Spinal cord injury remains a devastating complication of the treatment of extensive thoracoabdominal aortic aneurysms (TAAA), whether by open surgery or endovascular strategies. Although the incidence and pattern of occurrence of spinal cord injury have been changing in recent years, the threat of possible paraplegia or paraparesis continues to deter patients from undergoing elective operations to prevent rupture of extensive TAAA. A better understanding of the anatomy and physiology of the spinal cord circulation have in recent years led to a reduction in the risk of postoperative spinal cord ischemia; further insights should soon enable us to make postoperative paraplegia and paraparesis a very rare complication of TAAA treatment.
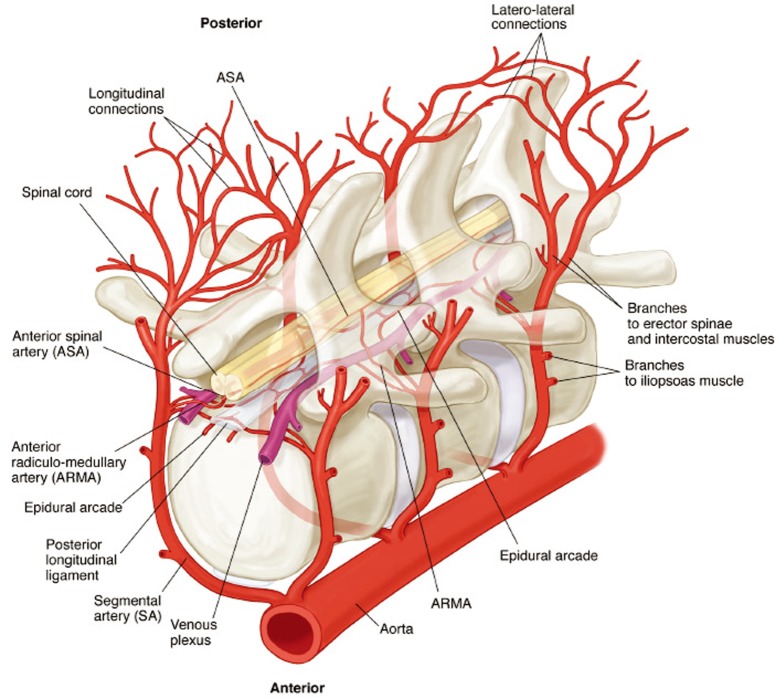
A number of clinical and experimental findings led us to question the traditional view that spinal cord perfusion is dependent principally upon a single prominent branch from the descending aorta with a distinctive hairpin configuration, the so-called artery of Adamkiewicz (1). We proposed instead the existence of an extensive collateral network that supports spinal cord perfusion. This collateral network includes all the segmental arteries (SAs), both intercostal and lumbar: they send branches to the anterior spinal artery (ASA), the major artery within the spinal canal. In addition to multiple SA inputs, the ASA is also potentially fed by an extensive epidural arterial network, and by a dense array of small vessels which supply the paraspinal musculature. All these vessels are interconnected, and have major anastomoses with the subclavian arteries cranially, and the hypogastric arteries caudally. This extensive collateral network allows compensatory flow to the spinal cord when some of the direct inputs to the ASA are compromised during repair of TAAAs.
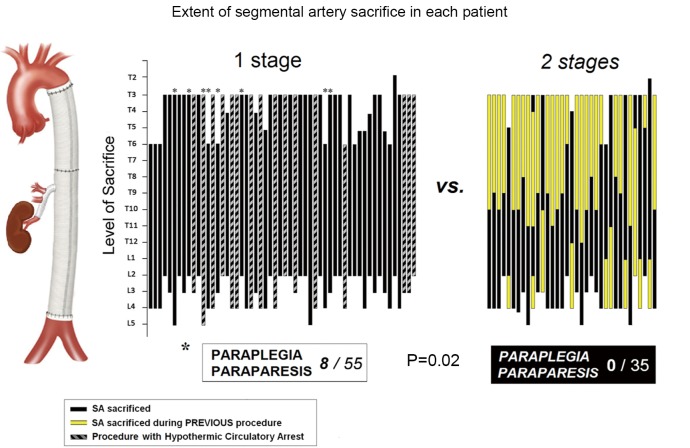
The observation that spinal cord injury occurs more frequently in TAAAs of great extent—type II in the Crawford classification—was documented more than 20 years ago (2). More recently, we and others noted that when such extensive resections are carried out in two stages rather than during a single procedure, the risk of spinal cord injury is reduced sharply, Figure 1 (3,4). Although in our retrospective study the two-stage procedures were dictated by the various circumstances of the evolution of each patient’s disease, we reasoned that the protection from spinal cord injury that resulted in each instance likely reflected a similar physiological adaptation by the collateral network to the loss of SA input. We have carried out a series of studies which have begun to illuminate the process by which complete recovery of spinal cord perfusion after extensive SA sacrifice is usually achieved, and to explain the mechanisms for the occasional occurrence of spinal cord injury following extensive TAAA resection.
Figure 1.
Extent of segmental artery sacrifice in a clinical series of thoracoabdominal aneurysm repairs. Each patient is represented by a single bar indicating the extent of the aneurysm resection. In the patients with repair in two stages, the extent of the first repair is indicated in yellow. Among the single stage repairs, those done using hypothermic circulatory arrest are distinguished from those carried out using left heart bypass. Patients who suffered paraplegia or paraparesis are indicated by asterisks. This figure is modified from reference (3)
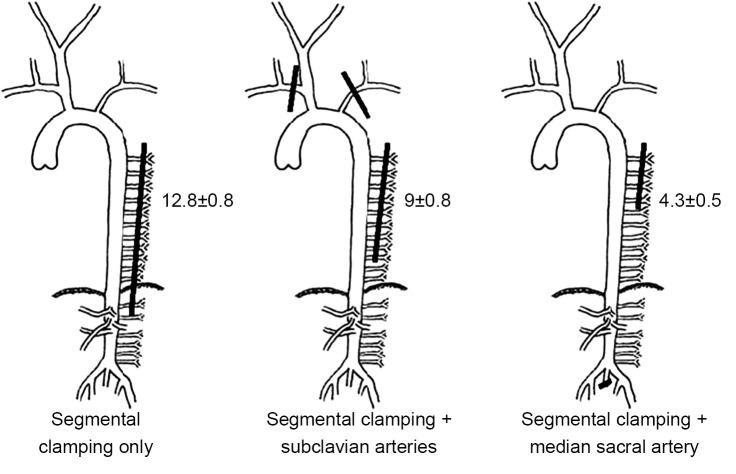
The first series of experiments in pigs with regard to the spinal cord circulation demonstrated that the subclavian and median sacral arteries (equivalent to the hypogastric arteries in humans) are important components of the collateral network. If the subclavian arteries or the median sacral arteries are eliminated as inputs to the collateral network, fewer SAs can be sacrificed during simulated TAAA resection without spinal cord ischemia [monitored by motor evoked potentials (MEP)] as shown in Figure 2. In the native pig, as many as 13 intercostal and lumbar artery pairs can be sacrificed without any impact, but fewer can be eliminated without MEP loss if there has been prior subclavian artery sacrifice, and fewer still after median sacral artery sacrifice (5).
Figure 2.
Shown are studies under various circumstances of the average numbers of segmental arteries¡ªintercostal or lumbar¡ªwhich could be serially clamped without provoking an ischemic change in the motor evoked potential signals in juvenile pigs. A higher number of segmental arteries can safely be clamped in the previously untouched control than after subclavian artery or after median sacral artery clamping. This figure is modified from reference (5)
The importance of the total extent of SA sacrifice as the principal predictor of the risk of subsequent paraplegia has been confirmed in two clinical studies. In the initial study, at a time when few intraoperative measures to enhance spinal cord protection were routine, paraplegia was very rarely seen if fewer than 11 segmental arteries were sacrificed during TAAA resection, Figure 3 (6). Up to the present day, based on 609 patients, the highest risk of paraplegia is incurred when more than 13 SAs are sacrificed. When between 8 and 12 SAs are involved, the risk of spinal cord injury depends upon their location, with the greatest vulnerability occurring with lower thoracic and lumbar SA sacrifice (Figure 4) (7).
Figure 3.
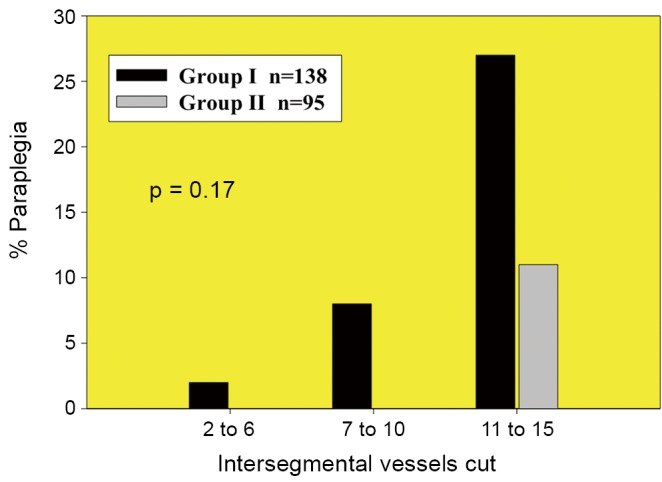
Comparison of the incidence of paraplegia before (in 138 patients) and after (in 95 patients) various adjuncts to increase spinal cord protection were routinely employed. Fewer cases of paraplegia were seen when adjuncts were utilized, but in both groups, cord injury depended upon the number of segmental arteries sacrificed. When fewer than 11 segmental arteries were sacrificed in the presence of adjuncts to protect the spinal cord, spinal cord injury did not occur. This figure is modified from reference (6)
Figure 4.
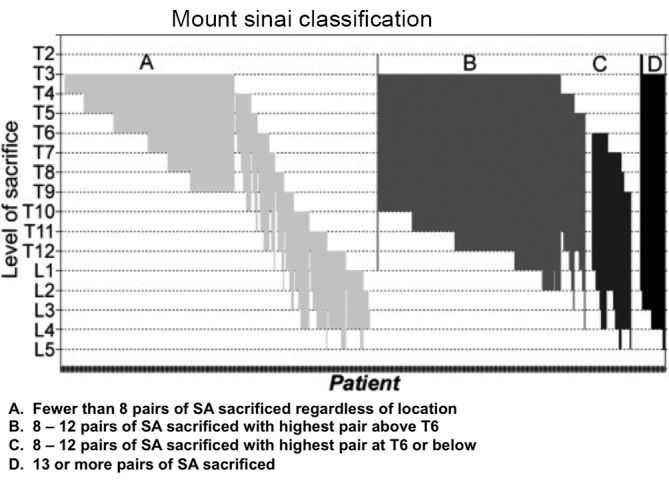
A proposed classification scheme for risk of paraplegia based on the extent and location of segmental artery (SA) resection during thoracic and thoracoabdominal aneurysm repair. For limited resections, especially those in the upper thoracic segments, the risk is quite low, but it rises sharply when more than 8 SAs are sacrificed, especially in the lower thoracic and lumbar area. The diagrams represent the extent of resection of the aneurysms in each group. The incidence of ischemic cord injury was 1.2% in Group A, 3.7% in Group B, 15% in Group C, and 12% in Group D. This figure is modified from reference (7)
Measurement of pressure in the collateral network—by means of a catheter in the stump of a divided SA—permits an appreciation of the dynamic response of the circulation to SA sacrifice. Pressure in the collateral network is 60-80% of mean arterial pressure (MAP) at baseline, and falls after extensive SA sacrifice, reaching its nadir about 5 hours later, during awakening from anesthesia. Gradual recovery of collateral network pressure begins within the first 24 hours. In the experiments shown in Figure 5, return to baseline pressure was invariably seen between 72 and 120 hours after complete SA sacrifice, even in pigs that showed functional evidence of spinal cord injury (8).
Figure 5.
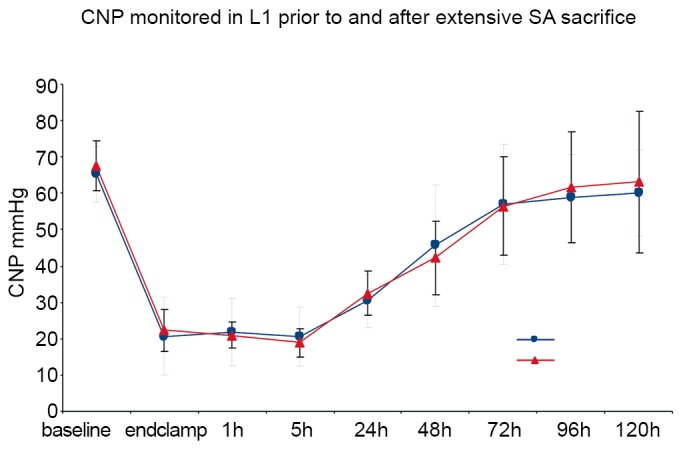
When pressure is monitored in the stump of a segmental artery (SA) after sacrifice of all SAs, the collateral network pressure (CNP) falls initially, reaches its lowest point about 5 hours after SA sacrifice, and recovers reliably between 72 hours to 5 days even in pigs that subsequently exhibit signs of functional spinal cord injury (8)
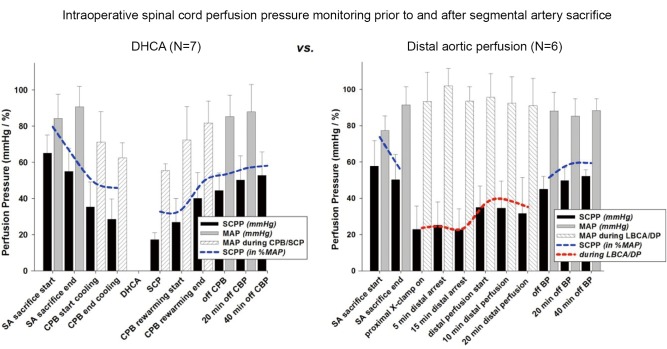
Intraoperative clinical measurement of collateral network pressures has also been possible in a few patients, Figure 6 (9). These clinical recordings reveal that, as in the pig, baseline collateral network pressure is less than baseline MAP. The perfusion pressure in the collateral network drops after SA sacrifice, and remains quite low until pulsatile perfusion is restored after discontinuation of cardiopulmonary bypass. Notably, perfusion pressure in the collateral network remains quite low despite what is generally considered effective partial left heart bypass. The lowest pressures, demonstrated in a few patients whose collateral network pressures were monitored for 24 hours postoperatively, occur¡ªas in the pig¡ªduring rewarming and awakening from anesthesia, several hours after completion of SA sacrifice. This may explain a recent clinical trend toward a preponderance of delayed rather than immediate paraplegia.
Figure 6.
Intraoperative collateral network pressure, thought to be roughly equivalent to spinal cord perfusion pressure (SCPP), can also be monitored clinically in patients undergoing resection of thoracic and thoracoabdominal aortic aneurysms. As in pigs, SCPP is initially less than mean MAP at baseline and falls with segmental artery (SA) sacrifice; SCPP is very low in the absence of pulsatile perfusion, including the interval of left heart bypass. Partial recovery is seen for the first 72 hours postoperatively (not shown) (9). CPB = cardiopulmonary bypass; DHAC = deep hypothermic circulatory arrest; DP = distal perfusion; LBCA = lower body circulatory arrest; SCP = selective cerebral perfusion
To document and possibly explain these physiological observations, we undertook a series of anatomical studies of the collateral network (10). In the initial studies, a mixture of methyl methacrylate was injected at physiologic pressure into the circulation of juvenile pigs in the native state, and subsequently in other pigs after sacrifice of all intercostal and lumbar SAs. The digestion of surrounding tissue revealed a cast of a vast network of interconnected small arteries and arterioles surrounding the spinal cord: a matrix of vessels connects the relatively modest spinal cord perfusion circuit with the much more extensive network of vessels supplying the paraspinal muscles, Figures 7 and 8 show the interconnecting vessels within the spinal canal which provide continuity between intraspinal and extraspinal circuits, and the presence of longitudinal as well as lateral interconnections. From this picture, it is possible to imagine how the loss of a few SAs can be compensated by flow via alternative routes within this rich anastomotic network.
Figure 7.
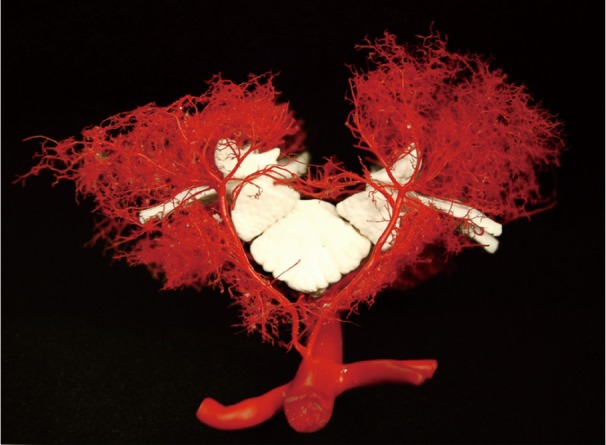
The perfusion of the spinal cord depends upon a vascular network which also provides flow to the paraspinal muscles, whose needs dwarf the flow to the spinal cord. In this view, the aorta is seen giving off paired segmental arteries which course around the vertebral body to supply the paraspinal muscles and, in the midline, the small anterior spinal artery
Figure 8.
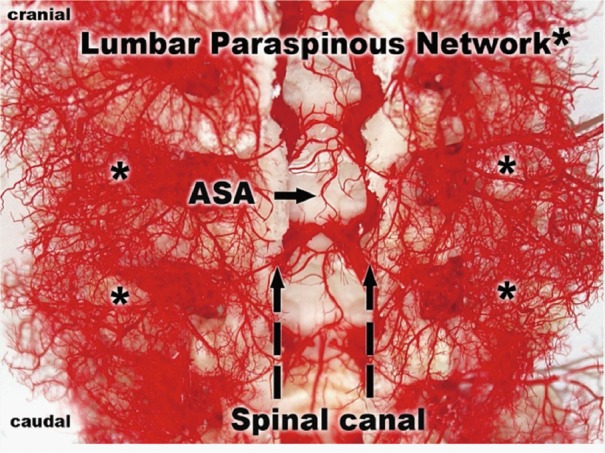
In this longitudinal section of a pig after injection of methyl methacrylate, the ASA is seen, with its multiple connections with the extensive vasculature of the paraspinal muscles adjacent to the spinal cord
An idealized reconstruction of the details of the structure of the spinal cord perfusion network can be seen in Figure 9 (10). There are multiple inputs into the ASA from the aorta via the SAs, but also connections to an extensive epidural network of vessels, which feature segmental epidural arcades which feed into the ASA periodically via radiculomedullary arteries (ARMAs).
Figure 9.
A diagrammatic reconstruction the blood supply to the spinal cord. The multiple inputs into the anterior spinal artery, and the rich matrix of longitudinal and lateral interconnections between the intraspinal and epidural systems are shown
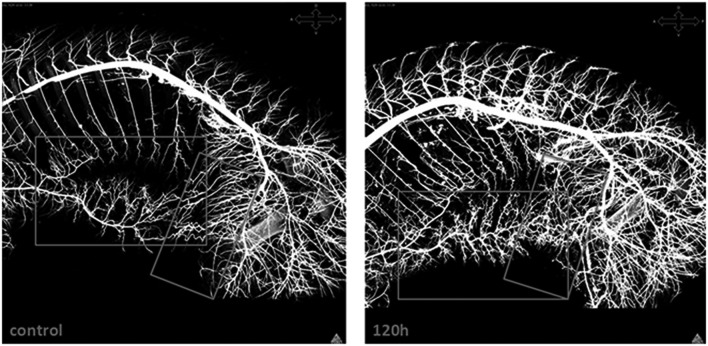
Anatomic studies of the changes in the circulation after extensive SA sacrifice in the pig reveal enlargement of the ASA within 24 hours, and more extensive expansion of various components of the intraspinal circulation by 5 days (11). A refinement of the injection technique allows visualization of the collateral network in situ by means of CT scanning, using a radiopaque viscous barium/latex mixture in place of the acrylic to outline even small vessels within the collateral network (12). In Figure 10, the ligated SAs are seen, filled retrograde to the radiopaque clips: the density of the collateral vasculature 5 days after SA sacrifice is clearly greater than in the accompanying picture from a native pig with intact SAs. These studies, taken together, confirm the enlargement and proliferation of the collateral network within 5 days in response to SA sacrifice. The presence of a greater number of vessels and an increased capacity within the collateral network explains the reliable return of a stable network collateral pressure 5 days after extensive SA sacrifice.
Figure 10.
A CT scan of a sagittal section of a juvenile pig infused with a barium/latex mixture shows the normal distribution of vessels in the collateral network surrounding the spinal cord, and then the increased numbers and density of these vessels in a pig 120 hours after interruption of all segmental arteries
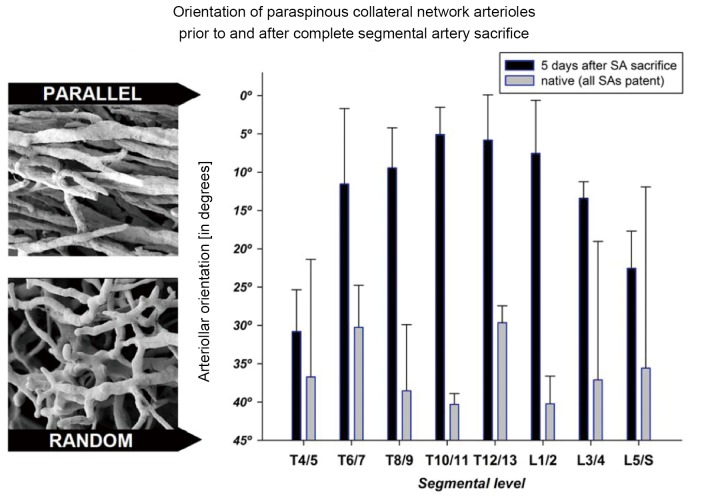
More intriguing than the enlargement and proliferation of the vessels is a series of observations made by scanning electron microscopy of the original methyl methacrylate casts. These pictures show a gradual reorientation of the proliferating vessels after extensive SA sacrifice: from the random orientation characteristic of the native state to a configuration with vessels more parallel to the spinal axis, Figure 11 (11). We speculate that this reorientation facilitates flow from the cranial and caudal inputs into the mid-thoracic region of the spinal cord circulation, which is the area most seriously deprived of its direct blood supply as the result of SA sacrifice. We anticipate that further refinements of imaging techniques will eventually allow assessment and monitoring of the spinal cord circulation in patients before and following SA sacrifice.
Figure 11.
The change in the orientation of the small arterial vessels of the paraspinous muscles within 5 days after complete sacrifice of all intercostal and lumbar segmental arteries (SA) in the pig is shown. A scanning electron micrograph (top) illustrates the more parallel orientation of the vessels after SA sacrifice, which is obviously different from the random arrangement in the native pig (below). The graph reflects the quantification of these changes in vessel orientation. This figure is modified from reference (11)
The mechanisms that provoke the anatomic changes which occur within the collateral network after extensive SA sacrifice remain to be elucidated. It is likely that there is vasodilatation to account for the early ASA enlargement, probably in response to mild ischemia. The subsequent proliferation of small vessels seems to involve generation of new vessels¡ªangiogenesis¡ªas well a transformation of smaller arterial conduits into larger ones by means of arteriogenesis. Clearly, an understanding of how this process is stimulated and controlled may allow manipulation to augment the response in advance of an aneurysm procedure, and thus reduce the chance of subsequent function-impairing spinal cord ischemia.
In the interim, knowledge of the anatomy and physiology of the spinal cord circulation can be helpful in directing the time course of staged interventions, and the duration and nature of measures to improve spinal cord protection. The vulnerability of the spinal cord circulation during the first few days after extensive SA sacrifice¡ªuntil the observed adaptations in the collateral network have occurred¡ªwarrants vigilance to avoid hypovolemia, hyperthermia, high venous pressures, and other threats which may upset the temporarily tenuous balance between spinal cord blood supply and demand which prevails after SA sacrifice. Once the anatomic adaptations to a reduced segmental blood supply have been made, the system is once again quite robust, and the threat of postoperative paraplegia recedes.
Acknowledgements
The work described was possible thanks to the support of grant # RO1HL45636 from the National Heart, Lung and Blood Institute of the NIH.
Disclosure: The authors declare no conflict of interest.
References
- 1.Adamkiewicz A. Die Blutgefasse des Menschlichen Ruckenmarkes. Krakau, 1881. [Google Scholar]
- 2.Svensson LG, Crawford ES, Hess KR, et al. Experience with 1509 patients undergoing thoracoabdominal aortic operations. J Vasc Surg 1993;17:357-68; discussion 368-70 [PubMed] [Google Scholar]
- 3.Etz CD, Zoli S, Mueller CS, et al. Staged repair significantly reduces paraplegia rate after extensive thoracoabdominal aortic aneurysm repair. J Thorac Cardiovasc Surg 2010;139:1464-72 [DOI] [PubMed] [Google Scholar]
- 4.Coselli JS, LeMaire CC, Miller ZC, et al. Mortality and paraplegia after thoracoabdominal aneurysm repair: a risk factor analysis. Ann Thorac Surg 2000;69:409-14 [DOI] [PubMed] [Google Scholar]
- 5.Strauch JT, Spielvogel D, Lauten A, et al. Importance of extrasegmental vessels for spinal cord blood supply in a chronic porcine model. Eur J Cardiothorac Surg 2003;24:817-24 [DOI] [PubMed] [Google Scholar]
- 6.Griepp RB, Ergin MA, Galla JD, et al. Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 1996;112:1202-13 [DOI] [PubMed] [Google Scholar]
- 7.Zoli S, Roder F, Etz CD, et al. Predicting the risk of paraplegia after thoracic and thoracoabdominal aneurysm repair. Ann Thorac Surg 2010;90:1237-44; discussion 1245 [DOI] [PubMed] [Google Scholar]
- 8.Etz CD, Zoli S, Bischoff MS, et al. Measuring the collateral network pressure to minimize paraplegia risk in thoracoabdominal aneurysm resection. J Thorac Cardiovasc Surg 2010;140:S125-30; discussion S142-S146. [DOI] [PubMed]
- 9.Etz CD, Di Luozzo G, Zoli S, et al. Direct spinal cord perfusion pressure monitoring in extensive distal aortic aneurysm repair. Ann Thorac Surg 2009;87:1764-73; discussion 1773-4. [DOI] [PubMed]
- 10.Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: a reassessment of the anatomy of spinal cord perfusion. J Thorac Cardiovasc Surg 2011;141:1020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Etz CD, Kari FA, Mueller CS, et al. The collateral network concept: remodeling of the arterial collateral network after experimental segmental artery sacrifice. J Thorac Cardiovasc Surg 2011;141:1029-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisbuesch S, Schray D, Bischoff MS, et al. Imaging of vascular remodeling following simulated thoracoabdominal aneurysm repair. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.08.062. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]