Abstract
Introduction
Off-pump transapical aortic valve implantation (TA-TAVI) through mini-thoracotomy was first performed in Vancouver, Canada in October 2005. The objective of this manuscript is to describe the Vancouver experience with 178 TA-TAVI cases in symptomatic severe aortic stenosis (AS) since 2005.
Methods
Baseline characteristics and in-hospital outcomes were calculated for the overall cohort. To compare pre-procedure and post-procedure echocardiographic measurements, one way analysis of variance was utilized, followed by Tukey’s HSD. In-hospital outcomes were compared between early and late cohorts using Z-test and chi-squared test as appropriate. Kaplan-Meier analysis was used to generate 3-year survival curves. The Log-rank test was used to compare survival between low-risk and high-risk groups, based on STS score.
Results
Between October 2005-March 2012, TA-TAVI was performed in 178 patients. Mean age was 80.5±8.6 years. Congestive heart failure and renal failure were more common in the late cohort, while previous myocardial infarction was more common in the early cohort (P<0.05). Eleven patients (6.2%) had significant intra-operative complications, including death (1.7%), conversion to CPB (3.9%), and valve embolization (1.1%). In-hospital survival was 87.9%. Mean length of hospital stay was 12.2±17.1 days. In-hospital incidence of stroke was 3.4%. Bleeding (20.2% vs. 4.7%) and vascular (14.6% vs. 3.5%) complications were more common in the early cohort. Echocardiography demonstrated a significant increase in aortic valve area and decrease in mean transvalvular gradient post-procedure. These improvements were maintained throughout the 3-year follow-up period. Overall survival was 72.1% at 6 months, 67.7% at 12 months, 55.5% at 24 months, and 47.3% at 36 months. Repeated Kaplan-Meier analysis for the 2 groups stratified by STS score, demonstrated improved survival in the low-risk group (Log-rank P=0.039).
Conclusions
Among patients with symptomatic severe AS at high risk for conventional AVR, TA-TAVI is a reasonable therapeutic option with acceptable risk. Long-term survival appears to be superior in lower-risk patients.
Keywords: Transapical aortic valve implantation (TA-TAVI), aortic stenosis (AS)
Introduction
Although conventional aortic valve replacement (AVR) remains the standard of care for symptomatic severe aortic stenosis (AS), transcatheter techniques have been introduced over the past decade as a treatment option for high-risk or non-operative individuals. The trans-apical approach (TA-TAVI) has been reserved for patients with poor peripheral arterial access, which often limits the transfemoral approach (TF-TAVI). In this manuscript we review the experience to date with TA-TAVI at St. Paul’s Hospital in Vancouver, British Columbia, Canada.
The first successful off-pump TA-TAVI case through a mini-thoracotomy was performed in Vancouver in October 2005, with a resulting aortic valve area (AVA) of 1.9 cm2 and trans-valvular mean gradient (MG) of 3 mmHg (1). Our subsequent experience with a total of 7 nonsurgical candidates was encouraging, with successful valve deployment in all patients, with no intra-procedural mortalities or major complications (2). There was a resultant increase in the median AVA from 0.7±0.1 to 1.8±0.8 cm2, with 85.7% survival at 3 months (2). Six month follow-up of this cohort continued to demonstrate TA-TAVI as a feasible option for nonsurgical candidates with symptomatic severe AS (3).
One-year follow-up of our first 26 TA-TAVI patients demonstrated a 30-day mortality of 23% (4). Among patients surviving beyond the first 30-days post-operatively, 1-year survival was 85% (4). Subsequent follow-up of our first 71 patients with a predicted operative mortality of 34.5±20.4% by logistic Euro-SCORE and 12.1±7.7% by the Society of Thoracic Surgeons risk calculator, demonstrated overall survival rates of 66.3±6.4% at 24 months and 58.0±9.5% at 36 months (5). A significant improvement in New York Heart Association functional class compared to pre-operative status was also noted at 24 months [(3.3±0.8) vs. (1.8±0.8) months] (5).
Despite these encouraging early results, the question remained regarding the comparison of TA-TAVI to conventional AVR. Propensity-matched comparison of 46 TA-TAVI to 46 high-risk AVR patients suggested no difference in peri-operative outcomes, although selection bias could not be completely eliminated from the study (6). The PARTNER A trial randomized high-risk or non-operative candidates to TAVI or AVR (7). Although the trial included 104 TA-TAVI patients, there was inadequate statistical power to make any direct comparisons between TA-TAVI and conventional AVR (7).
Overall, early results with TA-TAVI have positive, but the current literature includes relatively small numbers of TA-TAVI cases. Therefore, the objective of this manuscript is to summarize our experience at St. Paul’s Hospital in Vancouver, Canada with our first 178 TA-TAVI cases for symptomatic severe AS.
Methods
Patient selection
TAVI was considered in patients with symptomatic severe AS deemed not to be candidates for routine open-heart AVR. All patients were assessed independently by the Heart Team, with at least 2 cardiologists and 2 cardiac surgeons, and accepted for the procedure based on the consensus that conventional surgical intervention was excessively high risk in terms of anticipated mortality and morbidity. Patient or physician preference alone was not considered an adequate indication. Patients who were accepted for TAVI were initially assessed for suitability for TF-TAVI. Patients underwent preoperative work-up, including coronary and aorto-femoral angiography and transthoracic echocardiography. Annulus size was measured using computed tomography and intraoperative transesophageal echocardiography (8). TA-TAVI was recommended if aortofemoral angiographic analysis revealed unfavorable anatomy for the transfemoral approach, including small or diseased ilio-femoral vessels, excessive tortuosity, or previous peripheral vascular reconstruction with inadequate access. Other specific indications for TA-TAVI were the presence of a mitral prosthesis, and an unfolded or excessively tortuous thoracic aorta.
Operative procedure
Our approach to the TA-TAVI procedure has been described in detail in our previous publications (1-5). Briefly, patients were placed under general anesthetic in an operating room. The apex of the left ventricle was identified using a high-definition fluoroscopy and exposed through a 3 to 5 cm anterior mini-thoracotomy and a small pericardial incision. Two paired, orthogonal, U-shaped polypropelene sutures with pledgets were placed into the myocardium and passed through tensioning tourniquets. Epicardial pacing wires were placed for rapid ventricular pacing during aortic valvuloplasty and deployment of the prosthesis.
Heparin was administered to achieve an activated clotting time greater than 250 seconds. Using fluoroscopic, aortographic, and TEE imaging, balloon valvuloplasty and then deployment of the bioprosthesis were performed during rapid ventricular pacing to minimize ventricular ejection without cardiopulmonary bypass (CPB). Our experience was entirely with the Edwards balloon-expandable transcatheter bioprostheses. Patients were maintained on aspirin indefinitely and clopidogrel for 1-3 months. Warfarin was used in the first 8 patients routinely, but was abandoned due to gastrointestinal bleeding in 2 patients (25%) within 30 days. In addition, warfarin was prescribed to patients with chronic atrial fibrillation, a mechanical valve prosthesis, or venous thrombosis.
Follow-up and data collection
All patients were carefully followed up by cardiac surgeons, cardiologists, clinical fellows, and clinical research coordinators. Standard endpoint definitions, as previously defined by the Valve Academic Research Consortium, were used in monitoring for complications (9). AVA and MG were determined by echocardiography before discharge (143 patients) and at 1 (83 patients), 6 (34 patients), 12 (42 patients), 24 (13 patients), and 36 (11 patients) months. Echocardiograms were reviewed by our senior echocardiographers.
Statistical analysis
Baseline characteristics, in-hospital outcomes, echocardiographic measurements, and 3-year survival were calculated for the entire cohort. The cohort was then divided into 2 equal-sized groups: early cohort-first 89 patients to receive TA-TAVI, and late cohort-next 89 patients to receive TA-TAVI. Baseline characteristics and in-hospital outcomes were compared between the early and late cohort to identify improvements in peri-operative outcomes following the initial learning curve. Furthermore, in an attempt to identify differences in long-term survival between low-risk and high-risk patients, the cohort was divided into 2 equal-sized groups based on Society of Thoracic Surgery (STS) score.
Continuous variables are presented as mean ± standard deviation, and categorical variables are presented as number and percentage. The chi squared test was used to compare differences between dichotomous categorical variables. The Z-test was used to compare differences in mean values for continuous variables. To compare differences in pre-procedure and post-procedure echocardiographic measurements, one way analysis of variance (ANOVA) was utilized, followed by Tukey’s HSD to account for multiple comparisons. Kaplan-Meier analysis was used to generate the 3-year survival curves, and the Log-rank test was used to compare survival between the low-risk and high-risk groups.
Results
Patient characteristics
Between October 2005 and March 2012, isolated TA-TAVI was performed in 178 patients at St. Paul’s Hospital in Vancouver, Canada. The mean age was 80.5±8.6 years, and 55.3% of patients were female. Co-morbidities were common (Table 1), with a mean calculated STS risk score of 10.3±6.6%. Comparing the early and late cohort, congestive heart failure (CHF; P<0.0001) and renal failure (P=0.004) were more common in the late cohort, while previous acute myocardial infarction (AMI) was more common in the early cohort (P<0.0001). Otherwise, there were no significant differences in baseline characteristics between the two cohorts.
Table 1. Baseline characteristics of 179 patients accepted for isolated TA-TAVI between 2005-2012 at St. Paul’s Hospital in Vancouver, Canada.
| Parameter | Overall (n=178) | Early cohort (n=89) | Late cohort (n=89) | P value |
|---|---|---|---|---|
| Age | 80.5 (8.6) | 80.5 (8.0) | 80.5 (9.2) | 1.00 |
| Female | 99 (55.3) | 56 (62.9) | 43 (47.8) | 0.12 |
| Body mass index (kg/m2) | 25.0 (4.8) | 24.7 (4.3) | 25.3 (5.3) | 1.00 |
| Diabetes | 53 (29.8) | 22 (25.3) | 31 (34.4) | 0.37 |
| Dyslipidemia | 129 (72.1) | 66 (75.0) | 63 (70.0) | 1.00 |
| Hypertension | 147 (82.1) | 71 (80.7) | 76 (84.4) | 0.77 |
| Smoking | 96 (53.6) | 50 (56.8) | 46 (51.1) | 1.00 |
| Congestive heart failure | 136 (76.4) | 54 (62.1) | 82 (91.1) | <0.0001 |
| Pulmonary hypertension | 54 (30.5) | 25 (29.1) | 29 (32.6) | 1.00 |
| Cerebrovascular accident | 40 (22.9) | 20 (23.8) | 37 (41.6) | 0.03 |
| Pulmonary disease | 49 (27.4) | 20 (22.7) | 29 (32.6) | 0.31 |
| Dialysis | 5 (2.9) | 1 (1.2) | 4 (4.4) | 0.40 |
| Peripheral vascular disease | 103 (59.2) | 56 (67.5) | 47 (52.2) | 0.12 |
| Coronary artery disease | 141 (78.8) | 71 (80.7) | 70(77.8) | 1.00 |
| Previous myocardial infarction | 87 (48.6) | 57 (64.8) | 30 (33.3) | <0.0001 |
| Previous cardiac surgery | 79 (44.4) | 43 (49.4) | 36 (40.0) | 0.41 |
| Previous percutaneous coronary intervention | 41 (22.9) | 18 (20.5) | 23 (25.6) | 0.84 |
| Porcelain aorta | 39 (22.7) | 21 (25.9) | 18 (20.5) | 0.80 |
| Atrial fibrillation/Flutter | 62 (35.6) | 33 (39.8) | 29 (32.2) | 0.60 |
| Permanent pacemaker | 37 (21.0) | 20 (23.5) | 17 (19.1) | 0.95 |
| Renal failure | 60 (33.9) | 20 (23.3) | 40 (44.4) | 0.004 |
| Creatinine (μmol/L) | 122.5 (92.0) | 110.8 (62.7) | 135.0 (115.4) | 0.33 |
| GFR (mL/min) | 55.2 (22.6) | 56.9 (23.1) | 53.5 (22.0) | 1.00 |
| Hgb (mmol/L) | 120.6 (18.2) | 120.6 (16.0) | 119.4 (20.4) | 1.00 |
| Society of thoracic surgery risk score (%) | 10.3 (6.6) | 11.4 (7.1) | 9.0 (5.6) | 1.00 |
Parameters presented as mean (standard deviation) for continuous variables and number (percentage) for categorical variables. Early cohort – first 89 patients undergoing TA-TAVI; late cohort - next 90 patients undergoing TA-TAVI. P-value, comparing baseline characteristics between early and late cohort, calculated using the Z-test for continuous variables and two-tailed chi square test for categorical variables
Intra-operative outcomes
The catheter delivery system used initially was the transfemoral Retroflex 1 (7 patients; Edwards Lifesciences, Irvine, CA, USA), followed by Ascendra (130 patients; Edwards Lifesciences, Irvine, CA, USA), Ascendra2 (40 patients; Edwards Lifesciences, Irvine, CA, USA), and Novaflex Plus (1 patient; Edwards Lifesciences, Irvine, CA, USA). Cribier–Edwards transcatheter equine pericardial tissue valves (Edwards Lifesciences, Irvine, CA, USA) were used in the first 13 patients and SAPIEN (n=115) and SAPIEN XT (n=50) bovine pericardial tissue valves (Edwards Lifesciences, Irvine, CA, USA) were used in the remaining patients. The majority of patients received a 26 mm prosthesis (93 patients), with the remainder receiving 23 mm (72 patients), 29 mm (12 patients), or 20 mm (1 patient) prostheses.
There were 11 patients (6.2%) with significant intra-operative complications. Five of these occurred in the early cohort and 6 occurred in the late cohort. These intra-operative complications included 3 intra-operative deaths (1.7%), 7 conversion to CPB (3.9%), and 2 valve embolizations (1.1%). Three patients required a second valve (1.7%): 1 for severe transvalvular AI after deployment of the first valve, and 2 for valves that were malpositioned towards the ventricular side. There were 3 left main (1.7%) and 1 right (0.6%) coronary artery obstructions, with 2 patients requiring open heart surgery, and 2 receiving PCI.
In-hospital outcomes
Among the 178 patients, in-hospital survival was 87.9%. The causes of in-hospital mortality were intra-operative (n=3), CVA (n=3), multi-organ failure (n=2), pneumonia (n=1), pulmonary embolism (n=1), major bleeding (n=1), peripheral emboli (n=1), sepsis (n=1), ventricular fibrillation (n=1), renal failure (n=1), aortic rupture (n=1), respiratory failure (n=1), and unknown cause (n=4). The mean length of hospital stay was 12.2±17.1 days. The in-hospital incidence of stroke was 3.4%, with 4 major strokes and 2 minor strokes. The other in-hospital complications are listed in Table 2. Bleeding (20.2% vs. 4.7%, P=0.004) and vascular (14.6% vs. 3.5%, P=0.02) complications were more common in the early cohort. In the early cohort, there were 12 major and 1 minor vascular complications, and 10 major and 8 life-threatening bleeding complications. In the late cohort, there were only 2 major and 1 minor vascular complications, and 2 major and 2 life-threatening bleeding complications. There was a trend towards shorter hospital stays (13.7 vs. 10.6 days), decreased risk of stroke, decreased risk of prolonged ventilation, and decreased mortality in the late cohort (16.9% vs. 7.1%), although these results were not statistically significant.
Table 2. In-hospital outcomes for 179 patients undergoing TA-TAVI at St. Paul’s hospital in Vancouver, Canada.
| Outcome | Overall (n=178) | Early cohort (n=89) | Late cohort (n=89) | P value |
|---|---|---|---|---|
| Hospital length of stay (days) | 12.2 (17.1) | 13.7 (22.1) | 10.6 (8.7) | 0.98 |
| Stroke | 6 (3.4) | 4 (4.5) | 2 (2.3) | 0.84 |
| Bleeding | 22 (12.6) | 18 (20.2) | 4 (4.7) | 0.004 |
| Vascular complication | 16 (9.1) | 13 (14.6) | 3 (3.5) | 0.02 |
| Blood transfusion | 78 (45.1) | 43 (48.3) | 35 (41.7) | 0.76 |
| Myocardial infarction | 5 (3.0) | 2 (2.2) | 3 (3.9) | 1.00 |
| Major adverse cardiovascular event | 29 (16.9) | 17 (19.1) | 12 (14.5) | 0.83 |
| GI complication | 4 (2.5) | 3 (3.4) | 1 (1.4) | 0.79 |
| Atrial fibrillation/Flutter | 23 (13.8) | 13 (14.9) | 9 (12.3) | 1.00 |
| Pneumonia | 17 (10.4) | 10 (11.2) | 7 (9.5) | 1.00 |
| Pulmonary embolism | 2 (1.2) | 2 (2.2) | 0 (0) | 0.39 |
| Prolonged ventilation | 10 (6.1) | 7 (7.9) | 3 (4.1) | 0.63 |
| Sepsis | 5 (3.1) | 3 (3.4) | 2 (2.7) | 1.59 |
| Wound infection | 7 (4.3) | 3 (3.4) | 4 (5.3) | 1.00 |
| Permanent pacemaker | 17 (9.9) | 7 (7.9) | 10 (12.0) | 0.72 |
| Acute renal failure | 23 (14.1) | 11 (12.4) | 12 (16.2) | 0.96 |
| Dialysis | 8 (5.0) | 5 (5.7) | 3 (4.1) | 1.00 |
| Cardiac arrest | 10 (6.2) | 5 (5.7) | 5 (6.8) | 1.00 |
| Mortality | 21 (12.1) | 15 (16.9) | 6 (7.1) | 0.09 |
Outcomes presented as mean (standard deviation) for continuous variables and number (percentage) for categorical variables. Early cohort - first 89 patients undergoing TA-TAVI; late cohort – next 89 patients undergoing TA-TAVI. P-value, comparing outcomes in early and late cohort, calculated using the Z-test for continuous variables and two-tailed chi square test for categorical variables
Echocardiographic follow-up
Based on one-way ANOVA, and Tukey’s HSD test, echocardiography demonstrated a significant increase in AVA from 0.65±0.17 cm2 prior to the procedure, to 1.48±0.42 cm2 at time of discharge (P<0.05) and a significant decrease in MG from 41.85±16.0 mmHg prior to the procedure to 10.5±4.3 mmHg at the time of discharge (P<0.05). These improvements in echocardiographic measurements were maintained throughout the 3-year follow-up period (Figure 1).
Figure 1.
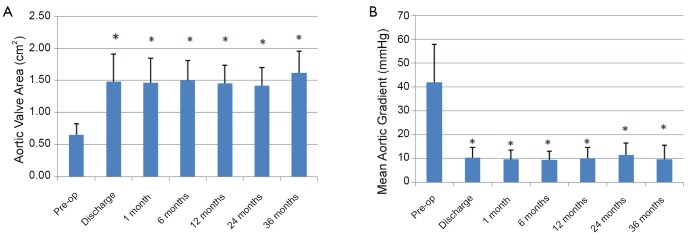
A. Echocardiographic measurements of aortic valve area pre- and post-TA-TAVI. *P<0.05 vs. pre-op measurement based on one-way ANOVA and Tukey’s HSD; B. Echocardiographic measurements of mean aortic valve gradient pre- and post-TA-TAVI. *P<0.05 vs. pre-op measurement based on one-way ANOVA and Tukey’s HSD
Survival
Based on Kaplan-Meier analysis, overall survival was 72.1% at 6 months, 67.7% at 12 months, 55.5% at 24 months, and 47.3% at 36 months (Figure 2). Kaplan-Meier survival analysis was repeated for all patients surviving beyond 30 days and for those patients who had follow-up beyond 30 days (Figure 3). Among this group, survival was 82.1% at 6 months, 77.1% at 12 months, 63.6% at 24 months, and 54.5% at 36 months.
Figure 2.
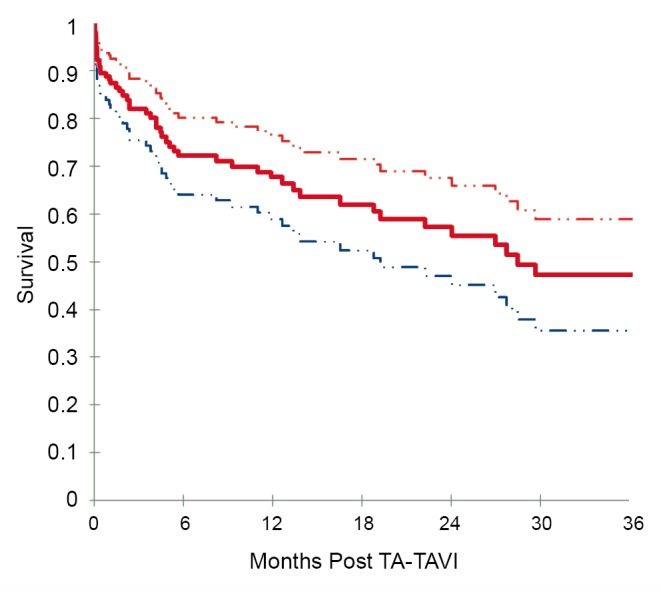
Kaplan-Meier survival analysis for 178 patients undergoing TA-TAVI
Figure 3.
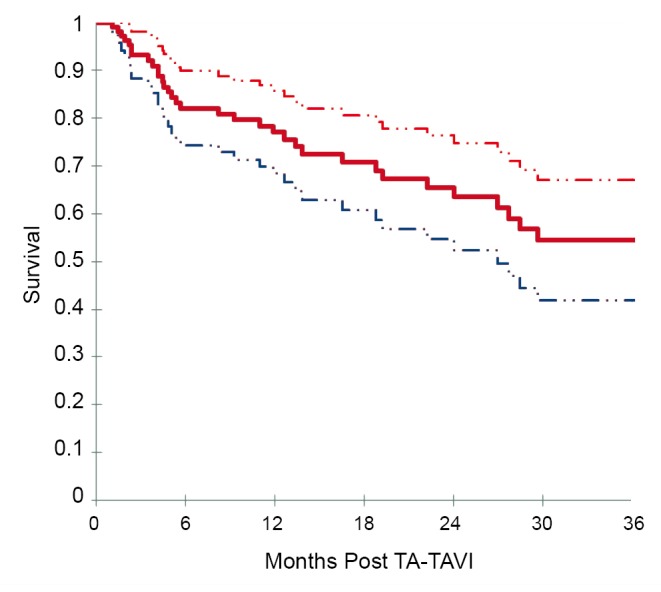
Kaplan-Meier survival analysis for TA-TAVI patients surviving beyond 1 month
The cohort was then divided into two equal groups based on STS score. The mean STS score in the low-risk group was 5.6±2.0%, and the mean STS score in the high-risk group was 15.0±6.3%. Figure 4 displays the results of the Kaplan-Meier survival analysis at 3 years. The calculated survival was 82.9% vs. 65.2% at 6 months, 82.9% vs. 58.3% at 12 months, 67.9% vs. 48.4% at 24 months, and 46.7% vs. 35.0% at 36 months, for the low-risk and high-risk cohorts respectively. Based on the Log-rank test, this improved survival among the low-risk cohort was statistically significant (P=0.039).
Figure 4.
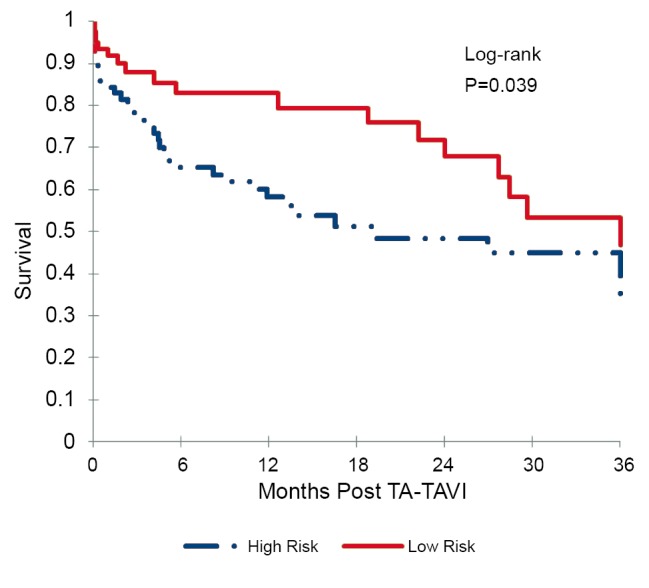
Kaplan-Meier survival analysis for low-risk versus high-risk patients, based on STS score
Discussion
Since the first TA-TAVI in October 2005 at St. Paul’s Hospital in Vancouver, Canada, 178 procedures have been performed at our centre for symptomatic severe AS in high-risk candidates for conventional AVR. This large series includes the first cases performed, and demonstrates low rates of intra-procedural mortality (1.7%) and in-hospital stroke (3.4%) among a high-risk group of patients with a large burden of pre-operative co-morbidities. Furthermore, this series demonstrates that the significant improvements in echocardiographic measurements following TA-TAVI are sustained during 3-year follow-up.
As the first centre to begin performing TA-TAVI, there was a learning curve associated with the procedure. With an increase in experience, complication rates, specifically bleeding (20.2% vs. 4.7%, P=0.04) and vascular complications (14.6% vs. 3.5%, P=0.02), dramatically declined, as demonstrated in the second half of this cohort. There were also decreases in the risk of in-hospital stroke (4.5% vs. 2.3%) and mortality (16.9% vs. 7.1%), although these differences were not statistically significant. There were few differences among the 2 cohorts prior to the procedure, and thus improved outcomes are likely due to improvements in pre-operative screening and patient selection, intra-operative technique and imaging, and peri-operative management. Longer follow-up may demonstrate long-term survival benefits as well among this later cohort. This learning curve, however, does not need to be repeated at all centres performing TA-TAVI. New sites wishing to perform TA-TAVI should receive on-site training and proctoring by experienced, well-qualified surgeons.
The Kaplan-Meier survival analysis demonstrated 3-year survival rates slightly lower than previously published by our group on the first 71 patients (58.0% vs. 47.3%) (5). However, a large number of patients were censored at time of hospital discharge in the current study and longer-term follow-up is still pending. When the current survival analysis was repeated for those patients surviving beyond 30 days and those patients with follow-up beyond hospital discharge, the 3-year survival improved to 54.5%. Finally, Kaplan-Meier survival analysis also demonstrated improved long-term survival at 6, 12, 24, and 36 months for “low-risk” patients, based on STS score (P=0.039). This may assist in future patient selection for TA-TAVI. The Partner II trial, which will include “operable” subjects with an STS score between 4-8%, randomized to AVR, TA-TAVI, or TF-TAVI, will hopefully provide further information regarding the safety and efficacy of transcatheter valve implantation in lower-risk patients (10).
In conclusion, among patients with symptomatic severe AS at high risk for conventional AVR, TA-TAVI is a reasonable therapeutic option with acceptable risk. Long-term survival appears to be superior in lower-risk patients, and future studies with longer follow-up may be indicated to appropriately determine which group of patients will benefit most from TA-TAVI.
Acknowledgments
Disclosures: Dr. Anson Cheung, Dr. Jian Ye, and Dr. John Webb are consultants to Edwards Lifesciences. There are no other disclosures or conflicts of interest.
References
- 1.Ye J, Cheung A, Lichtenstein SV, et al. Transapical aortic valve implantation in humans. J Thorac Cardiovasc Surg 2006;131:1194-6 [DOI] [PubMed] [Google Scholar]
- 2.Lichtenstein SV, Cheung A, Ye J, et al. Transapical transcatheter aortic valve implantation in humans: Initial clinical experience. Circulation 2006;114:591-6 [DOI] [PubMed] [Google Scholar]
- 3.Ye J, Cheung A, Lichtenstein SV, et al. Six-month outcome of transapical transcatheter aortic valve implantation in the initial seven patients. "Eur J Cardiothorac Surg 2007;31:16-21 [DOI] [PubMed] [Google Scholar]
- 4.Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: 1-year outcome in 26 patients. J Thorac Cardiovasc Surg 2009;137:167-73 [DOI] [PubMed] [Google Scholar]
- 5.Ye J, Cheung A, Lichtenstein SV, et al. Transapical transcatheter aortic valve implantation: Follow-up to 3 years. J Thorac Cardiovasc Surg 2010;139:1107-13 [DOI] [PubMed] [Google Scholar]
- 6.Higgins J, Ye J, Humphries KH, et al. Early clinical outcomes after transapical aortic valve implantation: A propensity-matched comparison with conventional aortic valve replacement. J. Thorac. Cardiovasc. Surg 2011;142:e47-52 [DOI] [PubMed] [Google Scholar]
- 7.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. NEJM 2011;364:2187-98 [DOI] [PubMed] [Google Scholar]
- 8.Willson AB, Webb JG, LaBounty TM, et al. 3-Dimensional Aortic Annular Assessment by Multidetector computed Tomography Predicts Moderate or Severe Paravalvular Regurgitation After Transcatheter Aortic Valve Replacement: A Multicenter Retrospective Analysis. J Am Coll Cardiol 2012; 59:1287-94 [DOI] [PubMed] [Google Scholar]
- 9.Leon MB, Piazza N, Nikolsky E, et al. Standardized Endpoint Definitions for Transcatheter Aortic Valve Implantation Clinical Trials: A Consensus Report from the Valve Academic Research Consortium. J Am Coll Cardiol 2011;57:253-69 [DOI] [PubMed] [Google Scholar]
- 10.ClinicalTrials.gov. The PARTNER II Trial: Placement of AoRTic TraNscathetER Valves. c2011 [updated 2012; cited 2012]. Available online: http://clinicaltrials.gov/ct2/show/NCT01314313


