Introduction
Minimally invasive video-assisted thoracic surgery (VATS) lobectomy has proven to be feasible and oncologically acceptable for non-small cell lung cancer (NSCLC) and a number of other conditions. Multiple studies have demonstrated clear benefits of VATs over a traditional thoracotomy approach, such as decreased length of stay, decreased short-term postoperative pain and fewer complications (1-4). Despite this, however, a VATS approach to anatomic resection is still not the current standard and is only slowly being implemented more widely. The explanation is likely multifactorial including: (I) technical issues, such as two-dimensional imaging and limited maneuverability of instrumentation; (II) lack of adequate training; and (III) concerns about the consequences of major vascular injury with a closed chest approach.
In order to address the perceived technical limitations of conventional minimally invasive platforms a master-slave robotic surgical system was developed (da Vinci Surgical System, Intuitive Surgical, Sunnyvale, California). The major advantages were in the three-dimensional visual system that re-establishes binocular vision and instrumentation capable of seven degrees of freedom enabling wristed movement for dissection. The initial intent for this robotic system was for use in closed chest coronary surgery, but this has not eventuated. Instead, the major applications have been for pelvic procedures, such as prostatectomy and hysterectomy. Use of robotics for general thoracic surgical procedures dates back to initial case reports in the early 2000’s, but it was not until 2004 and 2006 that actual series of robotic lobectomies were reported by Melfi and colleagues and Park and coauthors, respectively (5,6). These centers reported the initial technique and experience demonstrating feasibility and concordance of outcomes with the largest series of VATS lobectomies. However, long-term data are lacking in a larger cohort of patients.
Rationale and methods
Early in the development of thoracic robotic surgery it was clear that there were only a handful of centers throughout the world utilizing robotics for major pulmonary resection. In order to evaluate a large cohort of patients that underwent robotic lobectomy to analyze both the perioperative and long-term survival results a multicenter retrospective registry was created using prospectively collected data from the thoracic surgery divisions of three institutions active in robotic pulmonary resection: Memorial Sloan-Kettering Cancer Center, New York, New York, USA, The European Institute of Oncology, Milan, Italy and Ospedale Cisanello, Pisa, Italy. Eligible patients were those with biopsy-proven or suspected primary NSCLC isolated to the chest who subsequently underwent attempted robotic lobectomy for primary NSCLC. Patients with carcinoid tumor, small cell lung cancer, benign or metastatic lesions were excluded. Information regarding preoperative characteristics, operative details, hospital course, pathologic findings and postoperative follow-up were recorded prospectively and sent to one institution (Milan) for analysis.
Techniques of robotic lobectomy
One of the strengths of the study was that the patient selection and surgical approach was virtually uniform despite the retrospective design. The majority of patients had clinical early stage disease with no prior treatment, and patients gave informed consent to undergo robotic surgery. Each surgeon performed robotic lobectomy employing a technique that conformed to the CALGB 39802 consensus criteria for VATS lobectomy (7): use of non-rib-spreading incisions with a 3-4 cm utility incision, videoscopic guidance and traditional hilar dissection. Two of the surgeons employed a total of 4 incisions while the third used 3 incisions, and all phases of dissection were performed with robotic instrumentation. Patients underwent systematic hilar and mediastinal lymph node dissection. Operative times were measured from first incision to closure, and conversion was defined as use of a rib-spreading thoracotomy at any point after docking of the robot to the patient and initiation of robotic dissection.
Results
From November 2002 through May 2010 325 patients underwent robotic lobectomy for primary NSCLC at three centers. Sixty-three percent of the patients were male and 85% were former or current smokers. Fifty-one percent of the procedures were upper lobectomies (92 RUL, 75 LUL), and 40% were lower lobectomies (71 RLL, 57 LLL). The majority of cases were subtypes of adenocarcinoma (73%), and most patients were clinical stage I (247 IA, 63 IB) and had no preoperative therapy.
Median operative time was 206 minutes, ranging from 110 to 383 minutes. There were no intraoperative deaths and the conversion rate to thoracotomy was 8% (27/325). Three patients (0.9%) had conversion for minor bleeding that did not require intraoperative or postoperative transfusion. Overall morbidity rate was 25.2% (82/325), and 12 patients had major complications (3.7%), including bronchopleural fistula (2), pulmonary embolism (3), acute renal insufficiency (3), hemorrhage (2) and myocardial infarction (2). Supraventricular tachycardia was the most common postoperative complication, occurring in 37 patients (11.4%). Median chest tube duration was 3 days (range, 1-23 days) and length of stay was 5 days (range, 2-28 days). There was one in-hospital death in a patient that developed acute renal insufficiency followed by a pulmonary embolism and death on postoperative day 12, with a mortality rate of 0.3%.
Seventy-six percent (248/325) of patients were pathologic stage I (176 IA, 72 IB), and 68 (21%) patients were upstaged. The median tumor size was 2.2 cm (range, 0.7-10.2 cm) and the median number of lymph node stations dissected was 5 (range, 2-8). Sixty-one patients (19%) had metastatic nodal disease and 67 patients received adjuvant cytotoxic chemotherapy. At a median follow-up of 27 months 280 patients (86%) were without evidence of disease and 32 patients (10%) had recurred with 25 dead of their disease. The majority (72%) were distant (17 distant only, 6 locoregional + distant) and 28% (9/32) were locoregional only. Overall 5-year survival for the group was 80% (Figure 1) and stage-specific survival is shown in Figure 2.
Figure 1.
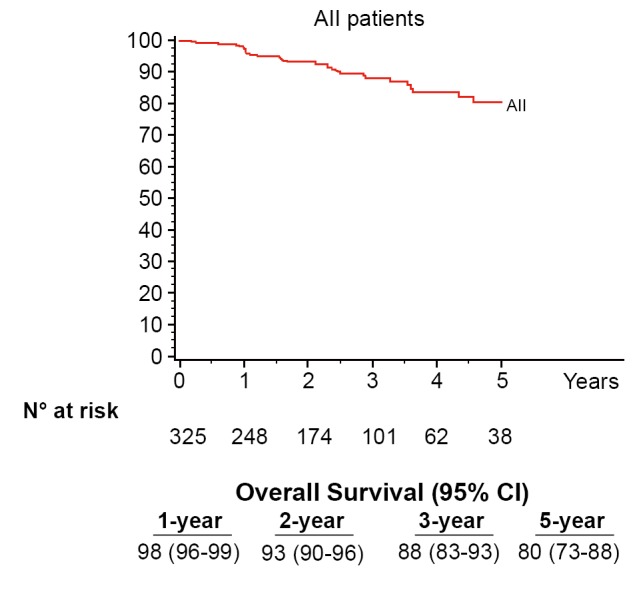
Overall survival for the group
Figure 2.
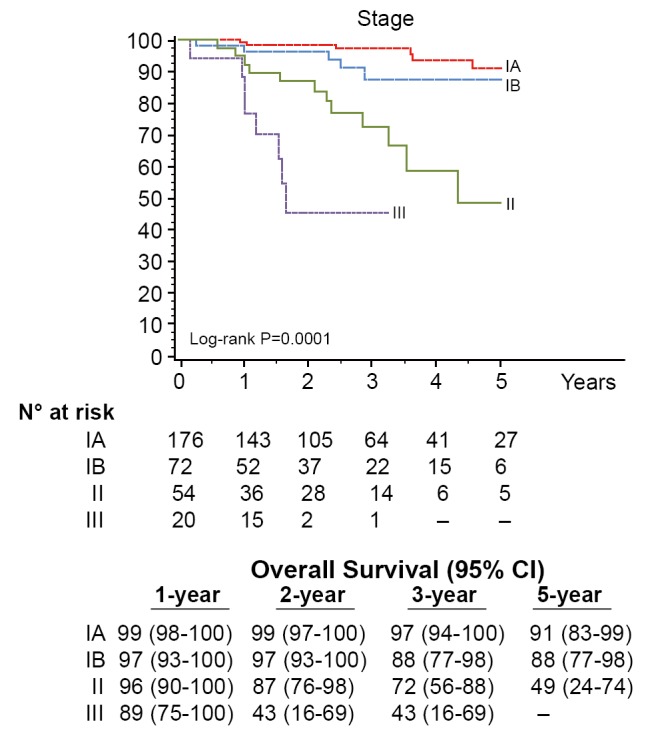
Overall survival for stage-specific survival
Impact and significance
This study is important for several reasons. First, it is the largest experience of totally robotic lobectomies reported to date. Previous initial feasibility studies had small numbers of patients, and like those this report shows perioperative results consistent with large VATS lobectomy experiences with short chest tube duration and length of stay, as well as low major morbidity (3.7%) and in-hospital mortality (0.3%) rates. Second, it is a multicenter, international experience with one center in the United States and two in Italy employing similar patient selection criteria, surgical technique and prospective evaluation of perioperative and long-term outcome. This demonstrates not only feasibility of the technique, but reproducibility as well. Third, this report is the first to look at the long-term oncologic outcome of robotic lobectomy for early NSCLC. The overall and stage-specific survivals are consistent with both the largest series of VATS lobectomies and the most recent data used for the revisions to the lung cancer staging system.
There are, however, limitations of this study and questions regarding the role of robotic technology in thoracic surgery. As this is a retrospective review, there are inevitable biases in patient selection and unknown differences between centers despite the fact that the patient characteristics and surgical techniques appear similar. Another limitation is the lack of other short- and long-term outcome measures, such as postoperative pain, respiratory function, rates of post-thoracoscopy pain and quality of life. Lastly, a comparative arm of VATS and/or thoracotomy patients is lacking. If utilization of robotic technology for thoracic surgical procedures increases, it will be important for future studies to attempt to discern differences between robotic and non-robotic approaches (VATS and thoracotomy) with respect to important outcomes, such as postoperative pain, quality of life and cost.
Robotic lobectomy is a feasible, safe and oncologically sound surgical treatment for early-stage lung cancer. The technique is reproducible across multiple centers and yields results consistent with the best seen with conventional VATS. It should not be considered experimental, but an accepted minimally invasive thoracic surgical technique. Future evaluation of differences between robotic versus VATS versus thoracotomy approaches to thoracic diseases is warranted.
Acknowledgements
Disclosures: The author is a speaker and proctor for Intuitive Surgical.
References
- 1.Park BJ, Melfi F, Mussi A, et al. Robotic lobectomy for non-small cell lung cancer (NSCLC): long-term oncologic results. J Thorac Cardiovasc Surg 2012;143:383-9 [DOI] [PubMed] [Google Scholar]
- 2.McKenna RJ, Jr, Houck W, Fuller CB. Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 2006;81:421-5; discussion 425-6 [DOI] [PubMed] [Google Scholar]
- 3.Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flores RM, Park BJ, Dycoco J, et al. Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2009;138:11-8 [DOI] [PubMed] [Google Scholar]
- 5.Melfi FMA, Ambrogi MC, Lucchi M, et al. Video robotic lobectomy. Video robotic lobectomy. Accessed 2011. Available online: http://mmcts.ctsnetjounals.org/cgi/content/full/2005/0628/mmcts.2004.000448
- 6.Park BJ, Flores RM, Rusch VW. Robotic assistance for video-assisted thoracic surgical lobectomy: technique and initial results. J Thorac Cardiovasc Surg 2006;131:54-9 [DOI] [PubMed] [Google Scholar]
- 7.Swanson SJ, Herndon JE, 2nd, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7 [DOI] [PubMed] [Google Scholar]


