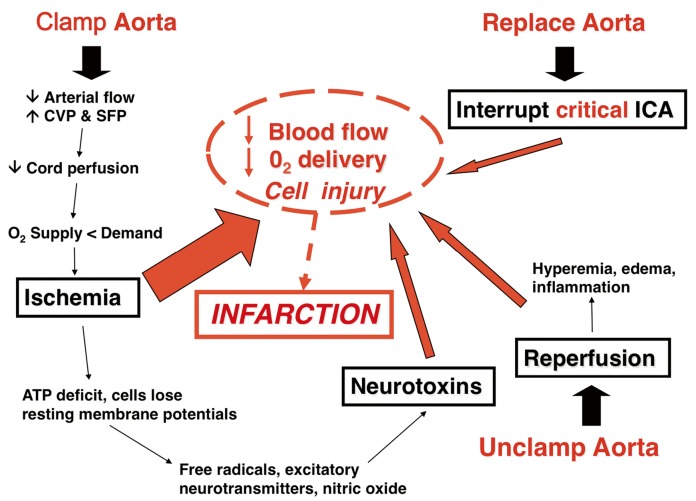
Spinal cord ischemia in thoracoabdominal aortic surgery is caused by the imbalance of oxygen demand and oxygen delivery produced by aortic occlusion. Ischemia and reperfusion initiate neurochemical cellular responses that can exacerbate ischemia, which may in turn progress to infarction (Figure 1). The most important factors in protecting the spinal cord during and after thoracic and thoracoabdominal aortic replacement are perfusion, metabolism, and oxygen delivery to the spinal cord during the vulnerable period of aortic occlusion, when spinal cord blood flow is significantly reduced (1,2) as well as after aortic replacement while the co-axial collateral network is recruited to return resting blood flow to near-normal levels (3). Assisted circulation and intercostal re-implantation have by themselves failed to reduce paraplegia risk both experimentally and clinically. This failure has been a source of frustration for surgeons as it runs counterintuitive to the anatomic paradigm for spinal cord preservation established by Adams and adopted by most surgeons for much of the last 60 years (4-8). However, reducing the vulnerability of the spinal cord during aortic replacement has always centered upon collateral circulation, increasing ischemic tolerance and protecting the spinal cord while collateral blood flow accommodates.
Figure 1.
Diagram of factors that are critical in spinal cord ischemia illustrating possible points of intervention to prevent paralysis
To reduce paraplegia risk in our patients we use the experimental literature to implement strategies validated as effective. In order to analyze our and others’ results we developed a predictive paraplegia model to calculate O/E ratios because percent paralysis is misleading by not accounting for patient mix (9). This model accounted for 99% of the variability in clinical reports of more than 5,000 patients. This analysis demonstrated that Crawford’s technique of repair, assisted circulation (AC), and now thoracic and thoracoabdominal endografts (TEVAR) have similar O/E ratios of approximately one if no protective adjuncts are used (Figure 2). This observation of parity implies the most important factor in paraplegia is interruption of intercostal blood flow, which lowers perfusion pressure in the axial collateral network reducing spinal cord blood flow and oxygen delivery regardless of technique of repair. This perfusion vulnerability is time dependent and influenced by other variables that positively or negatively affect blood flow, such as cardiac function (the strength of the pump) and arterial perfusion pressure (recruiting collateral flow); oxygen delivery (cardiac index, hemoglobin and pulmonary function); factors that increase ischemic tolerance of the spinal cord while perfusion is low, such as hypothermia and drugs that mitigate ischemic injury (steroids, excitatory neurotransmitters inhibitors and free radical scavengers).
Figure 2.
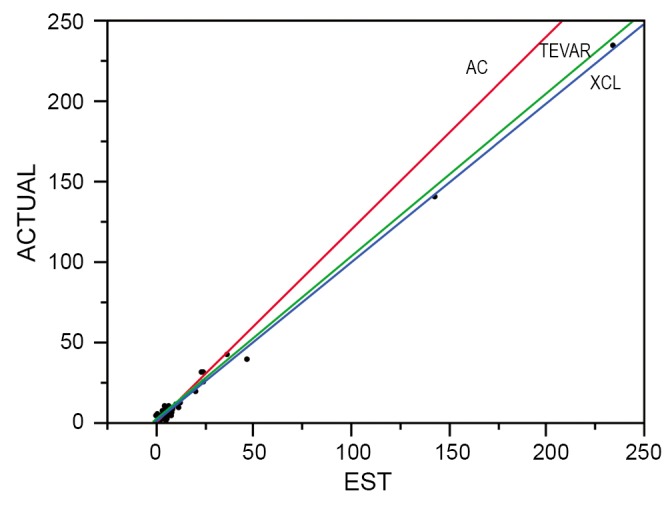
Clinical reports that appear to have different paralysis risk actually have the same risk when patient mix and clinical presentation are accounted for in our predictive model (9), which accounted for 99% of the variation in clinical reports. Assisted circulation (AC) and Crawford¡¯s technique of cross clamp without assisted circulation (XCL) and TEVAR had similar O/E ratios of about 1 in this regression plot of expected versus actual neurologic deficits (paraplegia)
Because we do not use assisted circulation (except hypothermic arrest for anatomic reasons) and added protective interventions sequentially we evaluated their impact both individually and cumulatively. From the beginning in 1983 we used moderate hypothermia, steroids (30 mg/kg methylprednisolone) (10), optimized cardiac index and proximal perfusion pressure (mean arterial pressure > 90 mmHg), and ligated intercostal arteries to improve perfusion pressure in the collateral bed during repair. We ceased using morphine after witnessing rapid onset paraplegia following a postoperative dose of intravenous morphine (11-13). With the addition of spinal fluid drainage (SFD) in 1987 we saw a 55% decline in paralysis rate (14,15). We added low dose naloxone after observing complete reversal of a profound deficit in a ruptured thoracoabdominal aortic aneurysm (TAAA) patient who had SFD, which resulted in an additional 30% decline in O/E ratio to 0.15 (16-20). The mechanisms of action of these strategies are thought to be reduction of spinal fluid pressure with SFD and endorphin receptor blockade with naloxone. However, the naloxone effect may suppress excitatory neurotransmitters and SFD may remove substances toxic to ischemic neurons (i.e., endorphins, excitatory neurotransmitters or other substances) thereby acting on the same final injury pathway (21,22). We added barbiturate burst suppression for its demonstrated protective effect and eliminated nitroprusside because of its negative impact on spinal cord blood flow and organ perfusion (1,23,24). With moderate hypothermia we achieved core temperatures below 34 oC prior to aortic occlusion, which decreased an additional 1.5-2 degrees to 32-33 oC with the infusion of 300 mL of cold renal perfusion solution into each renal artery for rapid cooling protection during aortic occlusion (lactated ringer solution with 12.5 g/L Mannitol and heparin 1,000 U/L at 4 oC) (25-28). In 2005 we added non-selective intercostal re-implantation because of the persistence of delayed paraplegia from hypoxia or hypotension from sepsis, myocardial infarction, bleeding or other causes. We reported the effect of intercostal replantation in 2008 which reduced paraplegia O/E ratios from 0.18 to 0.05, an additional 10-15% reduction from predicted for an overall reduction of 95% (Figure 3) (29). Since that report the effect of re-implantation has been less dramatic with O/E ratios reduced from 0.18 to 0.11 in the last 261 patients (Table 1). This intercostal effect is most noticeable in Crawford type 2 patients with the O/E ratio declining from 0.34 to 0.18.
Figure 3.
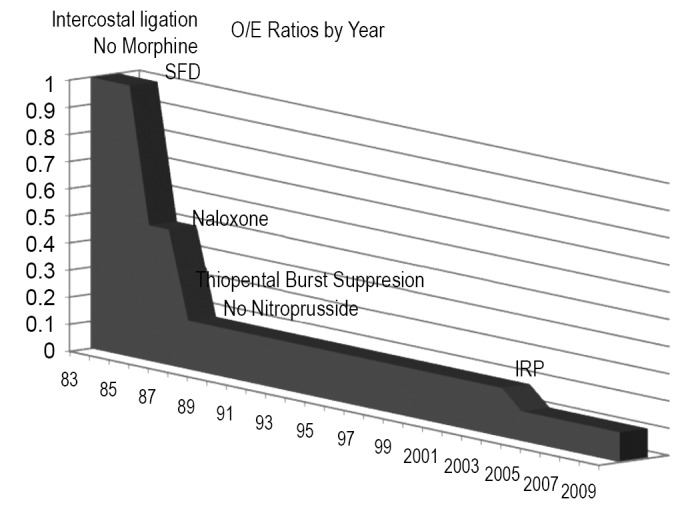
A plot of O/E ratios for paraplegia over time showing the effect of intervention. The incorporation of spinal fluid drainage (SFD) reduced paraplegia risk by about half. Low dose naloxone decreased the O/E ratio. Additional 30%, giving an 80-85% overall reduction in paralysis risk. Nonselective intercostal re-implantation (IRP) in high risk patients has added an additional 7-10% decrease for about a 90% reduction in paralysis risk overall
Table 1. Results in patients treated for thoracic and thoracoabdominal aneurysms. Hypothermic arrest was used when anatomy precluded aortic cross clamping and had higher mortality. Intercostal re-implantation was started in 2005 as was TEVAR, leading to lower paralysis risk and mortality.
| Group | No. | O/E | % Deficit | % Mortality (elective, acute) |
|---|---|---|---|---|
| All | 738 | 0.18 | 3.8 | 7.3 (2.8,15.7) |
| Intercostal re-implantation | 261 | 0.11 | 2.3 | 3.8 (0.6,9.6) |
| Hypothermic arrest | 33 | 0.24 | 9.1 | 18.2 (0.0,33.3) |
Other variables for paralysis risk identified in our patients by multivariate analysis are Crawford type 2 aneurysm, dissection and acute presentation, while our spinal cord protection protocol and increased cardiac index significantly preserve cord function (30) (Table 2). Cardiac index trumps both age and acuity, but as age increases cardiac index declines (Figure 4), which may explain why younger patients are at significantly less risk of paralysis with aortic replacement (Table 3).
Table 2. Increased cardiac index and spinal fluid drainage with naloxone were protective against paralysis. Crawford type 2 aneurysm, acute presentation and the presence of dissection increase the risk of paralysis. Factors such as age and temperature, significant by univariate analysis, were not as important as the other factors but when placed in a larger model with these factors cardiac index remained significant, while acute presentation was not significant.
| Variable | Odds ratio | P value |
|---|---|---|
| Crawford type 2 (yes) | 11.98 | <0.0001 |
| Spinal fluid drainage with naloxone (yes) | 0.294 | 0.0073 |
| Cardiac Index (per unit increase) | 0.624 | 0.0196 |
| Acute (yes) | 2.76 | 0.0127 |
| Dissection (yes) | 3.79 | 0.0024 |
Figure 4.
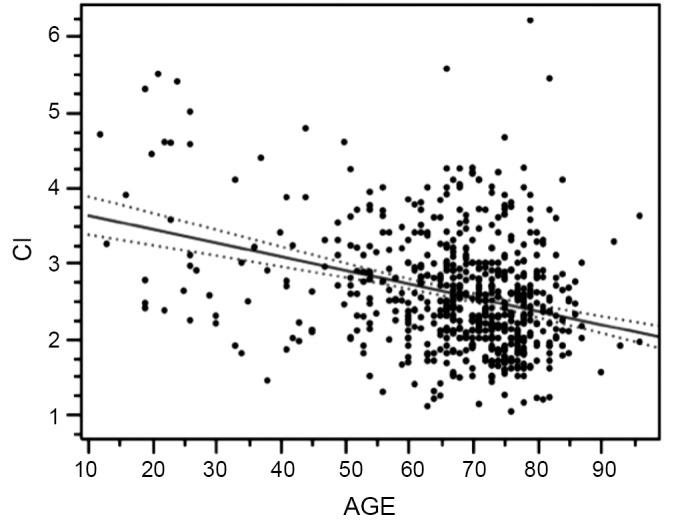
A plot of cardiac index (CI), a significant factor for paralysis risk, shows a significant decline with age that may explain and contribute to the increased paralysis risk associated with increased age
Table 3. Younger patients have less risk for paralysis and mortality but in a multivariate model age is less significant than cardiac index (Table 2), which may indicate that a good cardiac index is important in collateral perfusion dynamics.
| Group | Number of patients | O/E Ratio | % Paralysis | % Mortality |
|---|---|---|---|---|
| Age < 60 years | 153 | 0.06 | 1.4 | 0.7 |
| Age > 60 years | 617 | 0.28 | 5.7 | 8.9 |
The evolution of our neuroprotective strategies, based on the experimental literature and the concepts of perfusion, metabolism and oxygen delivery, and the resulting reduction in O/E ratios for paraplegia demonstrate that paralysis risk can be significantly reduced with experimentally validated interventions without using assisted circulation or selective intercostal artery re-implantation.
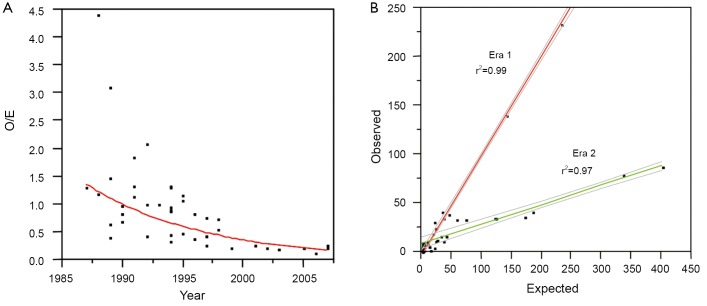
There has been a significant decline in paraplegia risk in clinical reports of thoracoabdominal surgery in the last 27 years and this decline is defined by two distinct eras: before 1997 and after 1997 (Figure 5). We studied the world literature (16,000 patients in approximately 100 reports) to understand why outcomes improved over time and between eras (31). We categorized patients by surgical technique: assisted circulation (AC), hypothermic arrest (HA) and cross clamp without assisted circulation (XCL). From this analysis the most important factor was the introduction of SFD whether the technique was AC, HA or XCL, with significant paraplegia reduction in all groups (Table 4). O/E ratios for AC, HA and XCL without SFD did not change between eras however O/E ratios for AC+SFD improved between Era 1 and Era 2. HA improved between eras because of SFD (Table 5). The improvement in AC+SFD O/E ratios between eras was because surgeons added hypothermia controlled spinal fluid pressure more aggressively, and with motor evoked potentials learned that maintaining high mean perfusion pressure, (>80 mmHg) improved spinal cord perfusion. Surgeons learned how to use AC in a way that was not as deleterious to spinal cord perfusion, metabolism and oxygen delivery.
Figure 5.
(A) We have modeled approximately 16,000 patients from clinical reports. A plot of the decline in O/E ratios for paraplegia over time shows a significant decline that can be divided into 2 distinct eras as shown in (B). The improvements in era 2 are not well documented in the reports themselves
Table 4. The addition of spinal fluid drainage (SFD) to assisted circulation, Crawford’s technique of cross clamp without AC, and hypothermic arrest has significantly reduced paralysis risk. This is also true for endovascular procedures.
| Group | Total Patients | O/E Ratio | % Deficit | P-value |
|---|---|---|---|---|
| Assisted circulation | 1,386 | 1.03 | 15.4 | <0.0001 |
| Assisted circulation + SFD | 5,773 | 0.24 | 4.3 | |
| Cross clamp | 4,485 | 0.91 | 13.9 | 0.0013 |
| Cross clamp + SFD | 1,384 | 0.23 | 4.1 | |
| Hypothermic arrest | 399 | 0.42 | 6.7 | 0.0065 |
| Hypothermic arrest + SFD | 463 | 0.15 | 2.8 | |
| Endovascular procedures | 733 | 1.12 | 6.9 | 0.0459 |
| Endovascular procedures + SFD | 1,423 | 0.61 | 4.2 |
Table 5. While the addition of spinal fluid drainage (SFD) significantly improved results (Table 4) which explains most of the improvement in paralysis risk between era 1 and era 2 (Figure 5B), Assisted circulation with SFD showed improved O/E ratios between eras, while assisted circulation with cross clamp and risk remained stable. The most likely factors for improvement for Assisted circulation + SFD across eras was the transition from normothermic to hypothermic assisted circulation and an emphasis on higher mean arterial pressures and more aggressive control of spinal fluid pressures.
| Group | Number of patients | O/E Ratio | % Paralysis | P-value |
|---|---|---|---|---|
| Assisted circulation 1 + SFD | 532 | 0.42 | 6.6 | 0.182 |
| Assisted circulation 2 + SFD | 5,321 | 0.23 | 4.2 | |
| Hypothermic arrest 1 | 297 | 0.44 | 7.1 | 0.0257 |
| Hypothermic arrest 2 + SFD | 505 | 0.16 | 3.3 | |
| Cross clamp 1 | 1,241 | 0.91 | 13.5 | 0.8073 |
| Cross clamp 2 | 230 | 0.92 | 14.3 | |
| Assisted circulation 1 | 4,273 | 1.03 | 15.3 | 0.5593 |
| Assisted circulation 2 | 152 | 0.94 | 13.9 |
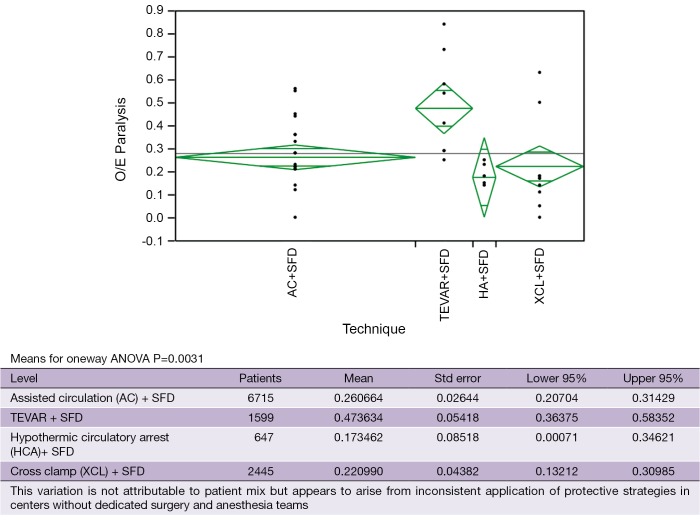
With the study of our and others¡¯ outcomes it is apparent that the anatomic paradigm is not the dominant paradigm for preventing paraplegia. This has been brought into sharper focus by the results of TEVAR and branched endograft use where the anatomic paradigm for spinal cord circulation is ignored and reliance on physiologic factors that affect spinal cord perfusion (MAP, SFD), metabolism and ischemic tolerance (steroids, naloxone, hypothermia), and oxygen delivery (hemoglobin, MAP, oxygen saturation, cardiac index) are the only tools to prevent paralysis. It is also apparent that patients treated with endografts are as vulnerable to paraplegia as patients having open surgery when extensive aortic coverage occurs and that this risk is predictable as is the effect of preventative interventions (Table 4). It is also evident that there is a range of effectiveness of applied strategies among treatment centers (Figure 6). What this variation tells us is that it is not enough to go through the motions in using hypothermia, draining spinal fluid, managing perfusion pressure and cardiac function, and applying neurochemical manipulation, but rather treatment protocols to optimize these factors must be followed consistently to achieve the best results. This lack of understanding is demonstrated in a recent paper by Greiner that focuses on using motor evoked potentials to selectively re-implant intercostal arteries (32). In their multicenter effort they indicate mean perfusion pressures of 60mmHg with no mention of hypothermia, and although they use SFD there is no detail about controlling spinal fluid pressure. Their O/E ratio for paraplegia is 0.50 which is what Safi, Svensson, Griepp and others had with SFD alone before adding hypothermia and MAP >85 mmHg, which brought O/E ratios down to the range of 0.1-0.2 (Table 5, Era 1 AC+SFD) (31). Greiner’s conclusion about the effectiveness of selective intercostal re-implantation using MEPs is belied by their reported results of 2 to 4 times the paraplegia risk that we and others have come to expect, and their results are consistent with the effect of SFD alone.
Figure 6.
A means plot of O/E ratios by technique of repair shows variation in outcomes using similar operative techniques
Unfortunately and indeed unnecessarily there continues to be confusion about paraplegia risk and prevention in the surgical treatment of the thoracoabdominal aorta. This confusion arises primarily from a perceived conflict between two paradigms of causation: the purely anatomic paradigm which postulates that paraplegia can only be prevented by reattaching the correct and specific intercostal arteries and a more complex paradigm of collateral blood flow and physiologic factors such as perfusion pressure, temperature, neurotransmitters, spinal fluid pressure, and cardiac function that is much more supported by the experimental literature and clinical observation. Equally distracting is the focus on surgical technique when equally good outcomes are possible with the application of physiologic principles, regardless of whether AC, HA or XCL is used for repair. The fact that TEVAR carries the same risk of paraplegia as open procedures supports the observation that spinal cord ischemia is primarily a matter of collateral blood flow and ischemic tolerance, rather than assisted circulation and selective intercostal reattachment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Gelman S, Reves JG, Fowler K, et al. Regional blood flow during cross-clamping of the thoracic aorta and infusion of sodium nitroprusside. J Thorac Cardiovasc Surg 1983;85:287-91 [PubMed] [Google Scholar]
- 2.Svensson LG, Rickards E, Coull A, et al. Relationship of spinal cord blood flow to vascular anatomy during thoracic aortic cross-clamping and shunting. J Thorac Cardiovasc Surg 1986;91:71-8 [PubMed] [Google Scholar]
- 3.Etz CD, Di Luozzo G, Bello R, et al. Pulmonary complications after descending thoracic and thoracoabdominal aortic aneurysm repair: predictors, prevention, and treatment. Ann Thorac Surg 2007;83:S870-6; discussion S890-2. [DOI] [PubMed]
- 4.Adams HD, van Geertruyden HH. Neurologic complications of aortic surgery. Ann Surg 1956;144:574-610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowell RC, Gloviczki P, Bergman RT, et al. Failure of selective shunting to intercostal arteries to prevent spinal cord ischemia during experimental thoracoabdominal aortic occlusion. Int Angiol 1992;11:281-8 [PubMed] [Google Scholar]
- 6.Griepp RB, Ergin MA, Galla JD, et al. Looking for the artery of Adamkiewicz: a quest to minimize paraplegia after operations for aneurysms of the descending thoracic and thoracoabdominal aorta. J Thorac Cardiovasc Surg 1996;112:1202-13 [DOI] [PubMed] [Google Scholar]
- 7.Svensson LG, Von Ritter CM, Groeneveld HT, et al. Cross-clamping of the thoracic aorta. Influence of aortic shunts, laminectomy, papaverine, calcium channel blocker, allopurinol, and superoxide dismutase on spinal cord blood flow and paraplegia in baboons. Ann Surg 1986;204:38-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maharajh GS, Pascoe EA, Halliday WC, et al. Neurological outcome in a porcine model of descending thoracic aortic surgery. Left atrial-femoral artery bypass versus clamp/repair. Stroke 1996;27:2095-100; discussion 2101 [DOI] [PubMed] [Google Scholar]
- 9.Acher CW, Wynn MM, Hoch JR, et al. Combined use of cerebral spinal fluid drainage and naloxone reduces the risk of paraplegia in thoracoabdominal aneurysm repair. J Vasc Surg 1994;19:236-46; discussion 247-8 [DOI] [PubMed] [Google Scholar]
- 10.Woloszyn TT, Marini CP, Coons MS, et al. Cerebrospinal fluid drainage and steroids provide better spinal cordprotection during aortic cross-clamping than does either treatment alone. Ann Thorac Surg 1990;49:78-82; discussion 83 [DOI] [PubMed] [Google Scholar]
- 11.Baskin DS, Kieck CF, Hosobuchi Y. Naloxone reversal and morphine exacerbation of neurologic deficits secondary to focal cerebral ischemia in baboons. Brain Res 1984;290:289-96 [DOI] [PubMed] [Google Scholar]
- 12.Doty S, Traber L, Herndon D, et al. Beta endorphin, a vasoconstrictor during septic shock. J Trauma 1988;28:131-9 [DOI] [PubMed] [Google Scholar]
- 13.De Riu PL, Petruzzi V, Palmieri G, et al. Beta-endorphin in experimental canine spinal ischemia. Stroke. 1989;20:253-8 [DOI] [PubMed] [Google Scholar]
- 14.Miyamoto K, Ueno A, Wada T, et al. A new and simple method of preventing spinal cord damage following temporary occlusion of the thoracic aorta by draining the cerebrospinal fluid. J Cardiovasc Surg (Torino) 1960;1:188-97 [PubMed] [Google Scholar]
- 15.Blaisdell FW, Cooley DA. The mechanism of paraplegia after temporary thoracic aortic occlusion and its relationship to spinal fluid pressure. Surgery 1962;51:351-5 [PubMed] [Google Scholar]
- 16.Rawal N, Schött U, Dahlström B, et al. Influence of naloxone infusion on analgesia and respiratory depression following epidural morphine. Anesthesiology 1986;64:194-201 [DOI] [PubMed] [Google Scholar]
- 17.Faden AI, Jacobs TP, Zivin JA. Comparison of naloxone and a delta-selective antagonist in experimental spinal stroke. Life Sci 1983;33:707-10 [DOI] [PubMed] [Google Scholar]
- 18.Faden AI, Jacobs TP, Smith MT, et al. Naloxone in experimental spinal cord ischemia: dose-response studies. Eur J Pharmacol 1984;103:115-20 [DOI] [PubMed] [Google Scholar]
- 19.Faden AI, Knoblach S, Mays C, et al. Motor dysfunction after spinal cord injury is mediated by opiate receptors. Peptides 1985;6:15-7 [DOI] [PubMed] [Google Scholar]
- 20.Acher CW, Wynn MM, Archibald J. Naloxone and spinal fluid drainage as adjuncts in the surgical treatment of thoracoabdominal and thoracic aneurysms. Surgery 1990;108:755-61; discussion 761-2 [PubMed] [Google Scholar]
- 21.Kunihara T, Matsuzaki K, Shiiya N, et al. Naloxone lowers cerebrospinal fluid levels of excitatory amino acids after thoracoabdominal aortic surgery. J Vasc Surg 2004;40:681-90 [DOI] [PubMed] [Google Scholar]
- 22.Wang YF, Gwathmey JK, Zhang G, et al. Cerebrospinal fluid may mediate CNS ischemic injury. Cerebrospinal Fluid Res 2005;2:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marini CP, Grubbs PE, Toporoff B, et al. Effect of sodium nitroprusside on spinal cord perfusion and paraplegia during aortic cross-clamping. Ann Thorac Surg 1989;47:379-83 [DOI] [PubMed] [Google Scholar]
- 24.Woloszyn TT, Marini CP, Coons MS, et al. Cerebrospinal fluid drainage does not counteract the negative effect of sodium nitroprusside on spinal cord perfusion pressure during aortic cross-clamping. Curr Surg 1989;46:489-92 [PubMed] [Google Scholar]
- 25.Black P, Markowitz RS. Experimental spinal cord injury in monkeys: comparison of steroids and local hypothermia. Surg Forum 1971;22:409-11 [PubMed] [Google Scholar]
- 26.Robertson CS, Foltz R, Grossman RG, et al. Protection against experimental ischemic spinal cord injury. J Neurosurg 1986;64:633-42 [DOI] [PubMed] [Google Scholar]
- 27.Vacanti FX, Ames A., 3rd Mild hypothermia and Mg++ protect against irreversible damage during CNS ischemia. Stroke 1984;15:695-98 [DOI] [PubMed] [Google Scholar]
- 28.Marsala M, Vanicky I, Yaksh TL. Effect of graded hypothermia (27 degrees to 34 degrees C) on behavioral function, histopathology, and spinal blood flow after spinal ischemia in rat. Stroke 1994;25:2038-46 [DOI] [PubMed] [Google Scholar]
- 29.Abitbol K, Acher F, Daniel H.Depression of excitatory transmission at PF-PC synapse by group III metabotropic glutamate receptors is provided exclusively by mGluR4 in the rodent cerebellar cortex. J Neurochem 2008;105:2069-79 [DOI] [PubMed] [Google Scholar]
- 30.Acher CW, Wynn MM, Hoch JR, et al. Cardiac function is a risk factor for paralysis in thoracoabdominal aortic replacement. J Vasc Surg 1998;27:821-8; discussion 829-30 [DOI] [PubMed] [Google Scholar]
- 31.Acher CW, Wynn M. A modern theory of paraplegia in the treatment of aneurysms of the thoracoabdominal aorta: An analysis of technique specific observed/expected ratios for paralysis. J Vasc Surg 2009;49:1117-24; discussion 1124 [DOI] [PubMed] [Google Scholar]
- 32.Greiner A, Mess WH, Schmidli J, et al. Cyber medicine enables remote neuromonitoring during aortic surgery. J Vasc Surg 2012;55:1227-32; discussion 1232-3 [DOI] [PubMed] [Google Scholar]