Editor’s Key Points.
1. In experienced hands, VATS lobectomy could be performed in majority of cases [70-80%] without compromising the completeness of resection and offering less morbidity while maintaining less than 1% 30-day mortality rate.
2. A standardized 3 port anterior approach is consistently used and structures are divided from anterior to posterior as they come across.
3. Patients with Tuberculosis, previous surgery, or chemo-radiotherapy are still considered as candidates for VATS approach.
4. Vascular control at hilum is relatively easier in anterior approach
5. Simulation and teaching is easier as surgeon and assistant stand on the same side
--A.H.
Introduction
Video-Assisted Thoracoscopic Surgery (VATS) lobectomy is now well established and performed all around the world. Formerly there was much debate about the feasibility of the technique in cancer surgery and proper lymph node handling. Although there is a lack of proper randomized studies, it is now generally accepted that the outcome of a VATS procedure is at least not inferior to a resection via a traditional thoracotomy. Several papers have concluded that there is no significant difference in survival rates and that there might even be a better outcome by VATS (1-3).
A VATS lobectomy and even more a VATS anatomical segmentectomy is a challenging and technically demanding procedure to perform; and yet there is still no consensus about the basic principles in the technique. Different techniques have been described including the simultaneously stapled lobectomy (4), a VATS assisted operation with some rib spreading (5) and a true VATS lobectomy defined by no rib spreading along with anatomical hilar dissection and only monitor based vision rather than looking through the utility incision. The procedure is performed with up to 5 incisions and is even reported with a uniportal approach (6). Different lobe specific approaches have been reported (7) and a wide variation in instruments and camera positions is seen.
At our institution we have a large experience with about 1,000 cases performed by a standardised three-port anterior approach with sequential division of the hilar structures, proper lymph node handling, no rib spreading and vision relying on the monitor only. This allows us to perform VATS lobectomies in the majority of the cases even if there are significant difficulties (8). We find that our standardized three-port anterior approach facilitates the VATS lobectomy, and it is our experience from visiting surgeons that our technique can easily be adapted by many surgeons, especially those who are used to an open anterior approach.
The major advantages of the standardized anterior approach are:
❖ The mini-thoracotomy is placed directly over the hilum and the major pulmonary vessels. Easy to clamp the major vessels in case of major bleeding
❖ No need of changing the surgeons’ position or the place of the incision if a conversion is required
❖ The first structures to be transected are the major vessels
❖ The same approach to all lobes makes it easy to reproduce and learn
❖ The lung tissue only pushed backwards gently with peanuts and never grasped with forceps and therefore not torn apart
❖ Easy to teach as the surgeon and the assisting surgeon stand on the same side and use the same monitor. They do not work opposite to each other and therefore maybe against one and another. This facilitates a fluid learning process
Indications for VATS lobectomy
VATS lobectomy is commonly performed for selected peripherally located T1 or T2 tumours and usually reserved for patient where complications are not expected. We think that the advantages of a minimally invasive approach would also benefit cases that are more advanced and therefore the question in our daily clinically practice is: Are there any contraindications to perform the planned lobectomy as a VATS procedure?
At present we find the following contraindications:
❖ T3 or T4 tumours.
❖ Tumours larger than 6 cm.
❖ Tumours visible in the bronchus by bronchoscopy within 2 cm of the origin of the lobe to be resected and where a possible Sleeve resection might be needed.
❖ Centrally placed tumours in the hilum and adherent to vessels.
This means that patients with former Tuberculosis, previous cardiothoracic surgery and patients who have received preoperative chemo-radiotherapy are still considered as candidates for a VATS lobectomy. All our patients have a preoperative examination with lung function testing, PET/CT, bronchoscopy and EBUS/mediastinoscopy for preoperative staging (unless it is a peripheral placed T1 tumour on PET). With growing experience, we perform VATS lobectomy in the majority of the cases at our institution, even if they do present with co-morbidity. In the last few years, between 70% and 80% of all cancer lobectomies in our institution were performed by VATS and we now perform well over 200 VATS lobectomies and quite a few anatomical VATS segmentectomies each year (17 in 2011) with a very low conversion rate (2% in 2011).
Operating room set-up and basic surgical principles
A standard set-up is with one monitor placed on each side of the table in front of the surgeons and the scrub nurse (Figure 1). Other screens in the room allow other persons in the theatre to follow the surgery. We have two dedicated VATS theatres designed by the author together with Olympus Inc at our clinic and these theatres are only used for thoracic procedures. The basic principle is that the theatre is symmetrical so it is suitable for both right and left sided procedures. The light setting is a dynamic and colored lighting that enhances the surgical ergonometry.
Figure 1.
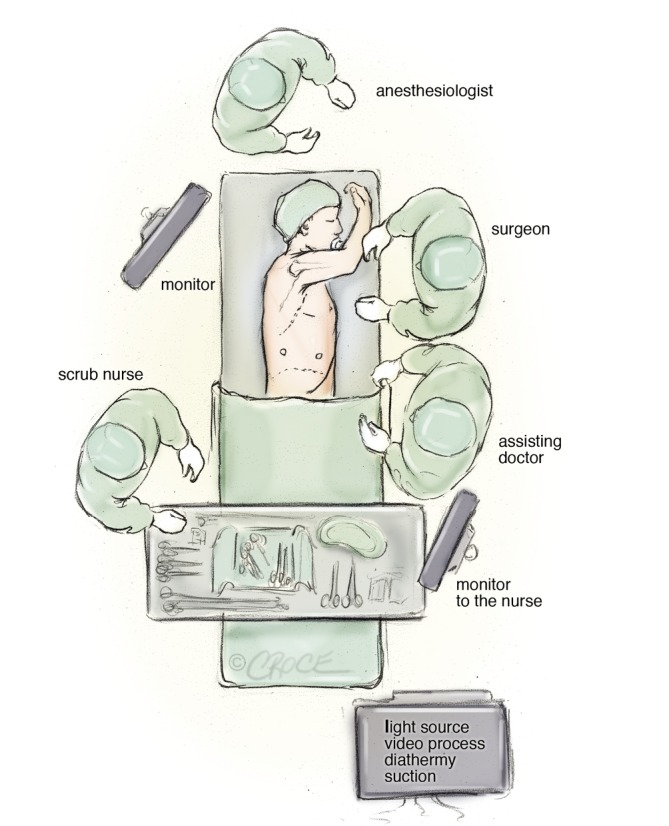
Operating room set-up for the anterior approach of video-assisted thorascopic lobectomy
All VATS lobectomies are performed with a 10 mm, 30 degree angled HD video-thoracoscope. The 30-degree angulation allows a superior view within the chest cavity. In a 10 mm camera, the power of the light source is stronger than the light source in the existing 5 mm cameras, and is not easily flooded by even a minor bleeding.
The surgeon and the assistant are positioned on the anterior (abdominal) side of the patient and with the surgeon cranially. The scrub nurse is opposite to the assistant and follows the operation on a separate screen and still positioned face to face with the surgeon (Figure 1). Initially, a 5 cm anterior utility incision is made without any tissue retractor or rib spreading. The wound is protected by a plastic soft tissue retractor kept in place by a ring in the chest cavity and one outside the skin (Alexis Retractor, Applied Medical USA). This incision is later used for specimen retrieval, and is positioned between the breast and the lower angel of the scapula in the fourth intercostal space just anterior to the latissimus dorsi muscle (Figure 2). In case of a conversion to open procedure, this incision can be easily expanded to a 10 to 15 cm muscle sparring thoracotomy within a few minutes.
Figure 2.
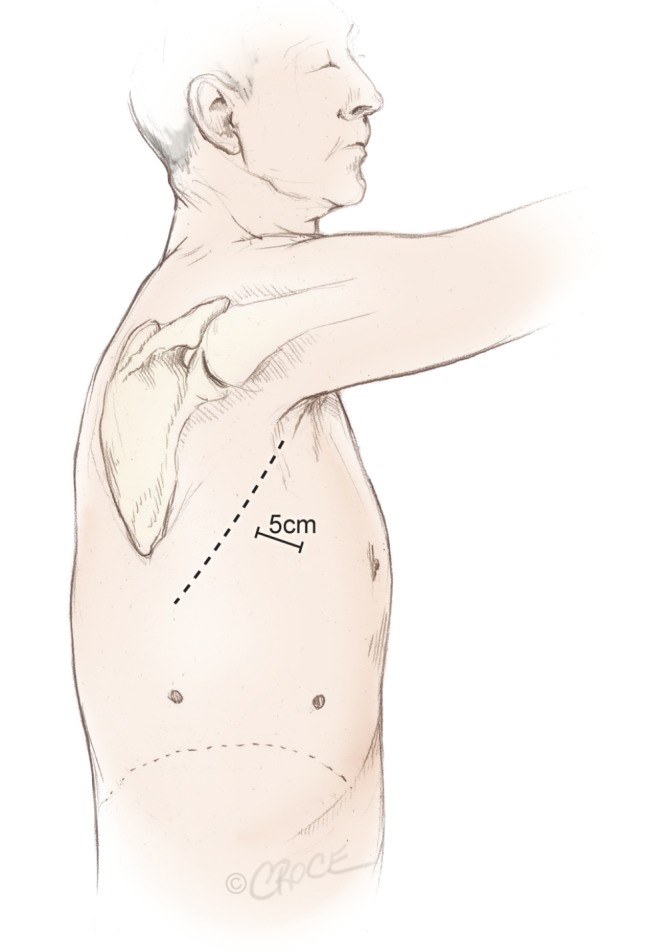
Three incisions made for the anterior approach forming a triangular configuration, with the utility incision at the apex of the triangle, measuring 5 cm in length
The cavity is evaluated with the camera through this incision looking for unexpected pathology, adhesions, and the level of the diaphragm. A low anterior 1 cm camera-port is positioned at the level of the top of the diaphragm and anterior to the level of the hilum and the phrenic nerve. The final 1.5 cm incision is positioned at the same level but more posterior in a straight line down from the scapula and anterior to the latissimus dorsi muscle. This results in a triangle with two approximately 10 cm limbs and the camera positioned at the apex, with a working channel on each side, which makes the procedure more easy and natural to the surgeon (Figure 2). The camera is in the lower anterior corner of the chest cavity with a good overview and it is usually not necessary to change camera port at any point of the procedure.
To palpate, free and prepare the structures, we use an array of a peanut or a sponge stick, an electrocautery blade hook controlled with a normal surgical handhold. The tip of the hook can be used to lift and divide the tissue. To present vessels and other structures to be divided we use an elastic vessel loop made of rubber, as slings of other materials present a risk of tearing, especially the fragile arteries to upper lobes. We do not place clamps on the vessels before stapling but two vessel clamps are ready on the table in case of an emergency bleeding and furthermore a set up for open surgery is present in the theatre.
The vessels, the fissures and the bronchus are divided sequentially, with appropriate endostaplers. For the vessels and thin parenchyma we use a tan Tri-stapler (Covidien, USA) and for poorly defined fissures and the bronchus, we transect with a purple Tri-stapler. Any specimen with suspicion of malignancy is removed with an endobag
Energy-based devices can also be used and we have some experience with an electro-thermal bipolar tissue sealing system (Ligasure, Valleylab Inc., USA) and find it useful to transect minor pulmonary arteries up to the size of 3-4 mm and they are very useful for lymph adenectomy where it facilitates an “en bloc” dissection.
Due to a high percentage of patients with prolonged air-leakage in our early experience, we have changed our strategy to a “fissure non-touch technique”. This means no dissection or use of electrocautery in the fissure. Instead the fissure is stapled with the visceral pleura intact as a seal above the parenchyma, giving a more tight closure within the stapling line, and no scars in the tissue next to the clips. To facilitate this, the fissure stapling is performed quite late in the procedure after the majority of the hilar structures are divided.
At the end, one intercostal drain is placed in through the camera incision. After surgery, the patient is transferred to an intermediate ward and next day to the normal ward. The patients are mobilised on the day of surgery and lung physiotherapy is provided for training. The tube is removed when there is no air-leakage and less than 500 cc of fluid in 24 hours. Patients are usually discharged one day after tube removal and seen ten days later in the outpatient clinic.
Operative techniques
After inspection of the cavity and confirmation of the indication for lobectomy, the structures are divided as you encounter them during the operation from anterior to posterior.
Right upper lobectomy
First the pleura over the anterior hilum and along the azygos vein are divided and next a thoracoscopic DeBakey forceps, introduced through the posterior port, is passed behind the superior pulmonary vein after clear identification of the middle lobe vein. The superior pulmonary vein is then encircled with a vessel loop and enough space to introduce a stapler around the vessel is created (Figure 3). The endovascular stapler is introduced through the posterior port and the thin blade is passed behind the superior pulmonary vein, which is then divided after removal of the vessel loop.
Figure 3.
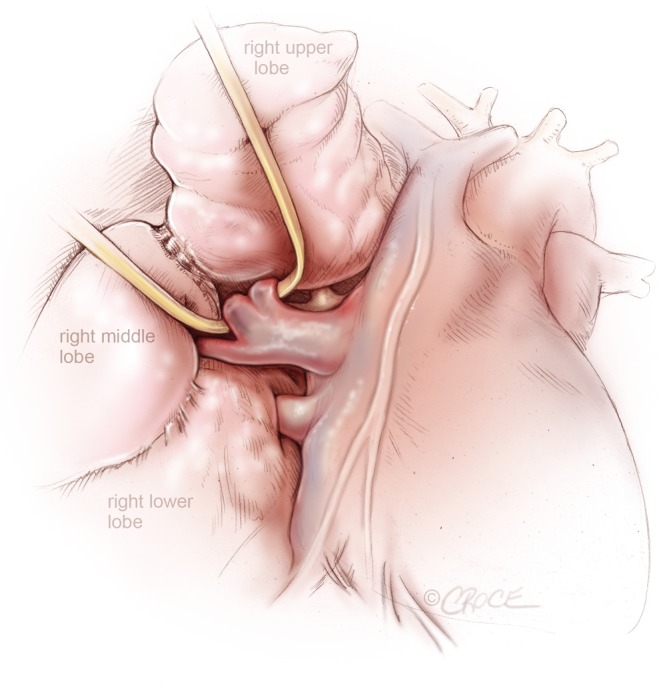
VATS right upper lobectomy: right superior pulmonary vein from right upper lobe is encircled by a vascular loop, while the pulmonary venous drainage from the right middle lobe is clearly seen
When the pulmonary vein is divided, the pulmonary artery and the truncus anterior are visualized and the superior trunk is transected in the same way as the vein. The pulmonary artery can then be visualized down to the branches to the middle lobe and the minor fissure is divided with endostaplers (Figure 4). The central landmark is the posterior border of the artery just above the middle lobe and below the divided vein to the upper lobe. After the transection of the fissure past the artery, the middle lobe drops down and exposes the posterior part of the artery and the remaining arterial branches to the upper lobe, which are then transected (Figure 4). Next the bronchus and the posterior part of the fissure is divided one by one. The bronchus is transected with a purple endostapler (in large-size patients a black Tristapler might be needed) and the device is closed and opened a few times to crunch the bronchus before firing to make the closure tighter. The lobe is then placed in an endobag and removed via the utility incision.
Figure 4.
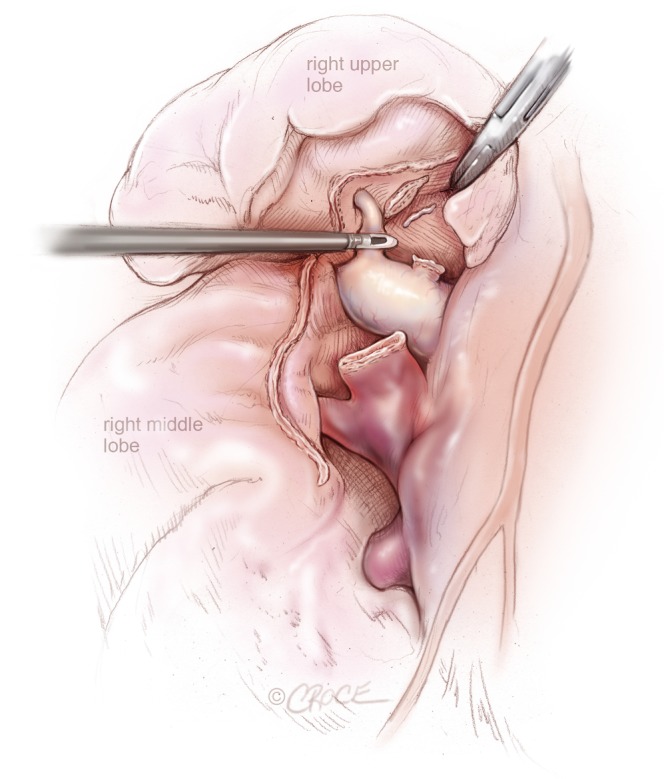
VATS right upper lobectomy: after division of right upper lobe pulmonary vein and truncus anterior, posterior ascending segmental artery to the right upper lobe is being divided by a Ligasure
Right middle lobectomy
The anterior part of the pleura over and between the veins is divided with the hook to expose and allow division of the middle lobe vein. Next, the middle lobe bronchus is exposed by blunt dissection in the hilum but sometimes the anterior part of the major fissure need to be transected. The bronchus is presented with a vessel loop and the stapler is introduced from the posterior incision (Figure 5). There will now be a good view of the artery and branches to the middle lobe are exposed (Figure 6), encircled and transected. The fissures are then completed beginning with stapling of the central part next to the artery via the posterior port.
Figure 5.
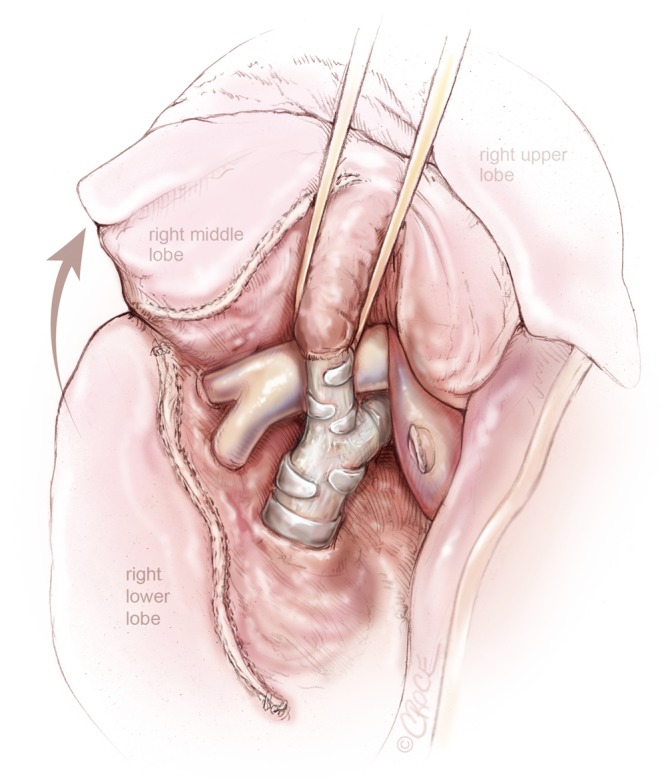
VATS right middle lobectomy: bronchus of the right middle lobe is presented through the oblique fissure after division of the right middle lobe vein
Figure 6.
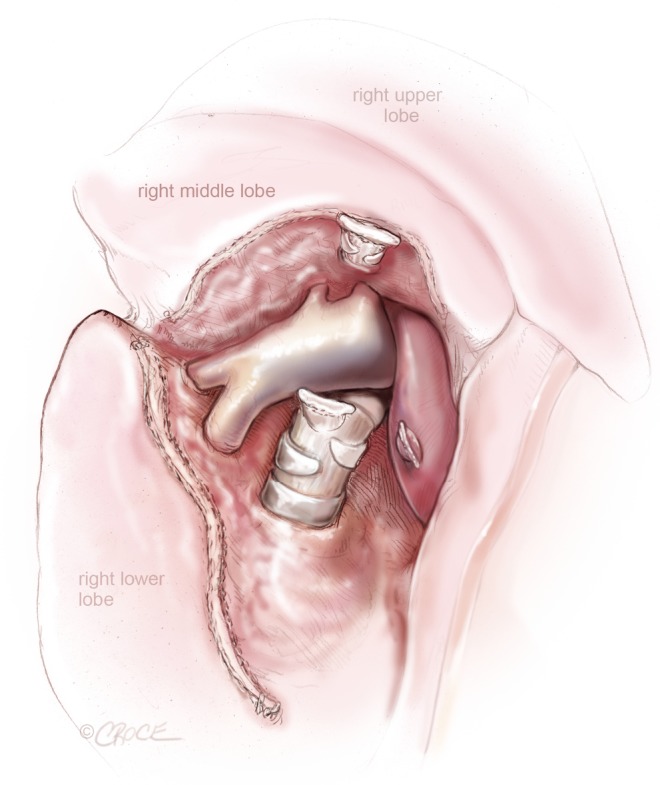
VATS right middle lobectomy: after the division of the right middle lobe vein and bronchus, pulmonary artery branches to the right middle lobe and lower lobe is exposed
Left upper lobectomy
The pleura over the hilum is divided and the artery and both veins are identified. The plane between the artery and the upper lobe vein is opened so the vein is exposed by a vessel loop coming from the anterior utility incision and it can then be transected with the stapler introduced from the posterior port. Next the superior branch of the pulmonary artery is divided in the same way and thereafter a plane between the artery and the bronchus can be created. The bifurcation of the left upper and lower lobe bronchi is identified, and the left upper lobe bronchus is transected from the posterior port by a purple Tristapler. The lobe is pushed posterior and the remaining branches on the pulmonary artery including the lingular artery are exposed and transected by stapler or a mixture of clips and energy based devices. The fissure is finally transected with endostaplers via the posterior port (Figure 7).
Figure 7.
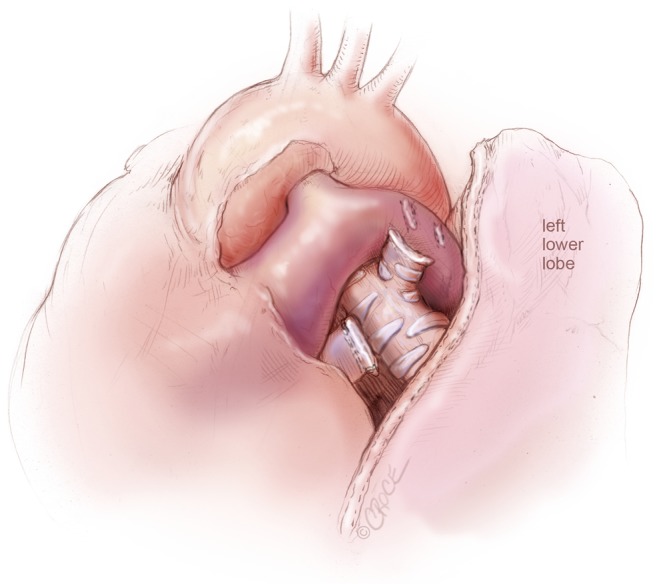
VATS left upper lobectomy: left upper lobe bronchial and vascular stumps are visualized, after left upper lobectomy and en bloc removal of Station 5 and Station 6 lymph nodes
Lower lobectomies
The lower lobe is retracted superiorly and the inferior pulmonary ligament is divided. This exposes the inferior pulmonary vein which can be encircled with a rubber loop and transected with the stapler from the utility incision. Next in the sequence is the artery where the pleura above are opened to allow the artery to be dissected in the sheath (Figure 8). The artery is lifted away from the parenchyma with a vascular sling and divided. In some cases, the superior segment of the right lower lobe artery needs to be divided separately, when it arises high up. The anterior part of the fissure is divided before or after the artery and the bronchus and the posterior fissure can be transected step by step.
Figure 8.
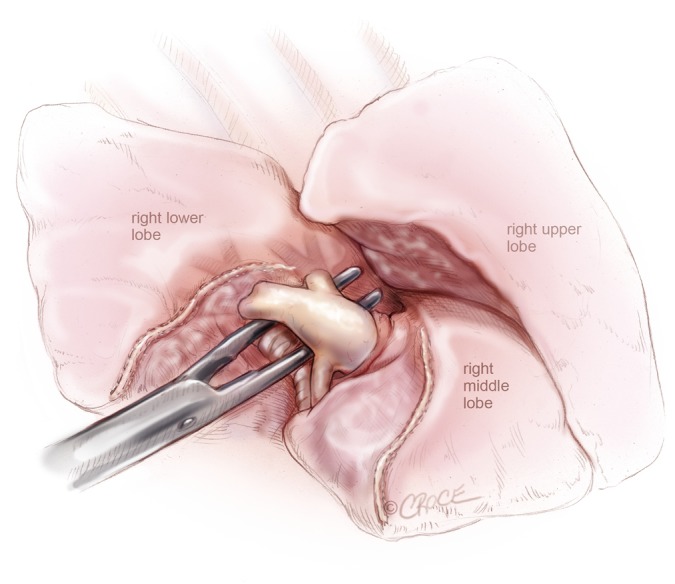
VATS right lower lobectomy: pulmonary artery to right lower lobe, including superior segmental artery is isolated, after dividing the oblique fissure anteriorly
One should be sure of the position of the middle lobe bronchus on the right side and when in doubt, the stapler should be closed at the site of the planned firing and the lung is then inflated to demonstrate airflow in the middle lobe.
Lymph node dissection
In our opinion it is doubtful whether a complete mediastinal lymph node dissection has a therapeutic benefit, or if the lymph node sampling only relates to stage identification. In all our cases we do a systematic lymph node dissection using the electro-thermal system (Ligasure) or electrocautery with removal of lymph nodes from at least 3 stations of N2 nodes according to the IASLC/Mountain classification.
In right-sided procedures, the nodes removed are from station 2R and 4R en-bloc. First, the pleura is opened above and under the azygos vein. The dissection begins at the tracheobronchial angle and progresses upwards under the azygos vein. After cleaning of the inferior part of the fatty tissue of the superior mediastinum the fatty tissue including the nodes is gripped from above and the dissection continues on so the level 2 nodes are included and the whole tissue packet is removed in one piece (Figure 9).
Figure 9.

VATS lymph node dissection: superior mediastinal lymph node dissection on the right side
To approach the subcarinal nodes the inferior ligament is divided and the pleura on the posterior limit of the lung is opened up to the azygos vein. The remaining lung is pushed anteriorly and the camera is angled so it looks along the oesophagus and the station 7 is exposed and removed so that the carinal bifurcation and the opposite bronchus are clean (Figure 10).
Figure 10.
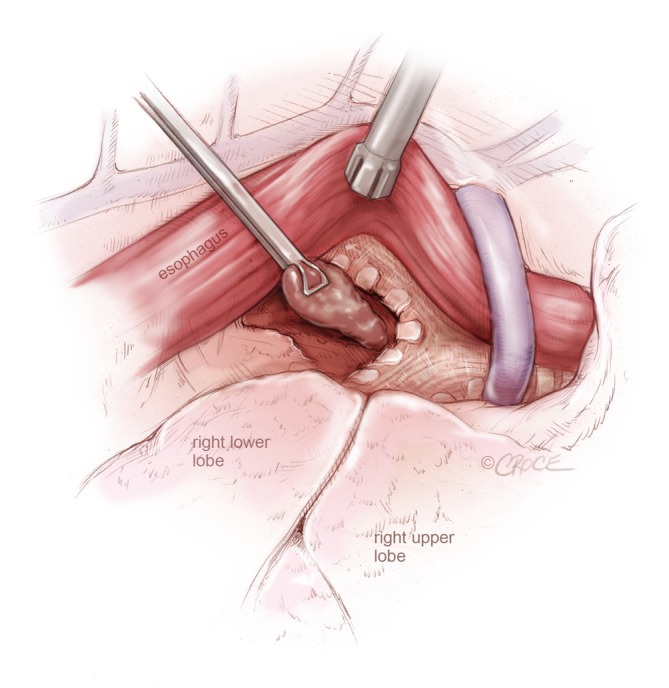
VATS lymph node dissection: subcarinal lymph node dissection from the right side, by retracting the esophagus posteriorly and the lung anteriorly to expose the membranous trachea and the subcarinal region
On the left side nodes are removed en-bloc from station 5 and 6 (as seen on Figure 7 between the aorta and the main pulmonary artery), station 7 is removed like on the right side. In lower lobe resections nodes from station 8 and 9 on the affected site are removed as well. Station 10 nodes are in upper lobe resections removed as part of the procedure to expose other structures.
Comments
We have a VATS lobectomy program that deals with the majority of our institution’s pulmonary resections. All operations are performed with a standardized three-port anterior approach independent of the procedure and the part of the lung to be addressed. There is a low conversion rate with a very low mortality (in the last few years <1% 30-day mortality) and a low morbidity.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Downey RJ, Cheng D, Kernstine K, et al. Video-Assisted Thoracic Surgery for Lung Cancer Resection. A Consensus Statement of the International Society of Minimally Invasive Cardiothoracic Surgery (ISMICS) 2007. Innovations (Phila) 2007;2:293-302 [DOI] [PubMed] [Google Scholar]
- 2.Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 2016-8. [DOI] [PubMed]
- 3.Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 200927:2553-62 [DOI] [PubMed] [Google Scholar]
- 4.Lewis RJ, Caccavale RJ, Sisler GE, et al. One hundred video-assisted thoracic surgical simultaneously stapled lobectomies without rib spreading. Ann Thorac Surg 1997;63:1415-21; discussion 1421-2 [DOI] [PubMed] [Google Scholar]
- 5.Okada M, Sakamoto T, Yuki T, et al. Hybrid surgical approach of video-assisted minithoracotomy for lung cancer: significance of direct visualization on quality of surgery. Chest 2005;128:2696-701 [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Rivas D, Paradela M, Fieira E, et al. Single-incision video-assisted thoracoscopic lobectomy: initial results. J Thorac Cardiovasc Surg 2012;143:745-7 [DOI] [PubMed] [Google Scholar]
- 7.Gossot D.Technical tricks to facilitate totally endoscopic major pulmonary resections. Ann Thorac Surg 2008;86:323-6 [DOI] [PubMed] [Google Scholar]
- 8.Hansen HJ, Petersen RH, Christensen M. Video-assisted thoracoscopic surgery (VATS) lobectomy using a standardized anterior approach. Surg Endosc 2011;25:1263-9 [DOI] [PubMed] [Google Scholar]


