Editor’s Key Points.
1. The main advantages of the Edinburgh posterior approach include: (I) easy access to posterior hilum including the bronchial branches and the pulmonary arteries; (II) lymph nodes are better seen and c) tips of the instruments are coming towards the camera, which provides safer dissection
2. In contrast to the anterior approach, the main differences in techniques of the posterior approach include: (I) the surgeons stand posterior to the patient; (II) utility incision is made at the 6th or 7th intercostal space anterior to latissimus dorsi muscle, instead of the 4th intercostal space; (III) camera port is made through the auscultatory triangle, instead of lower anterior incision; (IV) thorascoscopy is 0º rather than 30º; and (V)the order of dissection is from posterior to anterior, by opening up the fissure first to identify and isolate pulmonary arterial branches.
--A.H.
Introduction
Video-assisted thoracoscopic surgery (VATS) is now well established as an alternative to open thoracotomy for major pulmonary resections of bronchogenic carcinoma and benign disease (1,2). Compared to open surgery, the minimally invasive approach has a number of benefits in the immediate post-operative period that include reduced pain, better pulmonary function, shorter hospital stay, improved cosmesis and lower risk of developing pneumonia (3-7). VATS lobectomy is equivalent to open surgery in terms of oncological outcomes, is less immunomodulatory and enables more patients to commence and complete adjuvant chemotherapeutic regimens (7-10). Furthermore, minimally invasive techniques are cost effective and at least equivalent to open techniques in terms of long-term survival (11-13).
Whilst the anterior approach is preferred by many surgeons, the main advantages of the posterior approach in our experience is the excellent view that is obtained of the posterior hilum which facilitates dissection of the bronchi and branches of the pulmonary artery. In addition, the mediastinal node packets are clearly seen, allowing thorough lymphadenectomy. Importantly, in the posterior approach the tips of the instruments come towards the camera and are therefore easily seen whilst in use increasing the safety of dissection.
More than 800 VATS major pulmonary resections have been performed in our centre over the last 20 years (Video 1) and we here describe our method for fissure-based VATS lobectomy using a posterior approach.
Video 1.

Video-assisted thoracoscopic lobectomy - The Edinburgh posterior approach
Pre-operative assessment
Selection criteria
We have adopted VATS resecetion as the surgical strategy of choice for all cases of peripheral carcinoma of 5 cm or less in diameter and for suitable benign disease. Lobectomy and anatomic segmentectomy are standard procedures. It is possible to utilise VATS techniques in patients with more advanced disease such as moderate chest wall or pericardial involvement and, rarely, for pneumonectomy in patients with low bulk hilar involvement. However with the trend towards lung conservation strategies, we now reserve pneumonectomy for rare individuals in whom broncho-vascular reconstruction is not feasible.
Fitness for surgery
Baseline pulmonary function is assessed using a combination of spirometry and CO transfer factors for all patients. In addition, all patients undergo exercise testing. Cardiological assessment is carried out as relevant to the individual patient. Echo assessment of pulmonary (PA) pressure is undertaken in patients at risk of pulmonary hypertension (PAP>45 mmHg). Few patients are declined surgery on the basis of poor pulmonary function data. This serves as guidance for post-operative surveillance and respiratory management but our experience supports the view that even patients with a significantly reduced pulmonary function (>2 S.D. below normal) still fare well with a VATS approach.
Staging
In addition to a contrast-enhanced computed tomography scan of the head, chest, abdomen and pelvis, positron emission tomography-CT (PET-CT) with 18F-fluordeoxyglucose (18F-FDG) is performed in all patients with bronchogenic carcinoma under consideration for resection.
We have previously demonstrated that even in patients with no radiographic features of mediastinal lymph node spread, there is a 5% false negative rate for detection of lymph node metastases when compared to the gold standard of mediastinoscopy and lymph node biopsy (14). We therefore perform mediastinoscopy with sampling of ipsilateral stations 2 and 4, contralateral station 4 and the subcarinal (station 7) groups (consistent with current European Society of Thoracic Surgeons guidelines) in all patients being considered for VATS lobectomy for malignant disease in whom the procedure is not otherwise contraindicated.
In patients considered suitable for lobectomy, the VATS approach is attempted in all patients meeting size and stage criteria. The only absolute contraindications are those patients in whom the pleural cavity is obliterated on radiological grounds or who clearly have very proximal disease requiring a pneumonectomy. The requirement for sleeve lobectomy is a significant relative contraindication but not absolute. The conversion rate in our series is <7%.
Operative techniques
Anaesthesia and positioning
Following induction of anaesthesia, the patient is positioned in the lateral decubitus position. The hands are placed unsupported in the “prayer” position in front of the face and the operating table is manipulated to extend the thorax laterally opening up the intercostal spaces.
As soon as the double lumen endotracheal tube is confirmed to be in the correct position, whilst the patient is still in the anaesthetic room, ventilation is switched to the contralateral lung to optimise deflation of the lung that is to be operated upon. Suction is occasionally used if the lung does not deflate readily. The respiratory rate can be increased to 20 breaths/min or more in order to reduce the tidal volume and hence the degree of mediastinal excursion due to ventilation. This provides a more stable operating field. We rarely use central lines or urinary catheters but always use an arterial line and large bore venous cannulae.
Intercostal nerve blocks are used for perioperative analgesia in preference to epidural anaesthesia. Unless the parietal pleura has been disrupted, a local anaesthetic paravertebral catheter is placed at the end of the operation and remains in place for 48 hours. In addition, a patient-controlled morphine pump is supplied to the patient for post-operative analgesia.
The positioning of the surgical, anaesthetic and nursing teams and the equipment is illustrated in Figure 1. The surgeon and their assistant stand at the patient’s back with the screen directly across the table and the scrub nurse obliquely opposite. We utilise two additional large (55 inch) wall-mounted high definition screens. One is positioned opposite the scrub nurse and provides an operative view, which also allows anaesthetic staff, circulating nurses, students and observers to follow the progress of the operation. The other is positioned opposite the surgeon and provides large-scale high-definition radiology images, which the surgeon can view continuously in order to inform intra-operative decision-making.
Figure 1.
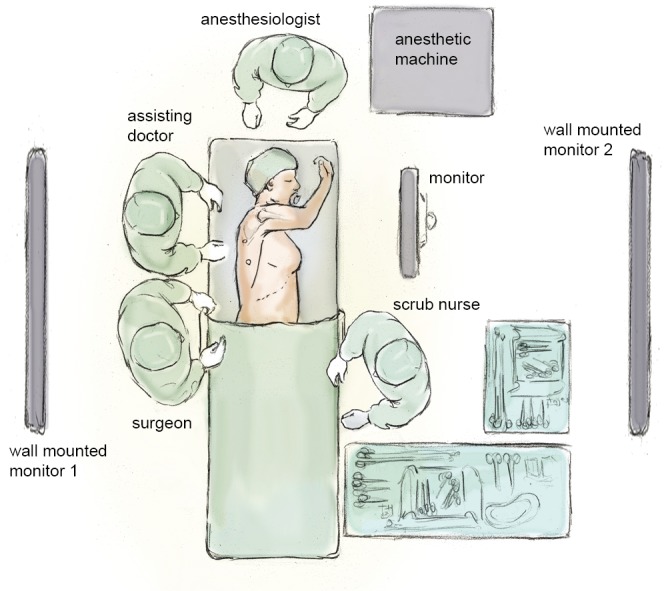
Schematic drawing of the surgical, anaesthetic and nursing teams and the equipment layout
Incisions
Three access ports are used and port position is standard irrespective of the lobe to be removed (Figure 2). A 5 cm utility port site incision is made in the sixth or seventh intercostal space (whichever is the larger) just in front of the anterior border of the latissimus dorsi muscle. The camera is temporarily introduced through this port to facilitate safe creation of a 1.5 cm incision posteriorly in the auscultatory triangle at the point nearest to the upper end of the oblique fissure. A port is inserted to accommodate the camera, which is positioned in this posterior port for the remainder of the procedure. A further 2 cm port is created in the midaxillary line level with the upper third of the anterior utility port. The anterior and posterior ports lie at opposite ends of the oblique fissure.
Figure 2.
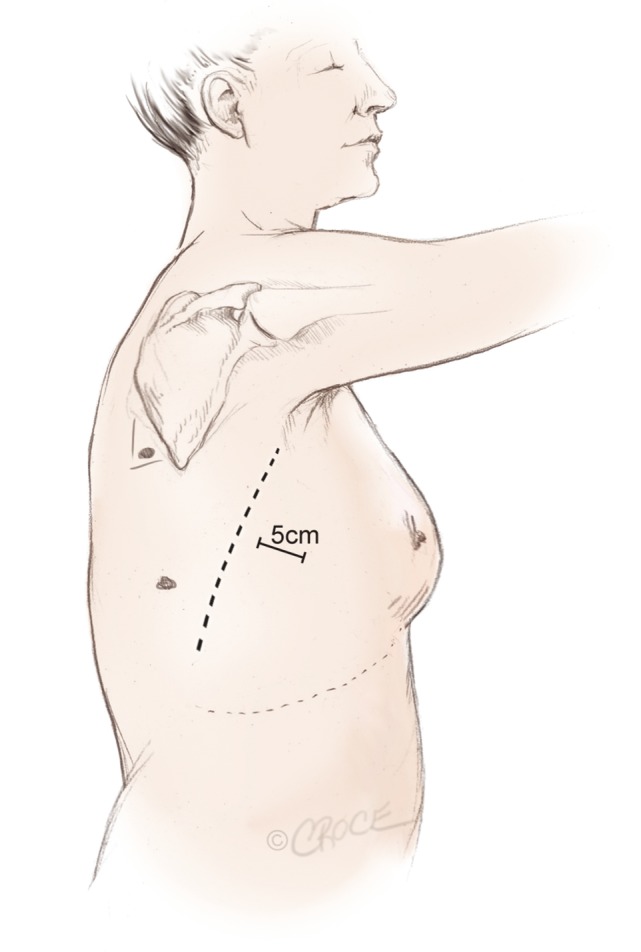
Incisions and port positions in relation to anatomical surface landmarks for the posterior approach, including a 5 cm utility incision anterior to the latissimus dorsi muscle
Instruments
We prefer a zero degree 10 mm high definition video thoracoscope as this provides a single axis view allowing easy correction of orientation. A combination of endoscopic and standard open surgical instruments is used. Lung retraction and manipulation are performed using ring-type sponge-holding forceps. Long artery forceps (30 cm) with or without mounted pledgets are employed for blunt dissection. These are particularly useful for exposing the PA at the base of the oblique fissure, cleaning structures and clearing node groups. A range of curved forceps and an endodissector are used gently as probes to create a passage between the lung parenchyma and major hilar structures. A right-angled dissector or long curved artery forceps is used to dissect out and pass slings around pulmonary arteries and veins. Endoscopic clips are used to ligate small vessels whilst large vessels and lung parenchyma are divided using endoscopic stapling devices to ensure haemostasis and aerostasis. We have found both endoscopic shears and specific VATS Metzenbaum type scissors to be helpful. The latter have the advantage of curved blade ends which reduce the risk of vascular injury.
Technique
A video-imaged thoracoscopic assessment is performed to confirm the location of the lesion, establish resectability and exclude unanticipated disease findings that might preclude resection. Video 1 is an edited video clip, which demonstrates several key points of VATS lobectomy via the Edinburgh Posterior approach.
The first step is to identify the PA within the central section of the oblique fissure. In some patients the PA is immediately visible, but in the majority of cases, the PA is revealed by separating the overlying pleura using blunt dissection with mounted pledgets (Figure 3). If the fissure does not open easily or is fused, an alternative approach utilising a fissure-last dissection as described below should be considered. Once the PA has been identified, the sheath of the artery is grasped with a fine vascular clamp or long artery forceps and an endoscopic dissector is used to enter the sheath defining the anterior and posterior margins of the artery. The apical lower branch of the PA is often exposed during this dissection.
Figure 3.
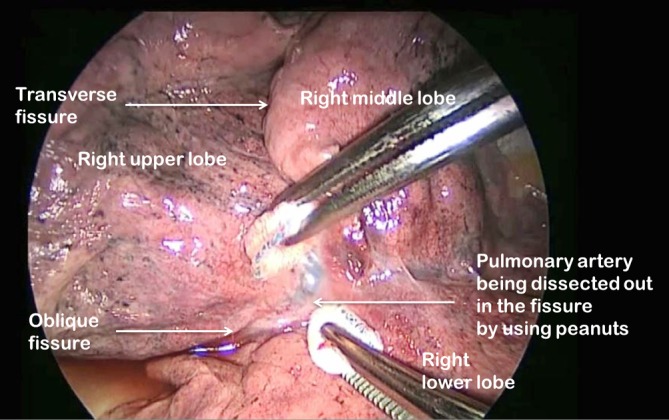
Pledget dissection of pulmonary artery in the oblique fissure on the right side
For all lobectomy procedures excepting middle lobectomy, the lung is then reflected anteriorly and the posterior pleural reflection is divided using sharp and blunt dissection. On the right this process should clear lung tissue away from the angle between the bronchus intermedius and the upper lobe bronchus exposing the lymph nodes in this position. On the left, the lung is swept away from hilum exposing the pulmonary artery (Figure 4). From the anterior port site, long artery forceps are then passed gently immediately posterior to the PA where it has been identified in the oblique fissure and central to the fused posterior fissure emerging through the incised posterior pleural reflection. On the right side care should be taken during this manoeuvre not to disrupt the lymph nodes lying along the bronchus intermedius. A sling is passed behind the posterior fissure, which is divided with an endoscopic linear stapling device. The PA is now clearly seen on the right side (Figure 5) and the distinction between the upper and lower lobes is established. Dissection then proceeds according to the lobe to be resected.
Figure 4.
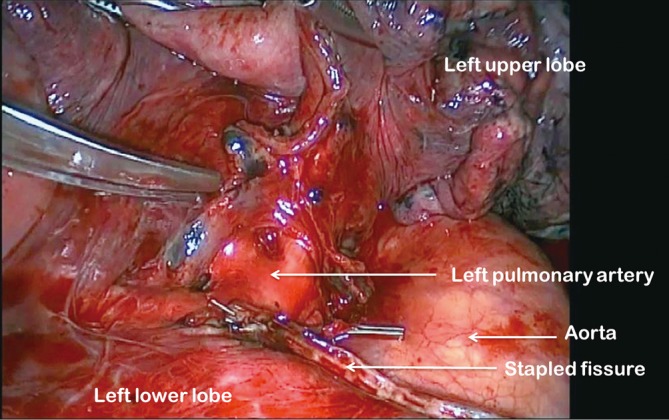
Left pulmonary artery exposed in oblique fissure
Figure 5.
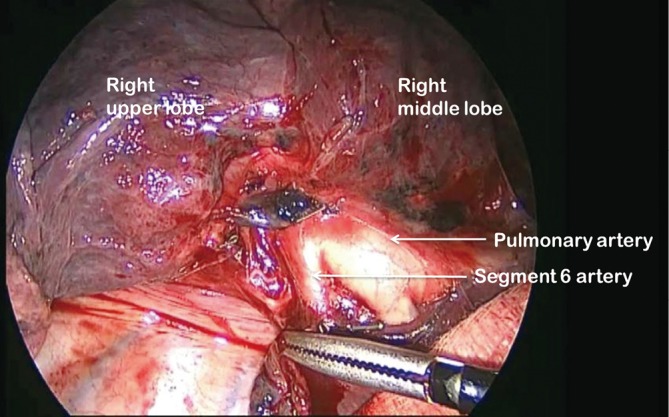
Right pulmonary artery exposed in oblique fissure
During division of the truncal pulmonary veins we place a central vascular clamp on the vein so that the vein will be secure and unable to retract away in the event of a mishap with the vascular endostaple.
All resected specimens are removed from the thoracic cavity in a retrieval bag to avoid contamination of the wounds with malignant cells.
Right upper lobectomy
Having divided the posterior fissure, the posterior ascending segmental branch of the PA is often evident and if it is should be divided at this stage. It is frequently small enough to clip. The upper lobe bronchus is then identified and dissected out. It is common to find a substantial bronchial artery running alongside the bronchus which should be ligated with clips and divided. Note that clips are only used on the proximal end and the distal end is not clipped since clips in this position may interfere with subsequent stapling of the bronchus.
The upper lobe is then retracted inferiorly and blunt dissection with mounted pledgets is used to free the cranial border of the upper lobe bronchus and define the apico-anterior trunk. The azygos vein is often closely related to the bronchus and can be pushed away using a gentle sweeping motion. A long artery forceps or vascular clamp is passed around the upper lobe bronchus close to its origin in the plane between the bronchus and the associated node packet (Figure 6). It should be appreciated that the apico-anterior trunk lies immediately anterior to the bronchus. The bronchus is transected at this level using an endoscopic linear stapling device. We do not find it necessary to inflate the lung to test that the correct bronchus is being divided as the vision is invariably excellent and the reinflated lung may then obscure the view for remainder of the resection. Following division of the bronchus the feeding vessels to the right upper lobe bronchus node packet are clipped and divided allowing the nodes to be swept up into the operative specimen.
Figure 6.

Right upper lobe bronchus ready to be stapled
Clasping the distal end of the transected bronchus with an endoscopic toothed grasper, the upper lobe can be reflected upwards. The posterior segmental artery is divided at this stage if not already dealt with and the apical and anterior segmental arteries or common stem artery are carefully cleaned, dissected out (Figure 7) and divided with an endoscopic stapler. Finally, the lung is retracted posteriorly facilitating dissection of the superior vein. This can be divided from either the posterior (Figure 8) or anterior aspect as convenient, taking care, in either case, to identify clearly and preserve the middle lobe vein. The transverse fissure is then divided. The middle lobe artery is most easily identified and protected if the stapling device is first passed through the inferior port and fired from posterior to anterior. Division of the transverse fissure is then completed passing the stapling device through the anterior port. The inferior pulmonary ligament is divided to facilitate expansion of the right lower lobe.
Figure 7.
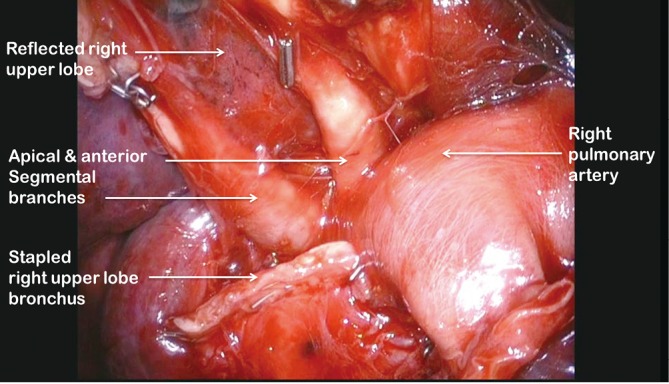
View of apical and anterior segmental arteries after dividing the right upper lobe bronchus and reflecting right upper lobe superiorly
Figure 8.
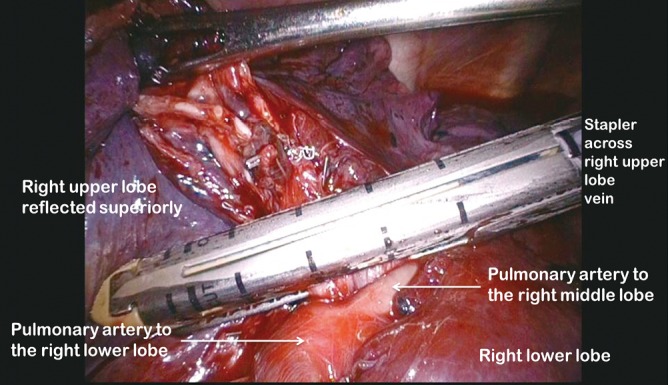
Right upper lobe vein divided using a stapler
Right lower lobectomy
Having identified the PA in the oblique fissure and divided the posterior oblique fissure, the pulmonary artery is then divided either in one or separately as a basal trunk artery and the apical segmental artery to the lower lobe. The space between the superior and inferior veins is developed and a long clamp is passed into this space emerging anterior to the PA in the oblique fissure. A sling is passed into this plane and the anterior oblique fissure is then divided. The lower lobe is mobilised by dividing the inferior pulmonary ligament. The inferior vein (Figure 9) is dissected free from surrounding tissue and divided using an endoscopic linear stapling device. The bronchus is identified and the bronchial vessels are clipped proximally. Lymph nodes are cleared from its medial and lateral margins. The lower lobe bronchus (Figure 10) is divided through its apical and basal branches preserving airflow to the middle lobe. The middle lobe bronchus must be visualised prior to stapling.
Figure 9.
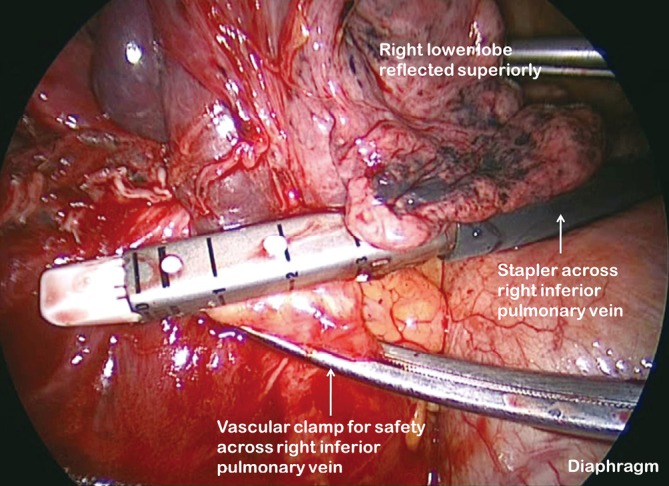
Right inferior vein divided with proximal vascular clamp
Figure 10.
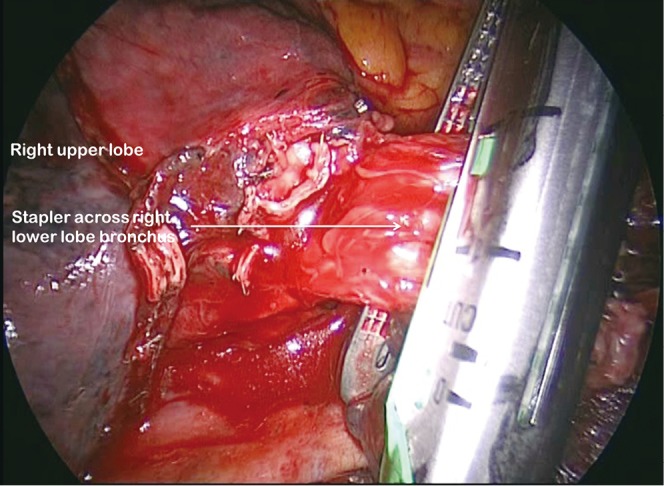
Division of right lower lobe bronchus across apical lower and basal trunk origins
Right middle lobectomy
The PA is identified and the anterior oblique fissure is divided as for right lower lobectomy. The vein, bronchus and arteries are then seen clearly and are divided in sequence. The transverse fissure is divided as described for right upper lobectomy.
Left upper lobectomy
The PA is identified in the oblique fissure and the posterior aspect of the oblique fissure (Figure 4) is divided in a similar way to the right side. The arterial branches to the left upper lobe are then divided sequentially. Division of the anterior aspect of the fissure is completed in similar manner to that on the right side. It is important to develop the space between the pulmonary veins and central to the fused anterior oblique fissure thoroughly. When passing a clamp through the utility incision and under the fused fissure, the surgeon will feel the lower lobe bronchus and should allow the clamp to pass superficial to that so preserving the airway to the lower lobe. Gentle blunt dissection is used to separate the superior pulmonary vein from the anterior surface of the bronchus. A long clamp is passed around the base of the bronchus taking particular care not to damage the PA. Retraction of the PA using a mounted pledget may be helpful. A sling is passed around the bronchus and used to elevate it (crane manoeuvre) in relation to the pulmonary artery and create a space via which an endoscopic stapling device can be inserted to divide the bronchus (Figure 11). The superior vein is cleaned and divided. The inferior pulmonary ligament is divided up to the level of the inferior vein to facilitate expansion of the lower lobe.
Figure 11.
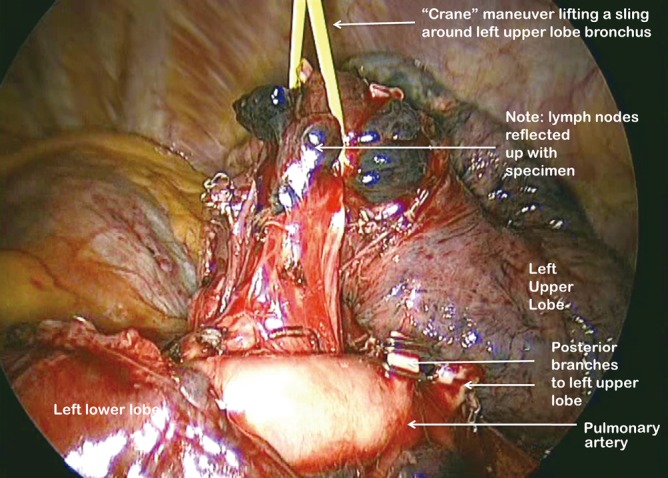
Use of “Crane” manoeuvre to elevate left upper lobe bronchus
Left lower lobectomy
As on the right side, having identified the PA and divided the posterior aspect of the oblique fissure, the arterial branches are identified (Figure 12). The anterior portion of the oblique fissure is divided as for left upper lobectomy and the arterial supply divided with an endostapler. The inferior pulmonary ligament is divided up to the level of the inferior pulmonary vein. The margins of the vein are clearly delineated and it is then divided. Bronchial vessels are clipped proximally and divided and the lymph node chains are cleared off the medial and lateral aspects of the bronchus, which is divided at its base.
Figure 12.
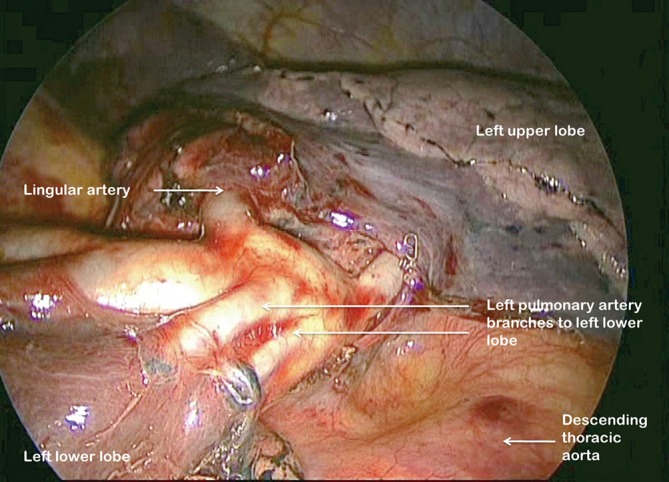
Left pulmonary artery branches to left lower lobe fully displayed
Strategies for dealing with challenging fissures
In some patients only a partial fissure is evident between the upper and lower lobes whilst in others the upper and lower lobes are completely fused. In such patients, the above techniques may have to be modified. Table 1 demonstrates a grading system for fissure quality (15).
Table 1. The Royal Infirmary Anatomical Classification of the Pulmonary Fissures.
| Grade I: The PA is readily visualised without dissection |
| Grade II: The PA is revealed following minimal dissection |
| Grade III: A shallow fissural cleft whereby a large amount of dissection is required to identify the PA. |
| Grade IV: There is no discernable fissural cleft at all on initial inspection |
It should be noted that there are important differences between the right and left oblique fissure. Fissural depth on the right is often not consistent so that while the fissure may be very thick in its upper part, the PA may be relatively easy to identify lower down where the basal trunk vessel is near the lung surface. In this situation the arterial sheath can be entered as described and the lung overlying the vessel divided retrogradely exposing the artery more centrally. On the left the PA can lie very posteriorly so that for upper lobectomy, the artery can be accessed simply by displacing the lung anteriorly.
We have developed two strategies for managing challenging fissures (Grade III/IV) in which the pulmonary artery is not accessible.
The first option is to reflect the lung anteriorly and divide the pleural reflection on the posterior aspect of the hilum. This allows the PA to be readily identified on the left and identified in the angle between the bronchus intermedius and upper lobe bronchus on the right. A clamp is then carefully placed adjacent to the PA and the fissure is draped over it tenting it up and thus identifying the point in the fissure that needs to be divided in order to let the clamp pass through. A sling is then passed through this space allowing the posterior part of the oblique fissure to be divided. The operation then proceeds as previously described.
The second alternative is to take a “fissure last” approach (Video 1). Beginning at the posterior pleural reflection, the artery, bronchus and vein are identified and divided first, with the fissure divided once these structures have been dealt with. This is a straightforward strategy for lower lobectomy as all the structures can be accessed by division of the pulmonary ligament and then serial division of the vein, bronchus and artery. It is not our first choice of approach, as we prefer to control the artery first if at all possible and to have optimum exposure of the bronchi in order to facilitate node management.
As a generalisation, in the very rare situation where the fissure is refractory to dissection we prefer the first strategy as it allows a standard dissection thereafter. When that seems technically awkward a fissure last approach is used. Sometimes, a hybrid approach based on the opportunities presenting as the procedure develops may be appropriate.
Lymph node management
All hilar level nodes relevant to the resected lobe are excised. At mediastinal level either extensive sampling or lymphadenectomy is preferred. We have utilised two strategies for lymphadenectomy either routine operative lymphadenectomy or prior mediastinoscopic lymphadenectomy, which typically accesses stations 2, 4, 7 with subsequent operative excision of the remaining mediastinal stations (Video 1). In our experience, prior video-mediastinoscopic adenectomy can result in significant tethering of the station 10 nodes and hilar fibrosis if there is any delay in proceeding to the VATS resection. As we prefer to await formal pathological analysis of the mediastinoscopic samples for staging purposes rather than rely on frozen section this induces a delay of several days in our management sequence and has made the VAMLA approach less attractive. We are currently examining our data and from this it is our evolving opinion that routine mediastinoscopy is probably sufficient to exclude station 2 and 4 disease but not for station 7. Thus preperative mediastinoscopy followed by routine formal adenectomy of station 7 and other node groups excluding stations 2 and 4 in this setting will likely be our future strategy.
Post-operative care
A size 32 Fr apical drain is placed through the mid-axillary line port site and is usually removed on the first post-operative day subject to a satisfactory chest radiograph and aerostasis. Patients are observed on a high dependency unit overnight and are typically nursed on the general thoracic ward thereafter. Analgesia is provided using a morphine patient controlled analgesia pump and a local anaesthetic paravertebral catheter placed under vision prior to port site closure. Early mobilisation is strongly encouraged with the availability of physiotherapy seven days per week, and discharge as early as post-operative day 3 is often possible.
Comments
We have presented a safe, reliable and reproducible approach to VATS lobectomy. VATS lobectomy has been shown to compare favourably with open thoractomy in terms of immediate post-operative recovery and is considered to be oncologically equivalent. We believe that the increasing use of minimally invasive techniques for lobectomy and other major pulmonary resections would be highly desirable.
Acknowledgments
Disclosure: The authors declare no conflict of interest.
References
- 1.Walker WS, Carnochan FM, Mattar S. Video-assisted thoracoscopic pneumonectomy. Br J Surg 1994;81:81-2 [DOI] [PubMed] [Google Scholar]
- 2.Walker WS, Codispoti M, Soon SY, et al. Long-term outcomes following VATS lobectomy for non-small cell bronchogenic carcinoma. Eur J Cardiothorac Surg 2003;23:397-402 [DOI] [PubMed] [Google Scholar]
- 3.Whitson BA, Andrade RS, Boettcher A, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 2007;83:1965-70 [DOI] [PubMed] [Google Scholar]
- 4.Handy JR, Jr, Asaph JW, Douville EC, et al. Does video-assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5 [DOI] [PubMed] [Google Scholar]
- 5.Lau KK, Martin-Ucar AE, Nakas A, et al. Lung cancer surgery in the breathless patient--the benefits of avoiding the gold standard. Eur J Cardiothorac Surg 2010;38:6-13 [DOI] [PubMed] [Google Scholar]
- 6.Nicastri DG, Wisnivesky JP, Litle VR, et al. Thoracoscopic lobectomy: report on safety, discharge independence, pain, and chemotherapy tolerance. J Thorac Cardiovasc Surg 2008;135:642-7 [DOI] [PubMed] [Google Scholar]
- 7.Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11 [DOI] [PubMed] [Google Scholar]
- 8.Jiang G, Yang F, Li X, et al. Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol 2011;9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim K, Kim HK, Park JS, et al. Video-assisted thoracic surgery lobectomy: single institutional experience with 704 cases. Ann Thorac Surg 2010;89:S2118-22 [DOI] [PubMed] [Google Scholar]
- 10.Whitson BA, D’Cunha J, Andrade RS, et al. Thoracoscopic versus thoracotomy approaches to lobectomy: differential impairment of cellular immunity. Ann Thorac Surg 2008;86:1735-44 [DOI] [PubMed] [Google Scholar]
- 11.Swanson SJ, Meyers BF, Gunnarsson CL, et al. Video-assisted thoracoscopic lobectomy is less costly and morbid than open lobectomy: a retrospective multiinstitutional database analysis. Ann Thorac Surg 2012;93:1027-32 [DOI] [PubMed] [Google Scholar]
- 12.Casali G, Walker WS. Video-assisted thoracic surgery lobectomy: can we afford it? Eur J Cardiothorac Surg 2009;35:423-8 [DOI] [PubMed] [Google Scholar]
- 13.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62 [DOI] [PubMed] [Google Scholar]
- 14.Carnochan FM, Walker WS. Positron emission tomography may underestimate the extent of thoracic disease in lung cancer patients. Eur J Cardiothorac Surg 2009;35:781-4;discussion 784-5 [DOI] [PubMed] [Google Scholar]
- 15.Craig SR, Walker WS. A proposed anatomical classification of the pulmonary fissures. J R Coll Surg Edinb 1997;42:233-4 [PubMed] [Google Scholar]


