicity: a clinical and neurophysiologic study. Neurology. 2004;62:2291–2293. doi: 10.1212/wnl.62.12.2291. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Jann S, Pace A, Plasmati R, Siciliano G, Briani C, Cocito D, Padua L, Ghiglione E, Manicone M, Giussani G. Multi-center assessment of the Total Neuropathy Score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11:135–141. doi: 10.1111/j.1085-9489.2006.00078.x. [DOI] [PubMed] [Google Scholar]
- Cavaletti G, Frigeni B, Lanzani F, Piatti M, Rota S, Briani C, Zara G, Plasmati R, Pastorelli F, Caraceni A, Pace A, Manicone M, Lissoni A, Colombo N, Bianchi G, Zanna C. The Total Neuropathy Score as an assessment tool for grading the course of chemotherapy-induced peripheral neurotoxicity: comparison with the National Cancer Institute-Common Toxicity Scale. J Peripher Nerv Syst. 2007;12:210–215. doi: 10.1111/j.1529-8027.2007.00141.x. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Rowinsky EK, Sartorius SE, Donehower RC, Cornblath DR. Peripheral neuropathy from taxol and cisplatin combination chemotherapy: clinical and electro-physiological studies. Ann Neurol. 1994;35:304–311. doi: 10.1002/ana.410350310. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Eisenberger MA, Sinibaldi VJ, Sheikh K, Griffin JW, Cornblath DR. A prospective study of suramin-induced peripheral neuropathy. Brain. 1996;119:2039–2052. doi: 10.1093/brain/119.6.2039. [DOI] [PubMed] [Google Scholar]
- Chaudhry V, Cornblath DR, Corse A, Freimer M, Simmons-O’Brien E, Vogelsang G. Thalidomide-induced neuropathy. Neurology. 2002;59:1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- Cornblath DR, Chaudhry V, Carter K, Lee D, Seysedadr M, Miernicki M, Joh T. Total Neuropathy Score: validation and reliability study. Neurology. 1999;53:1660–1664. doi: 10.1212/wnl.53.8.1660. [DOI] [PubMed] [Google Scholar]
- Diggle GE. Thalidomide: 40 years on. Int J Clin Pract. 2001;55:627–631. [PubMed] [Google Scholar]
- Harousseau JL, Attal M, Leleu X, Troncy J, Pegourie B, Stoppa AM, Hulin C, Benboubker L, Fuzibet JG, Renaud M, Moreau P, vet-Loiseau H. Bortezomib plus dexamethasone as induction treatment prior to autologous stem cell transplantation in patients with newly diagnosed multiple myeloma: results of an IFM phase II study. Haematologica. 2006;91:1498–1505. [PubMed] [Google Scholar]
- Jagannath S. Current standards for first-line therapy of multiple myeloma. Clin Lymphoma Myeloma. 2007;7:S207–S214. doi: 10.3816/clm.2007.s.024. [DOI] [PubMed] [Google Scholar]
- Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- Kane RC, Dagher R, Farrell A, Ko CW, Sridhara R, Justice R, Pazdur R. Bortezomib for the treatment of mantle cell lymphoma. Clin Cancer Res. 2007;13:5291–5294. doi: 10.1158/1078-0432.CCR-07-0871. [DOI] [PubMed] [Google Scholar]
- Kropff M, Bisping G, Schuck E, Liebisch P, Lang N, Hentrich M, Dechow T, Kroger N, Salwender H, Metzner B, Sezer O, Engelhardt M, Wolf HH, Einsele H, Volpert S, Heinecke A, Berdel WE, Kienast J. Bortezomib in combination with intermediate-dose dexamethasone and continuous low-dose oral cyclophosphamide for relapsed multiple myeloma. Br J Haematol. 2007;138:330–337. doi: 10.1111/j.1365-2141.2007.06656.x. [DOI] [PubMed] [Google Scholar]
- Mateos MV, San Miguel JF. Bortezomib in multiple myeloma. Best Pract Res Clin Haematol. 2007;20:701–715. doi: 10.1016/j.beha.2007.09.003. [DOI] [PubMed] [Google Scholar]
- Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, Diaz-Mediavilla J, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, Garcia-Larana J, Garcia-Sanz R, Blade J, Prosper F, Mateo G, Esseltine DL, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108:2165–2172. doi: 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Stocks EA, Hauer P, Cornblath DR, Griffin JW. Epidermal nerve fiber density: normative reference range and diagnostic efficiency. Arch Neurol. 1998;55:1513–1520. doi: 10.1001/archneur.55.12.1513. [DOI] [PubMed] [Google Scholar]
- Min CK, Lee MJ, Eom KS, Lee S, Lee JW, Min WS, Kim CC, Kim M, Lim J, Kim Y, Han K. Bortezomib in combination with conventional chemotherapeutic agents for multiple myeloma compared with bortezomib alone. Jpn J Clin Oncol. 2007;37:961–968. doi: 10.1093/jjco/hym126. [DOI] [PubMed] [Google Scholar]
- Plasmati R, Pastorelli F, Cavo M, Petracci E, Zamagni E, Tosi P, Cangini D, Tacchetti P, Salvi F, Bartolomei I, Michelucci R, Tassinari CA. Neuropathy in multiple myeloma treated with thalidomide: a prospective study. Neurology. 2007;69:573–581. doi: 10.1212/01.wnl.0000267271.18475.fe. [DOI] [PubMed] [Google Scholar]
- Richardson P, Hideshima T, Anderson K. Thalidomide: emerging role in cancer medicine. Annu Rev Med. 2002;53:629–657. doi: 10.1146/annurev.med.53.082901.104043. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed, refractory myeloma. N Engl J Med. 2003;348:2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24:3113–3120. doi: 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- Rosinol L, Oriol A, Mateos MV, Sureda A, Garcia-Sanchez P, Gutierrez N, Alegre A, Lahuerta JJ, de la Rubia J, Herrero C, Liu X, Van de Velde H, San Miguel J, Blade J. Phase II PETHEMA trial of alternating bortezomib and dexamethasone as induction regimen before autologous stem-cell transplantation in younger patients with multiple myeloma: efficacy and clinical implications of tumor response kinetics. J Clin Oncol. 2007;25:4452–4458. doi: 10.1200/JCO.2007.12.3323. [DOI] [PubMed] [Google Scholar]
- Schaumburg HH, Spencer PS. Toxic neuropathies. Neurology. 1979;29:429–431. doi: 10.1212/wnl.29.4.429. [DOI] [PubMed] [Google Scholar]
- Sheskin J. The treatment of lepra reaction in lepromatous leprosy. Fifteen years’ experience with thalidomide. Int J Dermatol. 1980;19:318–322. doi: 10.1111/j.1365-4362.1980.tb00342.x. [DOI] [PubMed] [Google Scholar]
- Spencer PS, Schaumburg HH. Pathology of neurotoxic axonal degeneration. In: S Waxman., editor. Physiology and Pathobiology of Axons. New York: Raven Press; 1978. p. 265. [Google Scholar]
- Wulff CH, Hoyer H, Asboe-Hansen G, Brodthagen H. Development of polyneuropathy during thalidomide therapy. Br J Dermatol. 1985;112:475–480. doi: 10.1111/j.1365-2133.1985.tb02323.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Sladky JT, Brown MJ. Dose-dependent expression of neuronopathy after experimental pyridoxine intoxication. Neurology. 1989;39:1077–1083. doi: 10.1212/wnl.39.8.1077. [DOI] [PubMed] [Google Scholar]