Abstract
Background
Liver biopsy has remained the gold standard for the diagnosis of chronic hepatitis C; even though, it has a low but non-negligible rate of both false negative and complications. Several authors have proposed noninvasive tools to diagnose cirrhosis. But none of them showed complete concordance with liver biopsy.
Objectives
To devise a score based on noninvasive routine parameters that discriminate between patients with a high risk, and those with a low risk of cirrhosis among patients with chronic hepatitis C without performing liver biopsy, and to compare this score with other ones using routine parameters devoted to this aim.
Patients and Methods
We reviewed the charts of patients with chronic hepatitis C who performed a liver biopsy between 2000 and 2004. Multivariate analysis was used to identify independent predictors of cirrhosis. An independent group of patients with chronic hepatitis C admitted for a liver biopsy between 2007 and 2012 constituted the validation set.
Results
We enrolled 249 patients who had complete laboratoristic data, and sufficient liver tissue for fibrosis staging. Age, AST, prothrombin activity, and platelets were identified as independent predictors of histological cirrhosis. We categorized these variables, and devised a novel score called CISCUN (Cirrhosis Score University of Naples), giving one point to each of the following predictors: age > 40 years; AST > 2 upper normal values; platelet count < 160.000/mmc; prothrombin activity < 100%. Cirrhosis rate was 2.9% for the 103 patients with a CISCUN = 0 or 1, 23.4% for the 124 patients with a CISCUN of 2 or 3, and 86.4% for the 22 patients with a CISCUN = 4. These results were confirmed in the independent validation group of 285 patients with similar characteristics.
Conclusions
Patients with chronic hepatitis C and with a CISCUN ≤ 1 had a very low rate of cirrhosis while those with a CISCUN = 4 had a high risk of cirrhosis. Patients with CISCUN = 2 or 3 had an intermediate rate of cirrhosis, and therefore needed to perform a liver biopsy to receive a reliable diagnosis.
Keywords: Liver Cirrhosis, Fibrosis, Biopsy, Noninvasive Ventilation
1. Background
Hepatitis C virus (HCV) is estimated to infect approximately 2.2–3.0% of the world’s population which corresponds to 130–170 million people (1). After penetrating in the host HCV gives rise to an acute infection which becomes chronic in about 70% of infected people (2, 3). About 25% of these patients would develop liver cirrhosis in about 20-30 years (4). About 4% per year of patients with cirrhosis would evolve toward a decompensated disease with an annual death rate between 15% and 30% (2). Finally about 1.6% of patients with cirrhosis develop hepatocellular carcinoma (HCC) yearly (2). Therefore the presence of cirrhosis is a main determinant of poor prognosis in patients with chronic HCV infection. Moreover, in the presence of cirrhosis, ultrasound screening for HCC, and upper endoscopy for varices detection are mandatory (5). Finally, the presence of cirrhosis influences decisions about antiviral treatment. Patients with cirrhosis have the most urgent need for treatment, but also a low response rate, and require a close monitoring during treatment (6). Liver biopsy is still considered the standard method for the assessment of liver cirrhosis, even though sampling errors and interpreter variability may reduce its diagnostic accuracy (7-10). Moreover liver biopsy is an invasive test, and therefore it has a small but not negligible rate of complications (0.3-0.8%), and death (0.01-0.3%) (11-14). Several groups have proposed noninvasive means to diagnose liver cirrhosis (15, 16). The most studied tools include the measurement of liver stiffness (17), and the use of a panel of selected blood tests, and a proprietary algorithm called Fibrotest (18) or a combination of both (19). Moreover several authors have proposed the use of nonroutinely available analytes (20-27) or routinely-available parameters to predict cirrhosis (6, 16, 28-40) or a combination of clinical and laboratoristic parameters (41). The accuracy of the noninvasive tools to predict cirrhosis in comparison to liver biopsy remains to be confirmed. The ideal noninvasive model that is easy-to-perform, accurately discriminate between patients with or without cirrhosis remains to be found.
2. Objectives
The aim of our study is to devise a score based on noninvasive routine parameters which can be helpful for physicians to identify both patients with a high risk and those with a low risk of having cirrhosis among a population of patients with chronic hepatitis C without performing liver biopsy (therefore that can safely avoid it), and to compare our score with other ones using routine parameters.
3. Patients and Methods
We reviewed the charts of all patients with chronic hepatitis C admitted for a liver biopsy to the Department of Public Medicine and Social Security – Section of Infectious Diseases (University of Naples “Federico II”, Italy), and to Department of Public Medicine, section of Infectious Disease (Second University of Naples) between 01.01.2000 to 31.12.2004. Liver biopsy was sampled through an 18G needle under ultrasound guidance. The liver specimens, in each case more than 2 cm in length, were fixed in 10% neutral buffered formalin, embedded in paraffin, and stained with hematoxylin and eosin, and with the Masson’s trichrome method. A sample of liver biopsies was examined by two pathologists who were unaware of the virological and clinical data, and who had a k index of 85.5%. Fibrosis was assessed according to the Ishak scoring system (42). Patients with stage 5 or 6 were considered having histological cirrhosis. Inclusion criteria were positivity for anti-HCV and HCV RNA in serum, no contraindication to liver biopsy. All the biopsies were performed at least 12 months after the completion of an eventual course of interferon therapy. Exclusion criteria were presence of other causes of liver disease; HBV or HIV coinfection, hepatocellular carcinoma, ascites, prior liver transplantation, insufficient liver tissue for staging of fibrosis, and incomplete data on blood counts or liver panel. Patients with a diagnosis of cirrhosis based on laboratory or ultrasound evaluation were excluded as well. Except for HCV genotype, only laboratory results performed within 1 month from the date of the liver biopsy were used. We recorded variables listed in Table 1. We also calculated the AST/ALT ratio. Splenomegaly was diagnosed when spleen length exceeded 12 cm at ultrasound examination (43). Alcohol consumption was defined as drinking more than 30 g per day of alcohol for at least 6 months. This was corroborated by the patient’s family. After the construction of the score, we validated it in an independent group of patients admitted for a liver biopsy to our department between 01.01.2007 and 30.06.2012. Inclusion and exclusion criteria were similar to those of the first group of patients.
Table 1. Demographic, Laboratory, Ultrasound, and Histological Features of the First Group of Patients (n = 249) .
| Items | Data |
|---|---|
| Age, y, No. (Median) | 48 (35-54.5) |
| Sex | |
| Male, % | 61.8 |
| Female, % | 38.2 |
| Alcohol consumption, % | 12 |
| Pervious Antiviral treatment, % | 13.2 |
| Splenomegaly at ultrasound, % | 23 |
| Iron, μg/mL, No. (Median) | 118 (85.5-148.5) |
| Glucose, mg/dL, No. (Median) | 90 (80.25-101) |
| Total Bilirubin, mg/dL, No. (Median) | 0.66 (0.50-0.87) |
| ASTa, U/L, No. (Median) | 62 (40.5-93) |
| ALTa, U/L, No. (Median) | 106 (59-176) |
| Alkaline phosphatase,U/L, No. (Median) | 149 (103.5-208) |
| Albumin, g/dL, No. (Median) | 4.4 (4.2-4.6) |
| Cholinesterase,U/L, No. (Median) | 10,754 (8,334-12613) |
| White blood cells,cells/ μL, No. (Median) | 6,400 (5,370-7700) |
| Red blood cells,cells/ μL, No. (Median) | 4,870,000 (4,625,000-5,190,000) |
| Platelets, elements/μL, No. (Median) | 193,000 (159,000-234,000) |
| Hemoglobin,g/dL, No. (Median) | 15.1 (14.2-15.9) |
| Prothrombin activity, % | 99 (90-107) |
| Alpha-fetoprotein,pg/mL, No. (Median) | 4 (2.3-8.1) |
| Ferritin,ng/mL, No. (Median) | 162 (80-265.75) |
| HCVaRNA, UI/mL, No. (Median) | 515,000 (200,000 – 850,000) |
| Cirrhosis at liver biopsy, % | 20.5 |
| Staging at liver biopsyb, % | |
| 0 | 1.6 |
| 1 | 34.5 |
| 2 | 21.7 |
| 3 | 12.4 |
| 4 | 9.2 |
| 5 | 7.2 |
| 6 | 13.3 |
aAbbreviations: ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; HCV, hepatitis C virus
bAccording to Ishak classification
3.1. Statistical Analysis
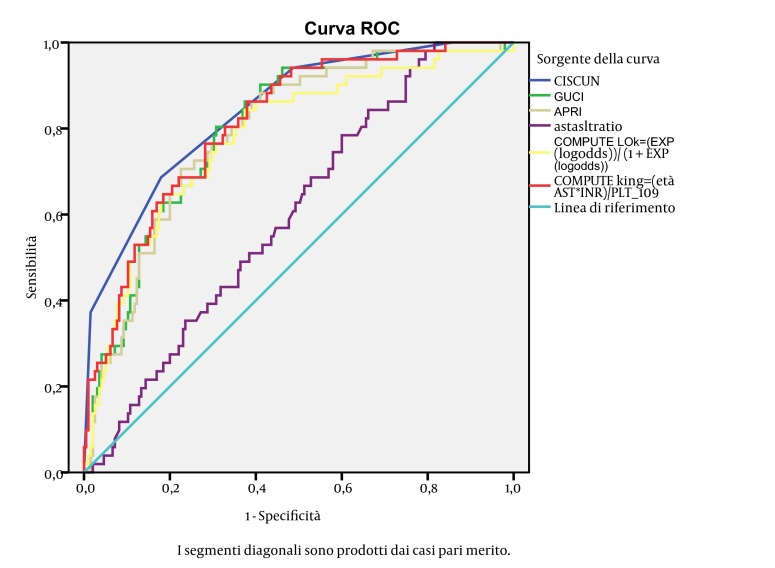
The Kolmogorov-Smirnov test was used to check quantitative variables for Gaussian distribution. In case of Gaussian distribution, data were reported as mean ± standard deviation (SD), while in case of non-Gaussian distribution they were reported as median and interquartile range (IQR). In case of Gaussian distribution, the Student’s t-test for unpaired variables was applied, while the Mann-Whitney U was used in case of non-Gaussian distribution. The chi-square test with Yates correction (or Fisher’s exact test where appropriate) was used for categorical variables. A P < 0.05 at two-sided test was considered statistically significant. Any independent variable statistically different in the two groups or with a P < = 0.2 at univariate analysis was included in binary logistic regression analysis using the forward conditional stepwise method. The cut-off values used for the stepwise method were: P = 0.05 for entry into the model, and P = 0.10 for its removal. We constructed a receiver operator characteristic (ROC) curve for each continuous variable. The curve shows the capacity of the variable to discriminate between patients with cirrhosis and those without it. The larger the area under the curve (AUC), the better the discriminating ability. An AUC above 0.7 is considered useful, and an AUC above 0.8 indicates excellent accuracy (44). All continuous variables were categorized to maximize easiness of use in clinical practice. Cutoffs were constructed by the means of ROC curve analysis based on the value that maximized the sum of sensitivity and specificity. AUCs of different ROC curves were compared according to the method of DeLong (45). We calculated specificity, sensitivity, positive predictive value, negative predictive value, diagnostic accuracy, positive likelihood ratio, negative likelihood ratio for our score, and compared these values with other scores available to the aim of predicting cirrhosis by noninvasive means. All statistical analyses were performed using the Statistical Package for the Social Sciences version 18.0 (SPSS Inc. Chicago, Ill).
4. Results
We enrolled 281 patients. We excluded 17 patients for insufficient liver tissue for staging of fibrosis, and 16 for incomplete biochemical or blood count data (one of them had also insufficient liver tissue). Therefore all analysis was performed on the remaining 249 patients. The main features of the 249 patients are shown in Table 1. In 51/249 patients (20.5%) histologic evaluation showed liver cirrhosis. Table 2 shows the demographic, laboratory, and ultrasound features of the 249 patients stratified according to the presence/absence of cirrhosis, and the results of univariate analysis. We put variables associated with presence of cirrhosis or with a P ≤ 0.2 at univariate analysis in a binary logistic regression analysis to identify independent predictors of cirrhosis. As shown in Table 3 higher age and AST, lower prothrombin activity and platelets were independent predictors of histological cirrhosis. We categorized these variables according to the ROC curves (see method section). The cutoff points generated were: age > 40 years; AST >2 upper normal values; platelet count < 160.000/mmc; prothrombin activity < 100%. We devised a score based on these predictors assigning 1 point per predictor. We called this novel score CISCUN (Cirrhosis Score University of Naples). Cirrhosis rate was 0% in the 28 patients with CISCUN = 0; 4% in the 75 patients with CISCUN = 1; 17.1% in the 76 patients with CISCUN = 2; 33.3% in the 48 patients with CISCUN = 3; and 86.4% in the 22 patients with CISCUN = 4 (see Figure 1). Therefore, as patients with CISCUN 0 or 1 had similar very low rate of cirrhosis, and those with CISCUN = 2 or 3 had similar intermediate risk compared to those with CISCUN = 4 which had a very different and high risk of cirrhosis, we decided to merge patients with CISCUN = 0 and 1, and consider them as patients at low risk, and to merge patients with CISCUN = 2 or 3 and consider them as patients in the “grey” area. Consequently, patients with a CISCUN of 0 or 1 had a very low risk of cirrhosis (2.9%) while patients with a score = 4 had a very high risk of cirrhosis (86.4%). Patients with a CISCUN of 2 or 3 showed an intermediate risk of cirrhosis (23.4%). We calculated the sensitivity (Se), specificity (Sp), positive predictive value (PPV), negative predictive value (NPV), diagnostic accuracy (DA), positive likelihood ratio (PLR), negative likelihood ratio (NLR) of the CISCUN using a cutoff of 1 (0-1 vs. 2-4), and using a cutoff of 3 (0-3 vs.4), and compared these with other scores predicting cirrhosis (see Table 4). We calculated AUC of the CISCUN and compared this value with other scores in the ability to predict cirrhosis (see Table 5). AUC of CISCUN was significantly higher than that of AST/AST ratio and Lok score. A trend toward higher AUC was observed for CISCUN compared to APRI and GUCI. In contrast AUC for CISCUN was not different from that of King’s college score. We validated CISCUN ability to discriminate between patients with and without cirrhosis in the independent set of patients with chronic hepatitis C. This group was composed of 285 patients admitted for a liver biopsy to our Departments between 01.01.2007 and 30.06.2012. Median age was 50 years (IQR: 39-58); median AST level was 52 U/L (IQR: 40-80); median Prothrombin activity was 101% (IQR: 94-108); median PLT levels was 217,000/µL (IQR: 178,000 – 279,500). Male represented 57.2% of subjects. Cirrhosis was identified in 27 (9.5%) cases. In these patients cirrhosis rate was 2.6% in the 39 patients with CISCUN = 0; 0.9% in the 117 patients with CISCUN = 1; 2.7% in the 73 patients with CISCUN = 2; 21.1% in the 38 patients with CISCUN = 3; and 83.3% in the 18 patients with CISCUN = 4. Therefore, even in this group it is confirmed that subjects with a CISCUN = 0 or 1 had a very low risk of cirrhosis (1.28%) while patients with a score = 4 had a very high risk of cirrhosis (83.3%). Patients with a CISCUN of 2 or 3 showed a non-negligible risk of cirrhosis (9%). Table 6 shows sensitivity, specificity, PPV, NPV, DA, PLR, and NLR of the CISCUN in the validation set.
Table 2. Demographic, Laboratory, Ultrasound Features of the First Group of Patients Stratified by Presence or Absence of Histological Cirrhosis (n = 249)b.
| Patients without cirrhosis (n = 198) | Patients with cirrhosis (n = 51) | P value | |
|---|---|---|---|
| Age, y, No (Median) | 46 (33-54) | 52 (45-56) | < 0.001 |
| Sex, % | 0.037 | ||
| Male | 58.6 | 74.5 | |
| Female | 41.4 | 25.5 | |
| Alcohol consumption, % | 13.4 | 17.5 | 0.500 |
| Pervious Antiviral treatment, % | 11.6 | 19.6 | 0.133 |
| Splenomegaly at ultrasound, % | 22 | 36.2 | 0.045 |
| Iron, µg/mL | 114 (83-143) | 151 (112-183) | < 0.001 |
| Glucose, mg/ dL | 88 (79-99) | 93 (86-109) | 0.002 |
| Total Bilirubin, mg/ dL | 0.61 (0.48-0.80) | 0.73 (0.59-1.04) | 0.002 |
| ASTa, U/L | 56 (39-83) | 94 (64-119) | < 0.001 |
| ALTa, U/L | 93 (54-159) | 139 (83-198) | 0.001 |
| Alkaline phosphatase , U/L | 147 (95-205) | 219.5 (130-296) | 0.001 |
| Albumin, g/ dL | 4.4 (4.2-4.6) | 4.3 (4.1-4.5) | 0.020 |
| Cholinesterase,U/L | 11,062 (8,799-12,996) | 8,949 (6,892-11,270) | 0.001 |
| White blood cells,cells/ μL | 6,450 (5,300-7,800) | 6,400 (5,500-7,300) | 0.541 |
| Red blood cells,cells/ μL | 4,860,000 (4,630,000-5,190,000) | 4,910,000 (4,570,000-5,190,000) | 0.765 |
| Platelets, elements/μL | 198,500 (171,750-245,000) | 149,500 (118,000-188,000) | < 0.001 |
| Hemoglobin,g/dL | 15.0 (14.1-15.9) | 15.4 (14.7-16.1) | 0.044 |
| Prothrombin activity, % | 101 (93-108) | 92 (86-99) | < 0.001 |
| Alpha-fetoprotein,pg/mL | 3.3 (2.2-5.8) | 8.1 (3.9-17.2) | < 0.001 |
| Ferritin, ng/mL | 137 (61-234) | 195 (130-445) | 0.037 |
| HCVaRNA, UI/mL | 511,000 (200,000–850,000) | 698,000 (126,500–980,000) | 0.541 |
aAbbreviations: ALT, Alanine Aminotransferase; AST, Aspartate Aminotransferase; HCV, hepatitis C virus
bData were compared using χ 2 test for categorical variables and Mann-Whitney U test for quantitative variables
Table 3. Independent Predictors of Cirrhosis at Logistic Regression Analysis in the First Group of Patients (n = 249).
| Regression coefficient | Standard Error | Odds Ratio, (95% CIa) | P value | |
|---|---|---|---|---|
| Age, y | 0.059 | 0.019 | 1.061 (1.021-1.102) | 0.002 |
| ASTa, U/L | 0.04 | 0.02 | 1.004 (1.001-1.008) | 0.046 |
| Prothrombin activity, % | -0.53 | 0.017 | 0.949 (0.919-0.980) | 0.002 |
| Platelets,103elements/μL | -0.014 | 0.004 | 0.986 (0.978-0.994) | 0.001 |
aAbbreviation: AST, Aspartate Aminotransferase; CI, Confidence Interval
Figure 1. Rate of Cirrhosis in Patients With CISCUN 0-4 in the First Group of Patients (n = 249).
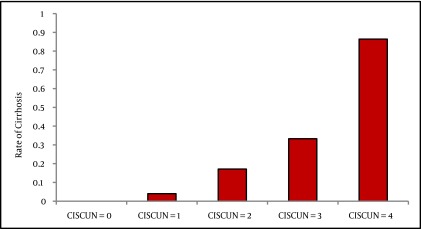
Table 4. Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, Diagnostic Accuracy, Positive Likelihood Ratio, Negative Likelihood Ratio for CISCUN, and For Other Noninvasive Scores Predicting Cirrhosis in the First Group of Patients (n = 249).
| Level | All Patients, No. | Patients. with cirrhosis, No. (%) | SEa, % | SPa, % | PPVa, % | NPVa, % | DAa, % | PLRa | NLRa | |
|---|---|---|---|---|---|---|---|---|---|---|
| CISCUN | 0-1 | 103 | 3 (2.9) | 94.1 | 50.5 | 32.9 | 97.1 | 59.4 | 1.90 | 0.12 |
| 2-4 | 146 | 48 (32.9) | ||||||||
| 0-3 | 227 | 32 (14.1) | 37.3 | 98.5 | 86.4 | 85.9 | 85.9 | 24.59 | 0.64 | |
| 4 | 22 | 19 (86.4) | ||||||||
| Lok score (6) | ≤ 0.2 | 99 | 6 (6.1) | 88.2 | 47 | 30 | 93.9 | 55.4 | 1.66 | 0.25 |
| >0.2 | 150 | 45 (30) | ||||||||
| ≤ 0.5 | 222 | 36 (16.2) | 29.4 | 93.9 | 55.6 | 83.8 | 80.7 | 4.85 | 0.75 | |
| > 0.5 | 27 | 15 (55.6) | ||||||||
| AST/ALTaratio (39) | < 1 | 227 | 46 (20.3) | 9.8 | 91.4 | 22.7 | 79.7 | 74.7 | 1.14 | 0.99 |
| ≥ 1 | 22 | 5 (22.7) | ||||||||
| GUCI (33) | ≤ 1 | 129 | 7 (5.4) | 86.3 | 61.6 | 36.7 | 94.6 | 66.7 | 2.25 | 0.22 |
| > 1 | 120 | 44 (36.7) | ||||||||
| King’s college (29) | < 16.6 | 135 | 9 (6.7) | 82.4 | 63.6 | 36.8 | 93.3 | 67.5 | 2.26 | 0.28 |
| ≥ 16.6 | 114 | 42 (36.8) | ||||||||
| APRIa (40) | ≤ 1 | 151 | 12 (7.9) | 76.5 | 70.2 | 39.8 | 92.1 | 71.5 | 2.57 | 0.34 |
| > 1 | 98 | 39 (39.8) | ||||||||
| ≤ 2 | 217 | 36 (16.6) | 29.4 | 91.4 | 46.9 | 83.4 | 78.7 | 3.43 | 0.77 | |
| > 2 | 32 | 15 (46.9) | ||||||||
| PLTa (35) | ≥ 150.000/mmc | 203 | 25 (12.3) | 51 | 89.9 | 56.5 | 87.7 | 81.9 | 5.05 | 0.55 |
| < 150.000/mmc | 46 | 26 (56.5) |
aAbbreviations: SE, Sensitivity; SP, Specificity; PPV, Positive Predictive Value; NPV, Negative Predictive Value; DA, Diagnostic Accuracy; PLR, Positive Likelihood Ratio; NLR, Negative Likelihood Ratio; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; GUCI, Goteborg University Cirrhosis Index; APRI, AST to platelet ratio index; PLT, Platelet Count
Table 5. Comparison of CISCUN With Other Cirrhosis Scores.
| AUCa | Standard Error | 95% CIa | P valueb | |
|---|---|---|---|---|
| CISCUN | 0.842 | 0.0300 | 0.790 to 0.885 | |
| APRIa | 0.793 | 0.0332 | 0.737 to 0.842 | 0.0604 |
| AST/ALT ratioa | 0.600 | 0.0405 | 0.536 to 0.662 | < 0.0001 |
| GUCIa | 0.799 | 0.0328 | 0.743 to 0.847 | 0.0800 |
| King | 0.813 | 0.0315 | 0.758 to 0.860 | 0.1496 |
| L ok | 0.781 | 0.0368 | 0.725 to 0.831 | 0.0493 |
aAbbreviations: AUC, Area Under the Curve; CI, Confidence Interval; APRI, AST to platelet ratio index; AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase; GUCI, Goteborg University Cirrhosis Index
bComparison with CISCUN
Table 6. Sensitivity, Specificity, Positive Predictive Value, Negative Predictive Value, Diagnostic accuracy, Positive Likelihood Ratio, Negative Likelihood Ratio for CISCUN in the Validation Set (n = 281).
| Level | All patients, No. | Patients with cirrhosis, No. (%) | SEa, % | SPa, % | PPVa, % | NPVa, % | DAa, % | PLRa | NLRa | |
|---|---|---|---|---|---|---|---|---|---|---|
| CISCUN | 0-1 | 156 | 2 (1.3) | 92.6 | 59.7 | 19.4 | 98.7 | 62.8 | 2.30 | 0.12 |
| 2-4 | 129 | 25 (19.4) | ||||||||
| 0-3 | 267 | 12 (4.5) | 55.6 | 98.8 | 83.3 | 95.5 | 94.7 | 47.78 | 0.45 | |
| 4 | 18 | 15 (83.3) |
aAbbreviations: SE: Sensitivity; SP: Specificity; PPV, Positive Predictive Value; NPV, Negative Predictive Value; DA; Diagnostic Accuracy; PLR, Positive Likelihood Ratio; NLR, Negative Likelihood Ratio
5. Discussion
In our study we identified 4 predictors of cirrhosis in a population of patients with chronic hepatitis C. These predictors were categorized to maximize the easiness of use in clinical practice. Patients with none or one of these predictors (CISCUN = 0 or 1) presented a very low risk of cirrhosis, while patients with all predictors (CISCUN = 4) had a high risk of cirrhosis. The patients in the “grey” zone (CISCUN 2 or 3) presented an intermediate risk of cirrhosis. As about a half of patients present a SCORE of 0, 1 or 4, liver biopsy may be avoided in about a half of patients with a low risk of misclassifying patients. We underline that the patients misclassified are 4.8% of patients with a score of 0, 1 or 4 and this Figure 2 is very low considering that liver biopsy itself has a rate of misclassification that reaches 11-20% in same studies (8, 46). These results were confirmed in an independent validation set of patients with HCV-related chronic liver disease. In this latter group more than 60% of liver biopsy (patients with CISCUN = 0, 1, or 4) may be avoided with an even lower risk of misclassification (2.9%). This is particularly interesting because the validation set showed a reduced rate of cirrhosis compared to the first group of patients. The diagnosis of cirrhosis remains a key element from both prognostic and therapeutic points of view. For example, the recent availability of protease inhibitors–containing combinations for patients infected with genotype 1, poses a series of problems. Costs and side effects of these drugs may represent a limitation to their use, considering that other and better tolerated drugs are in an advanced phase of clinical development. Decisions regarding immediate treatment or “wait and see” strategy cannot disregard a precise staging of the disease. The presence of cirrhosis is certainly a factor inducing an immediate treatment with the best options available, and liver biopsy still represents the gold standard to diagnose liver cirrhosis. However, liver biopsy is an invasive test associated with both complications and costs (11-14). Therefore a score able to diagnose cirrhosis by noninvasive means can be useful and cost-effective for the clinicians. Our score has several advantages over other ones devised for the same aim:
Figure 2. ROC Curves for the Scores Predicting Cirrhosis.
1) It includes parameters that are routinely available and unbiased in contrast with other studies that include analyses that are difficult-to-perform in clinical practice (20-27) or include subjective variables (41). Moreover the cost of the determination of our parameters is very low.
2) There is no need for mathematic formulae as in some other studies (6, 28-30, 32-34, 40). This represents a clear advantage for easiness which is a key element to make the score potentially widely used at the bedside
3) Our score showed a very high diagnostic performance in our cohort group of patients. In fact NPV (for CISCUN ≤ 1), and PPV (for CISCUN = 4) yielded the highest values among other scores devoted to cirrhosis prediction. It is noteworthy that the rate of false negative of CISCUN is very low (< 3%). This is a relevant point for a screening test.
Other approaches used to diagnose cirrhosis by noninvasive means include the measurement of liver stiffness by Fibroscan (17), and the employment of a proprietary algorithm on a panel of selected blood tests called Fibrotest (18). Both are useful predictors of cirrhosis. However, they are not universally available nor cost-saving tools compared to scores that use routine parameters. We underline that CISCUN includes only biologically plausible variables. In fact, age is associated with progression to cirrhosis (47). A low platelet count is associated with liver cirrhosis because of splenic sequestration due to portal hypertension. A reduced hepatic thrombopoietin production has also been claimed as a factor causing platelet decrease in patients with cirrhosis (48). It is noteworthy that platelet levels are included in almost all noninvasive scores predicting the presence of cirrhosis or esophageal varices (16, 49). AST levels are associated with liver cirrhosis as well. It is thought that this occurs because of a greater mitochondrial damage (and therefore to enhanced AST release) occurs in patients with cirrhosis. In our study AST/ALT ratio was not found to be associated with cirrhosis. Lastly prothrombin activity is directly related to hepatic synthetic function, and therefore worsens with progression of fibrosis and loss of hepatocytes (6). A potential limitation of our study (like all studies devoted to noninvasive prediction of cirrhosis) is that liver biopsy can provide additional information for physician such as quantify the degree of fibrosis, and show signs of overlapping conditions (e.g. NASH or iron overload).
In conclusion, we devised a novel score called CISCUN which is based on four predictors (Age > 40 years; AST > 2 upper normal values; platelet count < 160.000/mmc; Prothrombin activity < 100%), and assigns 1 point per predictor. CISCUN can identify patients with a very low rate of cirrhosis (CISCUN = 0 or 1), and patients with a high risk of having cirrhosis (CISCUN = 4) among subjects with chronic hepatitis C. Patients with a CISCUN = 2 or 3 (grey zone) present an intermediate risk of cirrhosis, and therefore should perform a liver biopsy to receive a reliable diagnosis.
Acknowledgments
The authors would like to thank all colleagues from the Section of Infectious Diseases of the Department of Clinical Medicine and Surgery.
Footnotes
Implication for health policy/practice/research/medical education:
The study provides a simple tool to identify patients with cirrhosis among those with chronic hepatitis C. The use of this novel noninvasive score, namely Cirrhosis Score University of Naples (CISCUN), based on routinely-available parameters may avoid safely about half biopsies to diagnose liver cirrhosis in this setting. It is noteworthy that results from a first group of patients were validated in an independent set of similar patients. The employment of CISCUN in clinical practice could reduce cost and rate of complications associated with liver biopsy. This article is intended for all medical doctors who manage chronic hepatitis C in their everyday clinical practice.
Please cite this paper as:
Gentile I, Coppola N, Pasquale G, Liuzzi R, Armiento MD, Di Lorenzo ME, et al. A Simple Noninvasive Score Based on Routine Parameters can Predict Liver Cirrhosis in Patients With Chronic Hepatitis C. Hepat Mon. 2013;13(5):e8352. DOI: 10.5812/hepatmon.8352
Authors’ Contribution:
I.G. conceived the original idea and the protocol, contributed to data analysis, and drafted the manuscript. N.Co. contributed to data analysis, and to the protocol development, and revised the manuscript. G.P. and M.D.A. were the pathologists of the study, and revised the manuscript. R.L. performed part of statistical analysis, and revised the manuscript. M.E.D.L. collected data of the patients, and revised the manuscript. N.Capol. collected data of the patients. A.B., E.S., F.M., N.Capor., E.S. contributed to protocol development and revised the paper. G.B. was the coordinator of the team and revised the manuscript.
Financial Disclosure:
The authors declare no conflict of interest regarding this manuscript.
Funding/Support:
No funding or sponsorship was involved in this study.
References
- 1.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29 Suppl 1:74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 2.Global burden of disease (GBD) for hepatitis C. J Clin Pharmacol. 2004;44(1):20–9. doi: 10.1177/0091270003258669. [DOI] [PubMed] [Google Scholar]
- 3.Coppola N, Pisapia R, Tonziello G, Masiello A, Martini S, Pisaturo M, et al. Improvement in the aetiological diagnosis of acute hepatitis C: a diagnostic protocol based on the anti-HCV-IgM titre and IgG Avidity Index. J Clin Virol. 2009;46(3):222–9. doi: 10.1016/j.jcv.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Hoofnagle JH. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26(3 Suppl 1):15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922–38. doi: 10.1002/hep.21907. [DOI] [PubMed] [Google Scholar]
- 6.Lok AS, Ghany MG, Goodman ZD, Wright EC, Everson GT, Sterling RK, et al. Predicting cirrhosis in patients with hepatitis C based on standard laboratory tests: results of the HALT-C cohort. Hepatology. 2005;42(2):282–92. doi: 10.1002/hep.20772. [DOI] [PubMed] [Google Scholar]
- 7.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38(6):1449–57. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Pagliaro L, Rinaldi F, Craxi A, Di Piazza S, Filippazzo G, Gatto G, et al. Percutaneous blind biopsy versus laparoscopy with guided biopsy in diagnosis of cirrhosis. A prospective, randomized trial. Dig Dis Sci. 1983;28(1):39–43. doi: 10.1007/BF01393359. [DOI] [PubMed] [Google Scholar]
- 9.Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97(10):2614–8. doi: 10.1111/j.1572-0241.2002.06038.x. [DOI] [PubMed] [Google Scholar]
- 10.Siddique I, El-Naga HA, Madda JP, Memon A, Hasan F. Sampling variability on percutaneous liver biopsy in patients with chronic hepatitis C virus infection. Scand J Gastroenterol. 2003;38(4):427–32. doi: 10.1080/00365520310000825. [DOI] [PubMed] [Google Scholar]
- 11.Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32(3):477–81. doi: 10.1053/jhep.2000.16602. [DOI] [PubMed] [Google Scholar]
- 12.Janes CH, Lindor KD. Outcome of patients hospitalized for complications after outpatient liver biopsy. Ann Intern Med. 1993;118(2):96–8. doi: 10.7326/0003-4819-118-2-199301150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Perrault J, McGill DB, Ott BJ, Taylor WF. Liver biopsy: complications in 1000 inpatients and outpatients. Gastroenterology. 1978;74(1):103–6. [PubMed] [Google Scholar]
- 14.Sagnelli E, Sagnelli C, Pisaturo MA, Coppola N, Pasquale G, Piccinino F. Liver biopsy in chronic hepatitis C: the experience of 15 Italian wards of infectious diseases. Infez Med. 2012;20(1):31–6. [PubMed] [Google Scholar]
- 15.Poynard T, Morra R, Ingiliz P, Imbert-Bismut F, Thabut D, Messous D, et al. Assessment of liver fibrosis: noninvasive means. Saudi J Gastroenterol. 2008;14(4):163–73. doi: 10.4103/1319-3767.43273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43(2 Suppl 1):S113–20. doi: 10.1002/hep.21046. [DOI] [PubMed] [Google Scholar]
- 17.Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41(1):48–54. doi: 10.1002/hep.20506. [DOI] [PubMed] [Google Scholar]
- 18.Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet. 2001;357(9262):1069–75. doi: 10.1016/S0140-6736(00)04258-6. [DOI] [PubMed] [Google Scholar]
- 19.Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee CM, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol. 2011;17(41):4581–9. doi: 10.3748/wjg.v17.i41.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berres ML, Schlosser B, Berg T, Trautwein C, Wasmuth HE. Soluble urokinase plasminogen activator receptor is associated with progressive liver fibrosis in hepatitis C infection. J Clin Gastroenterol. 2012;46(4):334–8. doi: 10.1097/MCG.0b013e31822da19d. [DOI] [PubMed] [Google Scholar]
- 21.Fortunato G, Castaldo G, Oriani G, Cerini R, Intrieri M, Molinaro E, et al. Multivariate discriminant function based on six biochemical markers in blood can predict the cirrhotic evolution of chronic hepatitis. Clin Chem. 2001;47(9):1696–700. [PubMed] [Google Scholar]
- 22.Ho AS, Cheng CC, Lee SC, Liu ML, Lee JY, Wang WM, et al. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci. 2010;17:58. doi: 10.1186/1423-0127-17-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovalenko E, Tacke F, Gressner OA, Zimmermann HW, Lahme B, Janetzko A, et al. Validation of connective tissue growth factor (CTGF/CCN2) and its gene polymorphisms as noninvasive biomarkers for the assessment of liver fibrosis. J Viral Hepat. 2009;16(9):612–20. doi: 10.1111/j.1365-2893.2009.01110.x. [DOI] [PubMed] [Google Scholar]
- 24.Mehta P, Ploutz-Snyder R, Nandi J, Rawlins SR, Sanderson SO, Levine RA. Diagnostic accuracy of serum hyaluronic acid, FIBROSpect II, and YKL-40 for discriminating fibrosis stages in chronic hepatitis C. Am J Gastroenterol. 2008;103(4):928–36. doi: 10.1111/j.1572-0241.2007.01761.x. [DOI] [PubMed] [Google Scholar]
- 25.Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol. 2005;11(4):476–81. doi: 10.3748/wjg.v11.i4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Selim HS, El-Barrawy MA, Taha HA, Abd El-Hafiz DA. Evaluation of Interleukin-18 as a Non Invasive Marker of Liver Fibrosis among Chronic Hepatitis C Virus Patients. J Egypt Public Health Assoc. 2009;84(5-6):391–403. [PubMed] [Google Scholar]
- 27.Valva P, Casciato P, Diaz Carrasco JM, Gadano A, Galdame O, Galoppo MC, et al. The role of serum biomarkers in predicting fibrosis progression in pediatric and adult hepatitis C virus chronic infection. PLoS One. 2011;6(8) doi: 10.1371/journal.pone.0023218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonacini M, Hadi G, Govindarajan S, Lindsay KL. Utility of a discriminant score for diagnosing advanced fibrosis or cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1997;92(8):1302–4. [PubMed] [Google Scholar]
- 29.Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King's Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21(7):730–8. doi: 10.1097/MEG.0b013e32830dfcb3. [DOI] [PubMed] [Google Scholar]
- 30.Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36(4 Pt 1):986–92. doi: 10.1053/jhep.2002.36128. [DOI] [PubMed] [Google Scholar]
- 31.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, et al. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163(2):218–24. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 32.Imperiale TF, Said AT, Cummings OW, Born LJ. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C. Am J Gastroenterol. 2000;95(9):2328–32. doi: 10.1111/j.1572-0241.2000.02322.x. [DOI] [PubMed] [Google Scholar]
- 33.Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol. 2005;40(7):867–72. doi: 10.1080/00365520510015674. [DOI] [PubMed] [Google Scholar]
- 34.Kim BK, Kim do Y, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546–53. doi: 10.1111/j.1478-3231.2009.02192.x. [DOI] [PubMed] [Google Scholar]
- 35.Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology. 2005;41(6):1376–82. doi: 10.1002/hep.20717. [DOI] [PubMed] [Google Scholar]
- 36.Luo JC, Hwang SJ, Chang FY, Chu CW, Lai CR, Wang YJ, et al. Simple blood tests can predict compensated liver cirrhosis in patients with chronic hepatitis C. Hepatogastroenterology. 2002;49(44):478–81. [PubMed] [Google Scholar]
- 37.Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis? J Gastroenterol Hepatol. 2000;15(4):386–90. doi: 10.1046/j.1440-1746.2000.02172.x. [DOI] [PubMed] [Google Scholar]
- 38.Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol. 2001;96(11):3142–6. doi: 10.1111/j.1572-0241.2001.05268.x. [DOI] [PubMed] [Google Scholar]
- 39.Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol. 1998;93(1):44–8. doi: 10.1111/j.1572-0241.1998.044_c.x. [DOI] [PubMed] [Google Scholar]
- 40.Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38(2):518–26. doi: 10.1053/jhep.2003.50346. [DOI] [PubMed] [Google Scholar]
- 41.Kaul V, Friedenberg FK, Braitman LE, Anis U, Zaeri N, Fazili J, et al. Development and validation of a model to diagnose cirrhosis in patients with hepatitis C. Am J Gastroenterol. 2002;97(10):2623–8. doi: 10.1111/j.1572-0241.2002.06040.x. [DOI] [PubMed] [Google Scholar]
- 42.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, et al. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22(6):696–9. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 43.Frank K, Linhart P, Kortsik C, Wohlenberg H. [Sonographic determination of spleen size: normal dimensions in adults with a healthy spleen]. Ultraschall Med. 1986;7(3):134–7. doi: 10.1055/s-2007-1011931. [DOI] [PubMed] [Google Scholar]
- 44.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 45.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–45. [PubMed] [Google Scholar]
- 46.Gentile I, Viola C, Graf M, Liuzzi R, Quarto M, Cerini R, et al. A simple noninvasive score predicts gastroesophageal varices in patients with chronic viral hepatitis. J Clin Gastroenterol. 2009;43(1):81–7. doi: 10.1097/MCG.0b013e318157464b. [DOI] [PubMed] [Google Scholar]
- 47.Pradat P, Voirin N, Tillmann HL, Chevallier M, Trepo C. Progression to cirrhosis in hepatitis C patients: an age-dependent process. Liver Int. 2007;27(3):335–9. doi: 10.1111/j.1478-3231.2006.01430.x. [DOI] [PubMed] [Google Scholar]
- 48.Peck-Radosavljevic M, Zacherl J, Meng YG, Pidlich J, Lipinski E, Langle F, et al. Is inadequate thrombopoietin production a major cause of thrombocytopenia in cirrhosis of the liver? J Hepatol. 1997;27(1):127–31. doi: 10.1016/s0168-8278(97)80291-7. [DOI] [PubMed] [Google Scholar]
- 49.Gentile I, Thabut D. Noninvasive prediction of oesophageal varices: as simple as blood count? Liver Int. 2010;30(8):1091–3. doi: 10.1111/j.1478-3231.2010.02317.x. [DOI] [PubMed] [Google Scholar]