Abstract
Survival of floret primordia initiated seems critical for the determination of grain number and yield in wheat, and understanding what determines floret mortality would help in the development of more robust physiological models of yield determination. The growth of the juvenile spikes has been frequently considered the determinant of grain number, implying that floret development would depend on resource availability and that the onset of floret death would be related to spike growth. However, this model has been recently challenged from a study concluding that floret death started when the most advanced floret primordia reached a particular developmental stage. As the few previous studies on this relationship involved photoperiod treatments which affect both floret development and the onset of spike growth, conclusions cannot be considered mechanistic. This comprehensive study analysed in detail floret development in wheat as affected by resource availability (mainly soil nitrogen levels) and found that the onset of floret death may occur when development of the most advanced florets ranged from stages 5 to 9 and that the average and standard deviation of floret developmental stage coinciding with the onset of floret death was not related to the level of availability of resources. These results provide further support to the model relating the onset of floret death with the initiation of active growth of the juvenile spike in which florets are developing.
Key words: Fertile floret, floret survival, grain number, nitrogen, spike dry weight, Triticum.
Introduction
To further increase wheat yield, it may be useful to understand the mechanisms controlling grain number determination (Slafer, 2003; Fischer, 2011; Reynolds et al., 2012). Grain number is related to the number of fertile florets (Kirby, 1988; Slafer and Andrade, 1993; Miralles et al., 1998, 2000) and the latter is the end result of the dynamics of floret primordia generation/degeneration during the stem elongation phase (Kirby, 1988). Most frequently, what determines the number of fertile florets is the survival of primordia initiated rather than the number of primordia produced (Ferrante et al., 2013 and references quoted therein). Understanding what determines floret mortality might be helpful to have a better physiological model of the process (Slafer et al., 2005).
As it has been shown several times that the numbers of both fertile florets and grains are related to spike dry weight at anthesis (Miralles et al., 1998; Acreche et al., 2008; Fischer, 2011; González et al., 2011; Ferrante et al., 2012), it would be reasonable to assume that floret development would depend on resource availability. In fact, there seemed to be a relationship between the fate of individual florets, determining floret survival, and spike growth dynamics as affected by photoperiod manipulation during stem elongation (González et al., 2003b, 2005; Serrago et al., 2008). The introgression of Rht genes did also seem to have increased grain number through increasing floret survival related to higher rates of spike growth before anthesis (Miralles et al., 1998). This model, assuming that the number of fertile florets is mainly the outcome of a simple trophic process, would be further supported if it is determined that what triggers floret mortality is also related to spike growth. In principle, and in line with the model of floret survival, it has been proposed that the onset of floret death would not take place before the beginning of active spike growth (Fig. 1, left). A tough problem to directly test the assumption is that determining the dynamics of floret initiation and death to assess the onset of floret death requires a huge amount of work (dissecting very often individual floret primordia to determine their developmental stage) and therefore most commonly the few results which are available are derived from the imposition of only few treatments (and the lower the number of treatments, the higher the uncertainty on the strength of the conclusions based on coordination, or lack of, between processes). The proposition that the onset of active spike growth would trigger floret death was based in a couple of studies by González et al. (2003b, 2005), in which plants were subjected to contrasting photoperiods during stem elongation. On the other hand, it has been recently concluded that the onset of floret death would be a ‘pure’ developmental process, unrelated to spike growth, governed by the stage of development of the most advanced floret primordium of the spike, the most proximal floret (F1) of central spikelets (Bancal, 2008, 2009; Fig. 1, right). Again, this conclusion was based on a rather small database (only three environmental treatments: a control, an extended photoperiod, and shading of 28% imposed on six cultivars). More recently, González et al. (2011) reanalysed a substantially larger database, in which timing of spike growth and development of florets were mainly, though not only, affected by photoperiod treatments, and concluded that onset of floret death was most likely related to the onset of spike growth at maximum rate. However, due to the nature of the treatments imposed, there was also a correlation between the onset of spike growth and floret developmental rates. As longer photoperiods advance both spike growth and floret development, resolving the issue would require treatments affecting growth but not development.
Fig. 1.
Diagram schematically describing two alternative models for processes and signals responsible for triggering the onset of floret death (downward arrows in the top panel): the trophic model (left side) in which floret death would be triggered by the dynamics of spike dry weight (SDW) between terminal spikelet initiation (TSI) and anthesis (A), and the ‘pure’ developmental model (right side) in which floret death would be triggered by the developmental stage of the most advanced floret primordium. Thus, floret death starts either at the onset of rapid spike growth in the trophic model, or when F1 of central spikelets reach the stage 8 of the Waddington et al. (1983) scale in the developmental model. Support for the trophic model comes from studies in which by reducing the length of the TSI–A period the onsets of both spike growth was floret death were advanced in parallel. Support for the developmental model comes from studies in which the onset of floret death was correlated with the timing required for F1 to achieve the stage 8 in the mentioned scale. The bottom part of the scheme represents the model of floret survival from onset of floret death to grain number, where there is consensus in the literature on the paramount relevance of floret survival in determining grain number in wheat.
This study undertook a number of experiments in which (i) wheat plants were exposed to treatments affecting crop growth but not crop development (mainly nitrogen fertilizations) and (ii) the developmental stage of floret primordia determined in detail at different positions in the spikes during stem elongation. This study aimed to determine whether the onset of floret death was related to the developmental stage of F1 in central spikelets by reanalysing the large database based on these experiments.
Materials and methods
General conditions
Six experiments were conducted, during four consecutive growing seasons from 2006–07 to 2009–10 (Table 1). Four experiments (experiments 1, 2, 3, and 6) were carried out outdoors in microcrops, within large cubic containers (1 m height and 1×1 m2 surface; details available in Ferrante et al., 2010) at the School of Agronomy, University of Lleida, Spain (41° 37′ 50′′ N 0° 35′′ E; altitude 180 m) while the other two were field experiments sown at Gimenells (41° 39′ 11′’ N 0° 23′ 28′′ E; altitude 258 m). In order to ensure a low nitrogen (N) availability in experiments 1, 2, 3, and 6, the containers were filled with a sand/soil mix (3:1 v/v). In each experimental unit, mineral N availability at sowing was equivalent to 70kg N ha–1 (experiment 1), 30kg N ha–1 (experiment 2), and 20kg N ha–1 (experiments 3 and 6). Before sowing, phosphorus fertilizer (triple superphosphate, 20kg P ha–1) was uniformly mixed within the upper 20cm of the soil mixture. Before tillering, micronutrients were added. Experiments 4 and 5 were conducted under field conditions in a soil classified as calcixerept petrocalcic (FAO, 1990). The main environmental differences between the two experiments was the soil-N contents and irrigation regime, although they also differed more slightly in sowing dates and soil depth (0.75 m in experiment 4 and more than 1 m in experiment 5).
Table 1.
Experimental details. Experiments: 1, factorial combinations of nitrogen; 2, water × N; 3, N × cultivars; 4 and 5, cultivars; and 6 cultivars × water × N. Experiments 1, 2, 3, and 6 were in large containers at Lleida and experiments 4 and 5 were under field conditions at Gimenells (NE Spain). Periodic irrigations throughout the growing season, from once a week in winter to every second day during grain filling; each microcrop was irrigated individually until water freely drained underneath the container or with sprinklers at mid-tillering (25 mm), jointing (30 mm), anthesis (60 mm), and mid-grainfilling (15 mm) under field conditions. Treatments in the containers were, watered only once at sowing to warrant germination and emergence, and when the minimal N doses was applied. Fertilization is given as decimal code 2.1 (Zadoks et al., 1974); fertilizer was applied by splitting the dose into two or three equal applications at the onset of tillering (DC 2.1), at mid-tillering (DC 2.3), and the onset of stem elongation (DC 3.1). Bold type indicate treatments within an experiment.
| Growing season | Experiment | Experimental design | Experimental approaches | Sowing date and density | Experimental treatments | |||
|---|---|---|---|---|---|---|---|---|
| Water regime | N availability | Cultivars | ||||||
| Soil N at sowing (kg N ha–1) | Fertilization (kg N ha–1) | |||||||
| 2006–07 | 1 | Completed randomized design (3 replicates) | Crops in large containers outdoors | 24 Nov 2006; 500 plants m–2 | Irrigated | 70 | — | Claudio |
| 70 | 50 DC21 + 50 DC31 | |||||||
| 2007–08 | 2 | Completed randomized design (3 replicates) | Crops in large containers outdoors | 14 Nov 2007; 300 plants m–2 | Irrigated | 30 | 20 DC21 | Claudio |
| 30 | 73.3 DC21 + 73.3 DC23 + 73.3 DC31 | |||||||
| Rainfed | 30 | 20 DC21 | Claudio | |||||
| 30 | 73.3 DC21 + 73.3 DC23 + 73.3 DC31 | |||||||
| 2008–09 | 3 | Completed randomized design (3 replicates) | Crops in large containers outdoors | 28 Nov 2006; 300 plants m–2 | Irrigated | 20 | 30 DC21 | Claudio, Donduro, Simeto, Vitron |
| 20 | 76.6 DC21 + 76.6 DC23 + 76.6 DC31 | Claudio, Donduro, Simeto, Vitron | ||||||
| 4 | Randomized block design (3 replicates) | Field | 24 Nov 2008; 300 plants m–2 | Rainfed | 130 | — | Claudio, Donduro, Simeto, Vitron | |
| 5 | Randomized block design (3 replicates) | Field | 12 Dec 2008; 300 plants m–2 | Irrigated | 580 | — | Claudio, Donduro, Simeto, Vitron | |
| 2009–010 | 6 | Completed randomized design (3 replicates) | Crops in large containers outdoors | 26 Nov 2009; 250 plants m–2 | Irrigated | 20 | 30 DC21 | Donduro, Vitron |
| 20 | 76.6 DC21 + 76.6 DC23 + 76.6 DC31 | Donduro, Vitron | ||||||
| Rainfed | 30 | 20 DC21 | Donduro, Vitron | |||||
| 20 | 76.6 DC21 + 76.6 DC23 + 76.6 DC31 | Donduro, Vitron | ||||||
All experiments were sown within the normal sowing dates of the region and at normal sowing densities (Table 1). Microcrops (experiments 1, 2, 3, and 6) were sown with a dedicated system to warrant perfectly uniformly distributed seedlings (as described and illustrated in Ferrante et al., 2010).
In all experiments, diseases and insects were prevented or controlled by spraying recommended fungicides and insecticides at the doses suggested by their manufacturers. In addition, weeds were removed by hand (microcrops) or controlled by spraying selective herbicides (field).
The mean daily global radiation (MJ m–2), mean temperature (°C), and accumulated precipitation (mm) were recorded daily in meteorological stations belonging to the Catalonian Agro-meteorological network, located close to the experimental sites, and are shown for the major developmental phases in each experiment in Table 2.
Table 2.
Daily global radiation, temperature, and accumulated rainfall during different phenological periods in all growing seasons.
| Sowing–emergence | Emergence–jointing | Jointing–anthesis | Anthesis–maturity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growing season | Experiment | Experimental approaches | Average temp. (ºC) | Daily global radiation (MJ m–2) | Rainfall (mm) | Average temp. (ºC) | Daily global radiation (MJ m–2) | Rainfall (mm) | Average temp. (ºC) | Daily global radiation (MJ m–2) | Rainfall (mm) | Average temp. (ºC) | Daily global radiation (MJ m–2) | Rainfall (mm) |
| 2006–07 | 1 | Micro-crop | 10.1 | 5.80 | 14.6 | 6.70 | 7.74 | 43.3 | 13.2 | 17.2 | 90.5 | 19.4 | 25.1 | 28.4 |
| 2007–08 | 2 | Micro-crop | 7.13 | 8.37 | 7.70 | 7.67 | 9.18 | 33.8 | 13.5 | 20.2 | 42.3 | 18.6 | 22.6 | 155 |
| 2008–09 | 3 | Micro-crop | 6.13 | 6.00 | 30.5 | 7.87 | 11.1 | 88.8 | 14.6 | 19.7 | 115 | 21.8 | 25.2 | 35.1 |
| 4 | Field | 4.82 | 6.24 | 24.2 | 6.38 | 9.13 | 67.5 | 11.9 | 19.1 | 134 | 17.9 | 24.6 | 4.93 | |
| 5 | Field | 3.15 | 4.40 | 29.8 | 8.50 | 12.7 | 40.4 | 12.9 | 20.2 | 140 | 20.8 | 24.3 | 20.7 | |
| 2009–10 | 6 | Micro-crop | 6.81 | 5.34 | 45.6 | 5.60 | 10.2 | 133 | 14.1 | 18.5 | 52.9 | 19.2 | 25.0 | 102 |
Treatments and experimental designs
Although treatments varied among experiments, in all there were different levels of N availabilities and/or different cultivars, in some cases combined with contrasting water regimes (Table 1).
During 2006–07 and 2007–08, cultivar Claudio (modern and well-adapted durum wheat) was grown in micro-crops with contrasting N (experiments 1 and 2) and water (experiment 2) availabilities (Table 1). In the third growing season (2008–09), the main treatments in the three experiments were four modern (released between 1987 and 2002) semidwarf durum wheat cultivars: Claudio, Donduro, Simeto, and Vitron (Table 1). In the experiment in large containers they were subjected to different N availabilities (experiment 3). The other two were field experiments with contrasting conditions: rainfed (experiment 4) and irrigated (experiment 5). In the fourth growing season (2009–10; experiment 6), two of these cultivars, Donduro and Vitron (selected because they were the two highest yielding cultivars in the third year, while exhibiting contrasting response to N in terms of spike fertility; Ferrante et al., 2012), were exposed to a factorial combination of two contrasting N and water availabilities.
Judged by the amount of certified seed produced, Claudio was the most widely grown cultivar in 2009/10 and is particularly adapted to the region; therefore, it used as a check for testing new cultivars in the northern growing area of Spain. Vitron and Simeto are cultivars of much wider adaptation than Claudio as they are checks not only for the northern but also the southern region of Spain (GENVCE, Group for the Evaluation of the New Cereal Varieties in Spain; Anonymous, 2008). Donduro is the newest cultivar and soon after its release it showed in comparative yield trials to perform consistently better than Vitron and Simeto over the wide range of conditions explored in that network (Anonymous, 2008). The four cultivars chosen are all modern and well adapted to the growing conditions and therefore their general phenological patterns are very similar and their plant heights are within the optimum maximizing yield (Ferrante et al., 2012).
Measurements and analyses
From terminal spikelet initiation to flowering, one plant per experimental unit was selected at random and harvested twice or thrice a week. The spike of the main shoot was dissected under a binocular microscope (Leica MZ 7.5, Leica Microsystems, Heerbrugg, Switzerland) to count the total number of floret primordia. In addition, at each sample the developmental stage (floral score) of each floret within particular spikelets was determined following the scale of Waddington et al. (1983), as described in Ferrante et al. (2010, 2013). The spikelets analysed were those in basal (fourth spikelet from the base), central (middle spikelet), and apical (fourth spikelet from the top) positions of the spike. Florets within the spikelets were numbered from F1 (floret primordium closest to the rachis) to Fn (the most distal floret primordium of the particular spikelet, this being normally F8). Floret primordia were considered as fertile florets when they were at W10 or immediately before that stage (when the stigmatic branches were curved with green anthers).
To determine the effect of treatments on the different variables, the data were subjected to analysis of variance and the relationships between variables were determined by regression analysis (SAS, 2002). Developmental score of F1 at the onset of floret death was determined, for each combination of resources availability and growing seasons. For this purpose, the number of living floret primordia was plotted against the stage of development of F1, and the stage of F1 at the onset of floret death was determined. For analysing all data from different spikelets and treatments together, the number of living floret primordia were standardized as a proportion of the maximum number of floret primordia initiated in each particular cases (González et al., 2011).
During the last growing season (2009–10), spike length and spike dry weight were also determined in each of the samples to describe floret development. The dynamics of spike length and dry weight were determined by fitting the data with a bilinear model and the onset of rapid spike growth was determined.
Results
Floret development
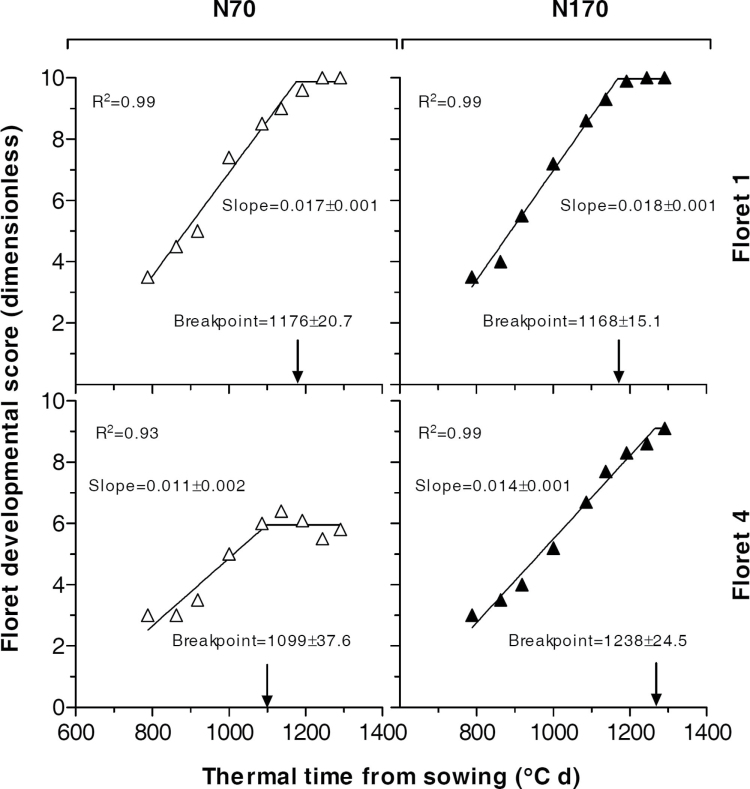
As expected, N did not affect the developmental rate of florets which are normally fertile at anthesis. This is illustrated in detail for the most proximal floret (F1) in central spikelets under low and high N availability (Fig. 2, upper panels). Development of F1 was never affected significantly by N availability (Supplementary Table S1, available at JXB online). Development of F2, which is also most frequently a fertile floret even under low N levels, did not respond to N either (data not shown). For more distal florets, like F4, which under some conditions becomes a fertile floret but under other conditions does not survive and dies, the effect of N on their development can be seen in some cases (Supplementary Table S1), mainly those in which F4 was fertile under high N but died at earlier stages under low N availability, as illustrated for F4 in experiment 1 (Fig. 2, bottom panels).
Fig. 2.
Relationship between floret developmental score (scale of Waddington et al., 1983) and thermal time from sowing of either the most proximal (and most advanced) floret primordia (F1) or the fourth floret primordia from the rachis, a relatively distal floret (F4) in central spikelets under contrasting N treatments in experiment 1.
As N did not affect the developmental pattern of the most proximal floret in central spikelets, it proved to be the type of treatment required to test whether the onset of floret death is actually determined by the timing of achieving a certain stage of development in these florets. If this is so, N availability (and the other treatments evaluated in the present study, Table 1) should have not affected the timing of the onset of floret death.
Dynamics of number of floret primordia
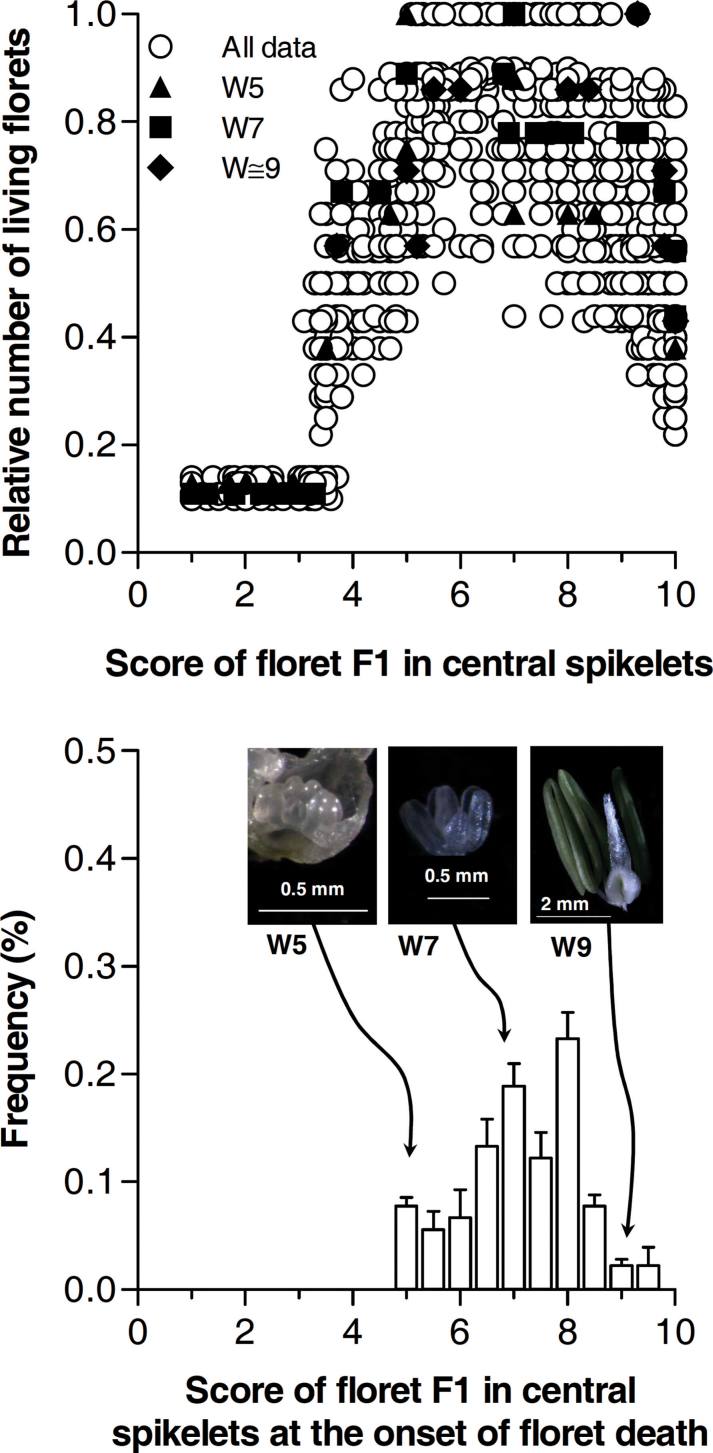
The dynamics of the relative number of living florets through time was very similar between all experimental years and spikelet positions. In all cases, the number of floret primordia increased sharply, reached a maximum, and then declined markedly to end up in a particular number of fertile florets, which normally ranged between 20 and 40% of the maximum number of floret primordia initiated. This process of floret generation/degeneration occurred while the proximal floret of the central spikelet progressed in its development (Fig. 3, top panel).
Fig. 3.
(Top) Relationship between the number of living florets, in relative terms to standardize for differences in maximum number of floret primordia depending on treatments and spikelet positions, and score of floret F1 in central spikelets. Open circles corresponding to the dynamics of all data (in each particular case unidentified in this figure always the values peak to 1 before the onset of floret death). To illustrate the range of differences in score of F1 in central spikelets at the onset of floret death in the wide range of conditions analysed, we identified the two extreme cases in which the onset of floret death coincided with F1 in central spikelets being at stages as early as a score of 5 (closed triangles) or as late as a score of 9 (closed rhomb). (Bottom) Frequency of developmental scores of the proximal florets (F1) of central spikelets at which floret death started considering the whole dataset analysed. Bars represent standard errors. Pictures show the appearance of the primordia of F1 in central spikelets corresponding to two extreme and one intermediate conditions.
Regardless of the noticeable similar general pattern, there was not a clear single developmental stage of the most proximal floret of central spikelet synchronic with the onset of floret death: depending on the particular case (experiment, treatments, spikelet positions), the onset of floret death coincided with a developmental score of the most proximal floret of central spikelets, ranging from 5 to 9 (Fig. 3, bottom panel).
In order to see whether the variation was induced by the different environmental conditions explored, the data were reanalysed within particular environments. For all, a strong association was found between the onset of floret death and the stage of development of the most advanced floret primordia in the spikes (Fig. 4, top panel). Again, within environmental conditions, floret death started when the developmental score of F1 of central spikelets ranged roughly from 5 to 9, depending on the cultivar and spikelet position considered (Fig. 4, bottom panels). In addition, similar to the finding using the developmental score of F1 in central spikelets, there was not a clear single floret development score associated with the onset of floret death when considered the most proximal floret of the corresponding spikelets (basal, central, and apical spikelet positions), either analysing all data pooled together (Supplementary Fig. S1) or analysing data within particular environmental conditions (Supplementary Fig. S2).
Fig. 4.
(Top) Number of living florets (relative to the maximum in each case) plotted against the developmental stage of the most advance floret (F1) in central spikelets for each of the three spikelet positions considered (upper panels) analysed for each of the six environmental conditions explored throughout the studies analysed independently. (Bottom) Frequency of developmental scores of the F1 of central spikelets at which the onset of floret death took place in each environmental conditions studied.
The developmental score of F1 of central spikelets that resulted most frequently related to the onset of floret death varied across experimental conditions, between 6.5 under low resources at the University of Lleida and 8 under high inputs in Gimenells (Fig. 4, bottom panels). However, the variation in stage of development of F1 at which floret death started did not seem to be consistently affected by the particular growing conditions (Fig. 5a and Supplementary Fig. S3a). To analyse this more quantitatively, assuming the yield broadly reflects the availability of resources, the mean score of F1 in central spikelets at the onset of floret death was related with the average yield in each of the conditions: there was no consistent trend (Fig. 5b and Supplementary Fig. S3b). There was no trend between the standard deviation in score of F1 at onset of floret death and yield in the particular conditions (Fig. 5c and Supplementary Fig. S3c), implying that the range in developmental score of F1 at onset of floret death was similar for the dramatically different growing conditions explored in this study.
Fig. 5.
(a) Boxplot of the stages of development of the most advanced floret (F1) in central spikelets at the onset of floret death for different experimental conditions (experiments and treatments). The horizontal line within each box is the median, and the bottom and top of each box represent the 25th and 75th percentiles, respectively. Crosses correspond to the mean. The whiskers represent the 10th and 90th percentiles, respectively and the values outside this range are individual outliers (closed circles). (b and c) Relationships between the average (b) and the standard deviation (c) of the scores of floret F1 in central spikelets at the onset of floret death in each environmental condition analysed and yield achieved by the crop in those conditions (as indicator of resource availability). Environmental conditions were (i) high- and low-nitrogen levels in the microcrops (closed and open symbols, respectively) under irrigated (circles) or rainfed conditions (triangles), and (ii) high- or low-inputs (closed and open squares, respectively) in the field experiments.
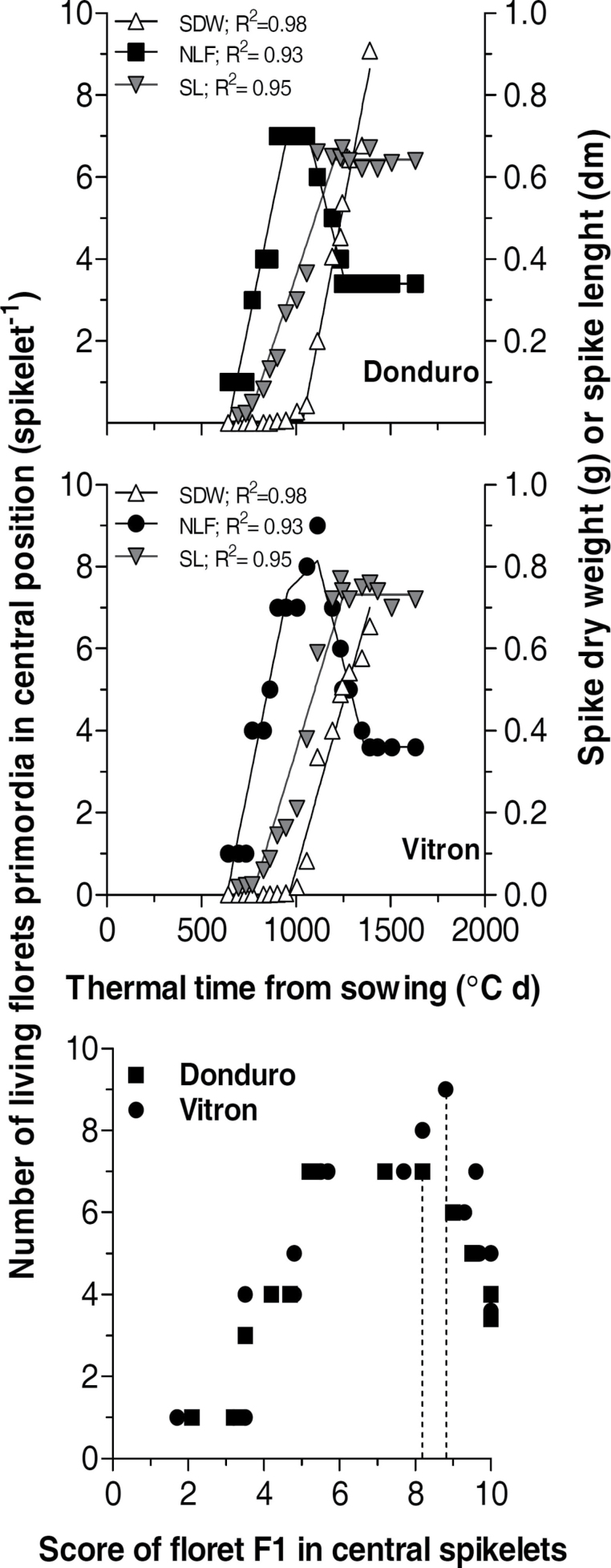
Spike length and spike dry weight
Although spike length and spike dry weight are acceptably related when both are determined at flowering, the dynamics of spike length and dry matter accumulation were rather different (Fig. 6). The onset of rapid spike lengthening took place at about 200 °C d before the onset of rapid spike dry matter accumulation (Fig. 6, top and middle panels). Thus, spike length can hardly be used as a proxy of spike dry matter if the aim is to relate early spike weight with dynamics of floret development. The only case in which spike dry weight was determined frequently between terminal spikelet and anthesis showed that the onset of floret death occurred soon after the onset of rapid dry weight accumulation on the juvenile spike (Fig. 6). In fact, both in Donduro and Vitron for the last experiment, the onset of floret death followed the onset of spike growth though due to their difference in development it was coincident with different stages of F1 development in central spikelets (W 8.2 and W 8.8 in Donduro and Vitron, respectively; Fig. 6, bottom panel).
Fig. 6.
Dynamics of the number of living floret primordia per spiketet (NLF, closed squares), spike length (SL, inverted grey triangles), and spike dry weight (SDW, open triangles) from terminal spikelet initiation to anthesis for Donduro (top panel) and Vitron (middle panel) under the irrigated and fertilized treatment of the last growing season. The bottom panel shows the relationship between the number of living floret primordia per spiketet and the developmental score of F1 in central spikelets for the same treatments. Dotted lines indicate the stage of F1 development at the onset of floret death.
Discussion
The main goal of this paper was to determine whether the onset of floret death was triggered by the achievement of a certain developmental stage (c.8 in the scale of Waddington et al., 1983), by the most advanced floret primordia (F1 of central spikelets) and independently of the growth of the spike. This was a conclusion derived from a study reported by Bancal (2009), implying that the onset of floret death would be, therefore, independent of spike growth during pre-anthesis (reinforcing a previous paper in which he inferred that spike growth may not determine number of fertile florets or grains; Bancal, 2008). If this were so, much of the most accepted physiological models for the determination of grain number, based on interplay between spike growth and floret survival (e.g. Slafer et al., 2005; Fischer, 2011), would need to be conceptually revised, and ideas on how manipulating genetically spike growth to maximize grain number in wheat (Miralles and Slafer, 2007; Foulkes et al., 2011; Reynolds et al., 2012) might be more doubtful. Therefore, testing the robustness of the relationship between the onset of floret death and achievement of a certain developmental stage in F1 of central spikelets is rather relevant. The mentioned studies by Bancal are extremely useful because of the detail provided for floret development under contrasting conditions but there are two major inconveniences to allow accepting their conclusions straightforwardly. First, the study included only very few environmental conditions to yield easily extrapolatable conclusions. Second, the likelihood of the onset of floret death be triggered by spike growth was discarded assuming that floret death should have started later than the timing when the rate of spike growth is maximized, which in the analysis occurred when spike dry weight was already c. 0.5 of the weight at anthesis (clearly much later than the actual onset of spike growth; see also discussion on this in González et al., 2011).
The present study comprehensively tested the existence of such developmental triggering of floret death with a comprehensive database of both dynamics of floret generation/degeneration (from where the onset of floret death could be determined) and detailed developmental progress of particular floret positions from terminal spikelet initiation to few days after anthesis, as affected by a number of treatments changing resource availability (mainly, but not only, soil-N availability levels), which are well recognized to greatly affect crop growth but not development. In agreement with González et al. (2011), who also analysed this relationship (but with a database mainly produced by manipulations of photoperiod), the current study found the onset of floret death to be rather independent of the developmental stage of the most advanced floret. In the database, floret death might start when F1 of central spikelets are anywhere between the stages 5 and 9 of the scale of Waddington et al. (1983); which might represent a difference in time of more than 200 °C d. The results of this study do therefore conflict severely with the conclusion that the onset of floret death may be simply a developmental process, giving further support then to the more-established model that the processes related to floret survival to determine number of fertile florets and grains are tightly related to the growth of the juvenile spikes during the stem elongation phase.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Relationships and frequencies equivalents to those shown in Fig. 3 but corresponding to bottom, central, and apical spikelets.
Supplementary Fig. S2. Number of living florets and frequencies equivalents to those shown in Fig. 4 but corresponding to bottom, central, and apical spikelets.
Supplementary Fig. S3. Boxplot and relationships equivalents to those shown in Fig. 5 but corresponding to bottom, central, and apical spikelets.
Supplementary Table S1. Spike dry weight at anthesis and parameters of the bilinear regression analysis of floret developmental stage vs. thermal time for the most proximal and one distal florets of central spikelets of wheat grown under a wide range of conditions.
Acknowledgements
The authors thank the team of the crop physiology Lab of the UdL for the technical assistance. Funding was provided by the Spanish Ministry of Science and Innovation through project AGL2009–11964. AF held a FPU, AP2006–03719, scholarship from the Spanish Ministry of Education.
References
- Acreche MM, Briceño-Félix G, Martín Sánchez JA, Slafer GA. 2008. Physiological bases of genetic gains in Mediterranean bread wheat yield in Spain. European Journal of Agronomy 28, 162–170. [Google Scholar]
- Anonymous. 2008. Report of new varieties of wheat and barley evaluated during the growing season 2007–2008. Accessible at: www.genvce.org. [Google Scholar]
- Bancal P. 2008. Positive contribution of stem growth to grain number per spike in wheat. Field Crops Research 105, 27–39. [Google Scholar]
- Bancal P. 2009. Early development and enlargement of wheat floret primordia suggest a role of partitioning within spike to grain set. Field Crops Research 110, 44–53. [Google Scholar]
- FAO. 1990. UNESCO soil map of the world: revised legend, World Soil Resources Report 60. Rome: FAO. [Google Scholar]
- Fischer RA. 2011. Wheat physiology: a review of recent developments. Crop and Pasture Science 62, 95–114. [Google Scholar]
- Ferrante A, Savin R, Slafer GA. 2010. Floret development of durum wheat in response to nitrogen availability. Journal of Experimental Botany 61, 4351–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante A, Savin R, Slafer GA. 2012. Differences in yield physiology between modern, well adapted durum wheat, cultivars grown under contrasting conditions. Field Crops Research 136, 52–64. [Google Scholar]
- Ferrante A, Savin R, Slafer GA. 2013. Floret development and grain setting differences between modern durum wheats under contrasting nitrogen availability. Journal of Experimental Botany 64, 169–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulkes MJ, Slafer GA, Davies WJ, Berry PM, Sylvester-Bradley R, Martre P, Calderini DF, Griffiths S, Reynolds MP. 2011. Raising yield potential of wheat. III. Optimizing partitioning to grain while maintaining lodging resistance. Journal of Experimental Botany 62, 469–486. [DOI] [PubMed] [Google Scholar]
- Ghiglione HO, Gonzalez FG, Serrago R, Maldonado SB, Chilcott Ch, Cura JA, Miralles DJ, Zhu T, Casal JJ. 2008. Autophagy regulated by daylength determines the number of fertile florets in wheat. The Plant Journal 55, 1010–1024. [DOI] [PubMed] [Google Scholar]
- González FG, Miralles DJ, Slafer GA. 2011. Wheat floret survival as related to pre-anthesis spike growth. Journal of Experimental Botany 62, 4889–4901. [DOI] [PubMed] [Google Scholar]
- González FG, Slafer GA, Miralles DJ. 2003a. Grain and floret number in response to photoperiod during stem elongation in fully and slightly vernalized wheats. Field Crops Research 81, 17–27. [Google Scholar]
- González FG, Slafer GA, Miralles DJ. 2003b. Floret development and spike growth as affected by photoperiod during stem elongation in wheat. Field Crops Research 81, 29–38. [Google Scholar]
- González FG, Slafer GA, Miralles DJ. 2005. Floret development and survival in wheat plants exposed to contrasting photoperiod and radiation environments during stem elongation. Functional Plant Biology 32, 189–197. [DOI] [PubMed] [Google Scholar]
- Kirby EJM. 1988. Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Research 18, 127–140. [Google Scholar]
- Miralles DJ, Katz SD, Colloca A, Slafer GA. 1998. Floret development in near isogenic wheat lines differing in plant height. Field Crops Research 59, 21–30. [Google Scholar]
- Miralles DJ, Richards RA, Slafer GA. 2000. Duration of stem elongation period influences the number of fertile florets in wheat and barley. Australian Journal of Plant Physiology 27, 931–940. [Google Scholar]
- Miralles DJ, Slafer GA. 2007. Paper presented at the International Workshop on Increasing Wheat Yield Potential, CIMMYT, Obregon, Mexico, 20–24 March 2006. Sink limitations to yield in wheat: how could it be reduced? Journal of Agricultural Science 145, 139–149. [Google Scholar]
- Reynolds M, Foulkes J, Furbank R, Griffiths S, King J, Murchie E, Parry M, Slafer G. 2012. Achieving yield gains in wheat. Plant, Cell and Environment 35, 1799–1823. [DOI] [PubMed] [Google Scholar]
- SAS. 2002. Statistics programme. Cary, NC: SAS Institute. [Google Scholar]
- Serrago RA, Miralles DJ, Slafer GA. 2008. Floret fertility in wheat as affected by photoperiod during stem elongation and removal of spikelets at booting. European Journal of Agronomy 28, 301–308. [Google Scholar]
- Sibony M, Pinthus MJ. 1988. Floret initiation and development in spring wheat (Triticum aestivum L.). Annals of Botany 61, 473–479. [Google Scholar]
- Slafer GA. 2003. Genetic basis of yield as viewed from a crop physiologist’s perspective. Annals of Applied Biology 142, 117–128. [Google Scholar]
- Slafer GA, Andrade FH. 1993. Physiological attributes related to the generation of grain yield in bread wheat cultivars released at different eras. Field Crops Research 31, 351–367. [Google Scholar]
- Slafer GA, Araus JL, Royo C, García Del Moral LF. 2005. Promising eco-physiological traits for genetic improvement of cereal yields in Mediterranean environments. Annals of Applied Biology 146, 61–70. [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. 1983. A quantitative scale of spike initial and pistil development in barley and wheat. Annual of Botany 51, 119–130. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. 1974. A decimal code for the growth stages of cereals. Weed Research 14, 415–421. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.