Introduction
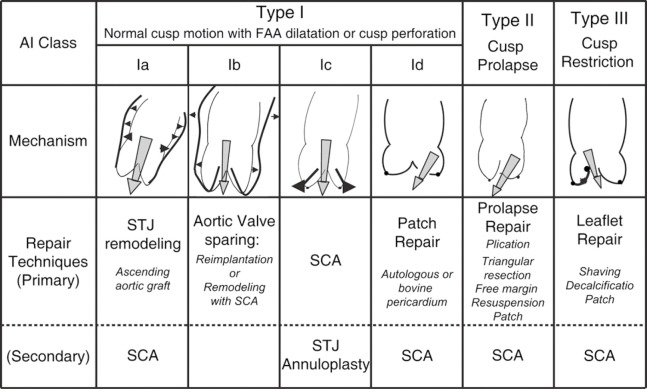
To understand the different mechanisms of aortic regurgitation (AR), the aortic valve needs to be considered as a functional unit composed of three structures: (I) the functional aortic annulus (FAA), comprising of the ventriculo-aortic junction (VAJ) and the sino-tubular junction (STJ) (Figure 1); (II) the leaflets with their attachment; and (III) the three sinuses of Valsalva. The different mechanisms of AR have been classified by our group in a repair-oriented classification, with particular importance given to the VAJ. Generally, along with the primary repair technique specific for each type of AR, a VAJ annuloplasty is required to restore the discrepancy between valve orifice and quantity of leaflet tissue (Figure 2) (1,2).
Figure 1.
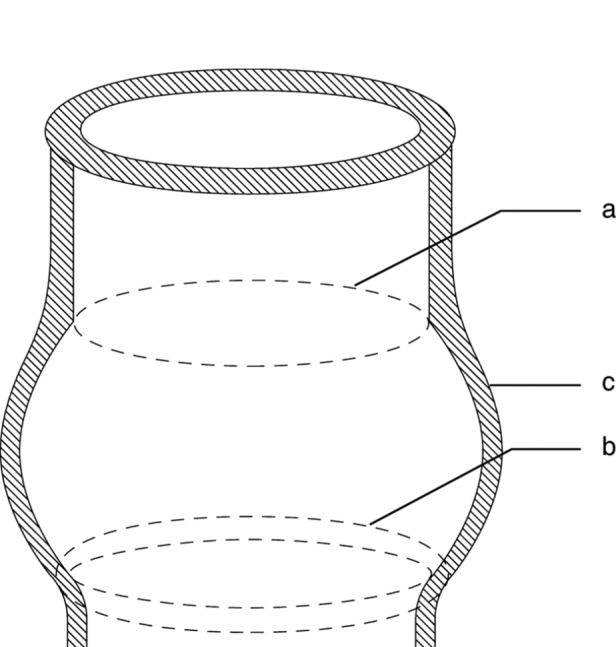
Diagrammatical representation of the aortic root. a. sinotubular junction; b. Ventriculo-aortic junction, also called basal ring or surgical annulus; c. the sinuses of Valsalva
Figure 2.
Repair-oriented functional classification of aortic insufficiency (AI) with description of disease mechanisms and repair techniques used. FAA, functional aortic annulus; STJ, sinotubular junction; SCA, subcommissural annuloplasty
In this review, we focus on the VAJ as a part of the FAA. We emphasize the role of the VAJ in the development of AR and the need for VAJ annuloplasty in AV repair. We describe the anatomy of the VAJ and the dynamics during the cardiac cycle. We also define its dimensions in normal and pathological aortic valve (AV). Finally, we analyze the different surgical solutions for VAJ annuloplasty.
The role of the VAJ in aortic regurgitation and the necessity of VAJ annuloplasty
In an echocardiographic study, Padial et al. analyzed aortic root sizes (i.e., VAJ, Valsalva sinus, STJ) in patients with chronic AR (3). They showed the correlation between root dilatation and the severity of AR, and the progression of root dilatation over time. Amongst the different levels of the aortic root, the STJ and Valsalva sinus dilatation showed the best correlation with the severity of AR. At the level of the VAJ, similar correlation was observed, but to a lesser extent. In another echocardiographic study including only patients with bicuspid aortic valve (BAV), Keane et al. have shown the correlation between aortic root sizes and the severity of AR, with a progressive and significant increase in all the aortic root diameters from no AR to severe AR (4).
The mechanism leading to AR when the FAA is dilated is the outwards displacement of the leaflets with a decrease of central coaptation. Whereas the surgical remodeling of STJ is relatively easy by the replacement of ascending aorta with tubular Dacron graft (5), the remodeling and stabilization of the VAJ is more complex because the structure is deeply embedded into the heart and close to delicate structures like the membranous septum and the conduction system. As a consequence, annuloplasty of the VAJ has frequently been ignored or neglected in AV repair (e.g., AV sparing with the remodeling technique) limiting the durability of the repair in patients with severe VAJ dilatation (6-10).
Anatomy of the VAJ
The terms, aortic annulus or basal ring, are frequently used to refer to the VAJ. They all describe the transition zone between the left ventricle and the aortic root but they have different definitions. For the echocardiographist, the aortic annulus corresponds to the plane passing through the nadir of the leaflet hinge-lines (11). For the surgeon, it generally corresponds to the leaflet hinge-lines, also called the surgical annulus, onto which the prosthetic valve is sewn. For the anatomist, the VAJ is where the ventricular myocardium terminates and gives way to the wall of aortic sleeve; yet most agree that no fibrous annulus can be isolated at this junction (12,13), even if controversy still animates the debate (14).
In the perspective of aortic valve repair, the VAJ as part of the FAA corresponds to a zone where an annuloplasty would restore the AV geometry and function. Thanks to the technique of valve sparing reimplantation wherein dissection of the proximal part of the root is necessary, surgeons have learned to consider not only the intraluminal side but also extraluminal side of the VAJ (Figure 3). Therefore, we feel it is important to include both aspects in the description of the VAJ.
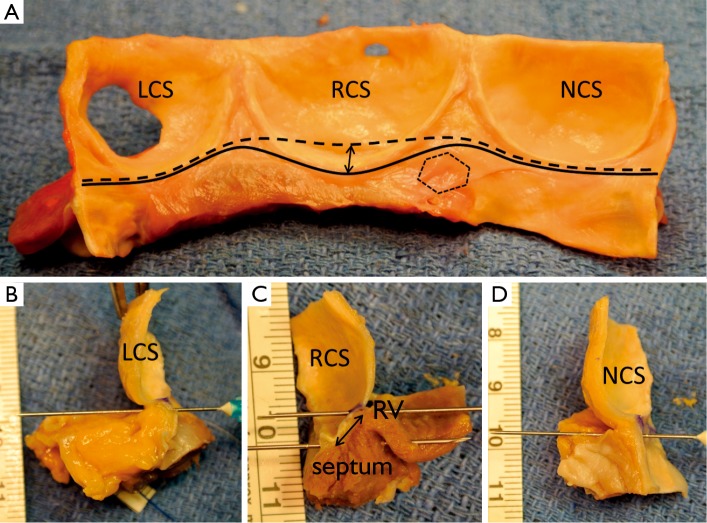
Figure 3.
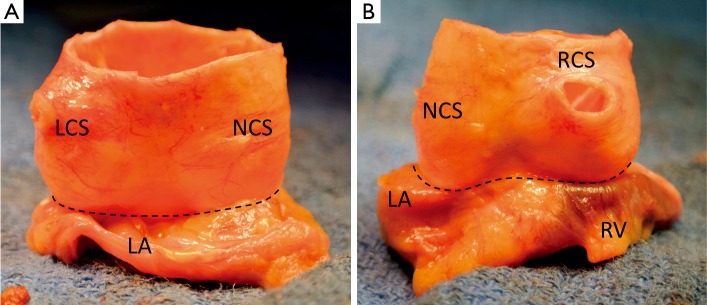
Pictures illustrating fresh cadaveric aortic root after proximal dissection to the junction between aortic wall and myocardial tissues (A. posterior view; B. anterior view; LCS, left coronary sinus; NCS, non coronary sinus; RCS, right coronary sinus; LA, left atrium; RV, right ventricle)
The VAJ is composed in approximately half of its circumference of a fibrous portion and in the other half of a muscular portion (15). The fibrous portion, posterior and right sided, consists in the aorto-mitral valvar continuity, the two trigones and the transition between the membranous septum and aortic wall. The fibrous portion is beneath the non-coronary sinus and half of the left coronary sinus. The muscular portion, anterior and left sided, consists of the interventricular muscular septum. This portion is under the right coronary sinus and half of the left coronary sinus. Variation exists in the architecture of the musculature supporting left and right sinuses (15).
On the intraluminal side of the aortic root, the nadirs of the leaflets hinge-lines cross the level of the VAJ (Figure 4). The upper parts of hinge-lines are above the VAJ; running along the aortic root wall, they end one or two millimeters below the STJ. On the extraluminal side of the aortic root, the VAJ corresponds to the zone where the root inserts in the myocardial tissues. This zone corresponds to the limit of aortic root dissection as performed in the AV sparing reimplantation technique. On the side of the non- and left-coronary sinus, the proximal limit of root dissection corresponds to the roof of the left atrium. This limit is at the same level or one or two millimeter below the nadirs of the corresponding leaflets hinge-lines (Figure 4B,D). On the side of the right coronary sinus, the proximal limit of root dissection corresponds to the myocardium coming from the interventricular septum and continuing laterally to the right ventricular outflow tract. This limit does not generally reach generally the level of the nadir of the right-coronary leaflet hinge-line. Effectively, the hinge-line sits slightly deeper on the ventricular septum (Figure 4C). In consequence, as described by Ho, a segment of myocardium is included into the base of the right aortic sinuses and the extent of myocardial inclusion varies from heart to heart (13). Relatively large myocardial inclusion can be seen in some patients with BAV for whom the term of “sinking sinus” has been used to describe that feature (Figure 5).
Figure 4.
A. Intraluminal view of longitudinally opened aortic root. Continuous black line delineates the ventriculo-aortic junction; the interrupted black line delineates the limit of proximal aortic root dissection; double black arrow show the segment of myocardium included into the base of the right coronary sinus; dotted line encircles the membranous septum (LCS, left coronary sinus; NCS, non coronary sinus; RCS, right coronary sinus); B. Longitudinal section through the nadir of the left coronary sinus; the limit of proximal root dissection reaches the level of the VAJ; C. Longitudinal section through the nadir of the right coronary sinus; the limit of proximal root dissection does not reach the level of the VAJ; double black arrow show the segment of myocardium included into the base of the right coronary sinus (RV, right ventricle; septum, interventricular muscular septum); D. Longitudinal section trough the nadir of the non-coronary sinus; the limit of proximal root dissection reach the level of the VAJ
Figure 5.
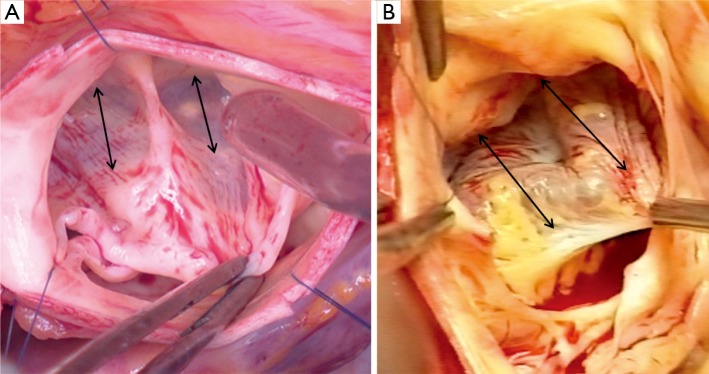
Intraoperative pictures showing the so called ‘sinking sinus’, showing large segment of myocardium included into the base of the right coronary sinus. (A. Type 1 bicuspid aortic valve; B. Type 0 bicuspid aortic valve, double arrows show the width of the myocardial inclusion)
Dynamic of the VAJ
The VAJ is not a rigid structure; it has, like the other parts of the aortic root and the leaflets, a specific kinematics during the cardiac cycle. Dagum and Lansac have both analyzed the aortic root dynamics in sheep models. In general, they have made quite similar observations (16,17). At the level of the VAJ, they have found that the area increases during the isovolumetric contraction and reaches a maximal expansion during first third of the ejection. Then, VAJ area decreases during the last two third of the ejection and during the isovolumetric relaxation. Finally, VAJ area increased during the diastole. Considering diameter instead of area, the difference between minimal and maximal expansion of the VAJ during the cardiac cycle was about 10% following Lansac et al. (17). Dagum et al. have shown that circumferential deformation of the VAJ was heterogeneous with a relatively greater deformation within the muscular portion compared to the fibrous portion of the VAJ (16).
Regarding that data, some authors have raised concerns over the durability of leaflet preservation if VAJ distensibility was lost. Effectively, studies have shown that with valve sparing reimplantation the VAJ distensibility is lost, whereas it is preserved with the remodeling technique in which valve and root dynamics are close to native AV (18). However, despite the haemodynamic advantages of remodeling, both techniques have shown similar long-term results (19,20).
VAJ dimensions in normal and pathological aortic valve
In the late 80s, Roman et al. analyzed aortic root dimensions in healthy children and adults (11). Aortic root measurements were performed by transthoracic echocardiography using M-mode tracings and 2-dimensional guidance to identify the different levels of the root. In adults, VAJ diameter was measured with a mean of 23±2 mm in women and 26±3 mm in men; indexed values were 13±1 mm/m2 in both genders (11). Body surface area and age had the most influence on VAJ size; whereas body mass index and blood pressure had the least influence on it (11,21). Several subsequent studies, based on 2-dimensional echocardiographic measurements, have reported a mean VAJ size of 23 mm in the adult population with normal AV (21,22). In those studies, the VAJ was measured on long-axis view of the aortic root. The measurement was taken with the inner edge to inner edge technique at the level of the leaflet hinge point. Anatomically, this measurement corresponds approximately to an antero-posterior diameter passing through the right coronary leaflet on one side and to the left or the non-coronary leaflet on the other side.
Despite the abundant literature on AV repair surgery, few studies reported the size of the VAJ in pathologies like AR or BAV disease. In patients with chronic AR, Padial et al. found an indexed VAJ diameter of 13 mm/cm2 with mild AR and of 14 mm/cm2 with moderate to severe AR (3). In that study, one-sixth of the patient had BAV and the remaining had tricuspid aortic valve (TAV). In another study, Lansac et al. found a mean VAJ diameter of 27.6±2.5 mm in a cohort of patients operated on for AR and root aneurysm, including 23% of BAV (23). In our cohort of adult patients having AV repair for similar indication, we confirm the relative VAJ enlargement with a mean VAJ diameter of 26 mm in TAV and 28 mm in BAV.
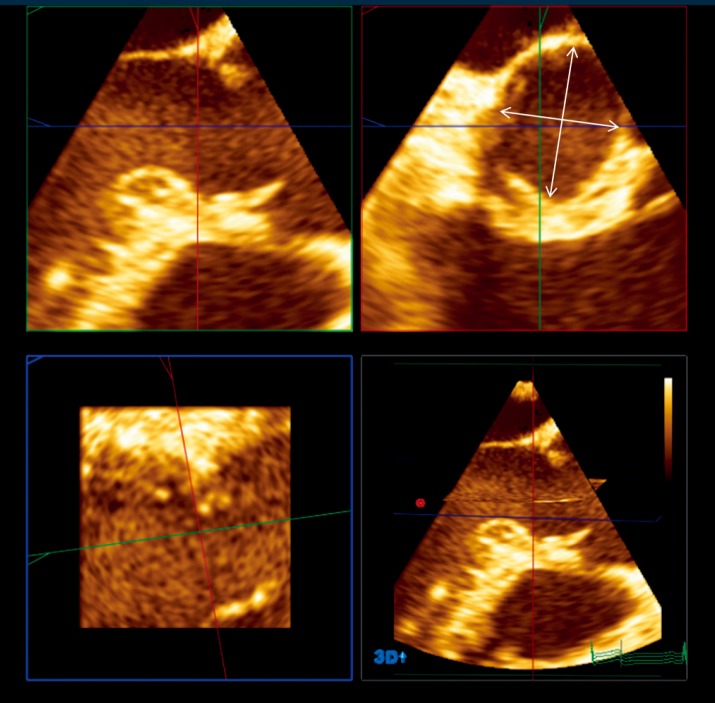
Recently, the advent of 3-dimensional imaging technology, such as 3-D echocardiography and CT scanner, has brought additional data on VAJ size and morphology. Most of those studies have analyzed the VAJ size in AV stenosis for transcatheter AV implantation. In that context, both echocardiographic and CT scanner studies have shown that the VAJ is often oval shaped (Figure 6) (24,25). Tops et al. reported an oval configuration of the VAJ in 50% of patients evaluated for transcatheter AV implantation, with a difference of 3±2 mm between coronal and sagittal measurements (24). Delgado et al. reported also an oval configuration of the annulus in patients with severe aortic stenosis with a significant difference between coronal (25±2.4 mm) and sagittal (23±2 mm) measurements (25). Finally, Rankin et al. have confirmed that in healthy adults the base of aortic valve was oval with a minor to major diameter ratio of 0.66 (26).
Figure 6.
This picture illustrates the 3 orthogonal views (sagittal, upper left quadrant; coronal, upper right quadrant; frontal, lower left quadrant; multiplanar reconstruction, lower right quadrant) issued from 3-dimensional echocardiography in a 58 years old patient with BAV, no AR and root aneurysm. The VAJ is analyzed in the coronal view, which is adjusted to correspond to the intersection of both the sagittal and the frontal views. In this patient, largest VAJ diameter was 28 mm and smallest was 21 mm
Surgical solutions for VAJ annuloplasty
In the 60s, Taylor was the first to describe a VAJ annuloplasty to treat AR (27). His technique consisted of circumclusion of the VAJ with a suture surrounding the base of the aorta. Several years later, different annular plication techniques have been described successively by Cabrol at the level of the interleaflet triangle (28), by Frater at the level of the Valsalva sinus (29) and by Duran in the subvalvular position (30). Of those, the Cabrol stitch, also called commissural plasty or subcommissural annuloplasty, has gained the most popularity because of its efficacy in increasing leaflet coaptation and its simplicity (2,31). During the last two decades, several other techniques using partial or circumferential bands have been used in clinical practice. In 1996, David et al. described a partial external band placed from trigone to trigone to support the fibrous portion of the annulus (32). In 2002, Izumoto et al. described a subvalvular circumferential ring (33). In 2005, Lansac et al. described an external circumferential aortic ring (34). Currently, despite all those techniques, the management of the VAJ has yet to be standardized, and other devices, like internal rigid rings developed by Rankin et al., are still under investigation (35).
The studies on VAJ annuloplasty devices generally report the results of one technique in series of variable size and follow-up. All of those techniques have been shown to be relatively safe with positive immediate- to mid-term results. Very few studies have analyzed the effect of each technique on valve function and geometry, or have compared these techniques in clinical or experimental setups. Based on those studies, we can elaborate the following analysis on the main concepts of VAJ annuloplasty (i.e., partial annuloplasty, internal ring, external ring). In general, annuloplasty techniques have shown to be able to reduce AR, alone or in association with other techniques of FAA and leaflet repair. In an in vitro study, we have shown that circumferential internal or external ring-based annuloplasties have a greater potential of VAJ reduction in comparison to non-circumferential stitch-based annuloplasty (i.e., the subcommissural annuloplasty) (36). In another study, we have showed that non-circumferential stitch-based annuloplasty can lose its remodeling effect over time in BAV repair (9). Therefore, we suggest the degree and extent of VAJ remodeling obtained with circumferential prosthetic-based annuloplasty is one of the factors that explain the better outcomes in BAV repair with valve sparing reimplantation compared to subcommissural annuloplasty (9).
Comparison between external and internal ring has been made by Scharfschwerdt et al. and by our group in vitro on porcine aortic valve. These studies have shown that both techniques can reduce AR with slightly better efficacy in favour of the internal ring (37). We have shown that in the porcine aortic valve, the external ring has a more a perivalvular position than a subvalvular position like the internal ring (36). While we recognize an important limitation of this study of the anatomical differences between porcine and human AV, it raises concern regarding potential misplacement of an external ring in respect to the right coronary cusp, especially in presence of a “sinking sinus”. Nevertheless, the external ring presents obvious advantages in comparison to internal rings, such as easier implantation and the absence of contact with the leaflets and the blood stream.
Conclusions
The VAJ is a three-dimensional oval-shaped structure deeply embedded into the heart structure that supports the base of leaflet hinge-lines. In normal aortic valves, the VAJ size is closely related to the body surface area. In pathological circumstances like chronic aortic regurgitation, aortic aneurysm and bicuspid aortic valve, the VAJ undergoes a degree of enlargement, thus losing its proportionality to body size. Different annuloplasty techniques and devices have shown to be effective in remodelling the VAJ and treating AR. Those techniques are applied to either the internal or external aspect of the VAJ. However, the management of the VAJ in aortic valve repair is far from standardized. Additional studies are necessary to evaluate the final impact on valve function, the long-term results of those techniques, and to establish the role these would play in the spectrum of aortic valve repair.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21 [DOI] [PubMed] [Google Scholar]
- 2.Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94 [DOI] [PubMed] [Google Scholar]
- 3.Padial LR, Oliver A, Sagie A, et al. Two-dimensional echocardiographic assessment of the progression of aortic root size in 127 patients with chronic aortic regurgitation: role of the supraaortic ridge and relation to the progression of the lesion. Am Heart J 1997;134:814-21 [DOI] [PubMed] [Google Scholar]
- 4.Keane MG, Wiegers SE, Plappert T, et al. Bicuspid aortic valves are associated with aortic dilatation out of proportion to coexistent valvular lesions. Circulation 2000;102:III35-9 [DOI] [PubMed] [Google Scholar]
- 5.David TE. Editorial Comment: Remodelling of the sinotubular junction to correct aortic insufficiency. Eur J Cardiothorac Surg 2012;42:1016-7 [DOI] [PubMed] [Google Scholar]
- 6.de Oliveira NC, David TE, Ivanov J, et al. Results of surgery for aortic root aneurysm in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2003;125:789-96 [DOI] [PubMed] [Google Scholar]
- 7.Erasmi AW, Sievers HH, Bechtel JF, et al. Remodeling or reimplantation for valve-sparing aortic root surgery? Ann Thorac Surg 2007;83:S752-6; discussion S785-90. [DOI] [PubMed]
- 8.Patel ND, Weiss ES, Alejo DE, et al. Aortic root operations for Marfan syndrome: a comparison of the Bentall and valve-sparing procedures. Ann Thorac Surg 2008;85:2003-10; discussion 2010-1. [DOI] [PubMed]
- 9.de Kerchove L, Boodhwani M, Glineur D, et al. Valve sparing-root replacement with the reimplantation technique to increase the durability of bicuspid aortic valve repair. J Thorac Cardiovasc Surg 2011;142:1430-8 [DOI] [PubMed] [Google Scholar]
- 10.Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85 [DOI] [PubMed] [Google Scholar]
- 11.Roman MJ, Devereux RB, Kramer-Fox R, et al. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol 1989;64:507-12 [DOI] [PubMed] [Google Scholar]
- 12.McAlpine WA. eds. Heart and coronary arteries. Berlin: Springer-Verlag, 1975:9-26. [Google Scholar]
- 13.Ho SY. Structure and anatomy of the aortic root. Eur J Echocardiogr 2009;10:i3-10 [DOI] [PubMed] [Google Scholar]
- 14.Berdajs D, Lajos P, Turina M.The anatomy of the aortic root. Cardiovasc Surg 2002;10:320-7 [DOI] [PubMed] [Google Scholar]
- 15.Sutton JP, 3rd, Ho SY, Anderson RH. The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 1995;59:419-27 [DOI] [PubMed] [Google Scholar]
- 16.Dagum P, Green GR, Nistal FJ, et al. Deformational dynamics of the aortic root: modes and physiologic determinants. Circulation 1999;100:II54-62 [DOI] [PubMed] [Google Scholar]
- 17.Lansac E, Lim HS, Shomura Y, et al. A four-dimensional study of the aortic root dynamics. Eur J Cardiothorac Surg 2002;22:497-503 [DOI] [PubMed] [Google Scholar]
- 18.Erasmi A, Sievers HH, Scharfschwerdt M, et al. In vitro hydrodynamics, cusp-bending deformation, and root distensibility for different types of aortic valve-sparing operations: remodeling, sinus prosthesis, and reimplantation. J Thorac Cardiovasc Surg 2005;130:1044-9 [DOI] [PubMed] [Google Scholar]
- 19.Aicher D, Langer F, Lausberg H, et al. Aortic root remodeling: ten-year experience with 274 patients. J Thorac Cardiovasc Surg 2007;134:909-15 [DOI] [PubMed] [Google Scholar]
- 20.David TE, Maganti M, Armstrong S. Aortic root aneurysm: principles of repair and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S14-9; discussion S45-51. [DOI] [PubMed]
- 21.Kim M, Roman MJ, Cavallini MC, et al. Effect of hypertension on aortic root size and prevalence of aortic regurgitation. Hypertension 1996;28:47-52 [DOI] [PubMed] [Google Scholar]
- 22.D’Andrea A, Cocchia R, Riegler L, et al. Aortic root dimensions in elite athletes. Am J Cardiol 2010;105:1629-34 [DOI] [PubMed] [Google Scholar]
- 23.Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring to standardise aortic valve repair: preliminary results of a prospective multicentric cohort of 144 patients. Eur J Cardiothorac Surg 2010;38:147-54 [DOI] [PubMed] [Google Scholar]
- 24.Tops LF, Wood DA, Delgado V, et al. Noninvasive evaluation of the aortic root with multislice computed tomography implications for transcatheter aortic valve replacement. JACC Cardiovasc Imaging 2008;1:321-30 [DOI] [PubMed] [Google Scholar]
- 25.Delgado V, Ng AC, van de Veire NR, et al. Transcatheter aortic valve implantation: role of multi-detector row computed tomography to evaluate prosthesis positioning and deployment in relation to valve function. Eur Heart J 2010;31:1114-23 [DOI] [PubMed] [Google Scholar]
- 26.Rankin JS, Bone MC, Fries PM, et al. A refined hemispheric model of normal human aortic valve and root geometry. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.06.043. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Taylor WJ, Thrower WB, Black H, et al. The surgical correction of aortic insufficiency by circumclusion. J Thorac Surg 1958;35:192-205 passim [PubMed] [Google Scholar]
- 28.Cabrol C, Cabrol A, Guiraudon G, et al. Treatment of aortic insufficiency by means of aortic annuloplasty. Arch Mal Coeur Vaiss 1966;59:1305-12 [PubMed] [Google Scholar]
- 29.Frater RW. Aortic valve insufficiency due to aortic dilatation: correction by sinus rim adjustment. Circulation 1986;74:I136-42 [PubMed] [Google Scholar]
- 30.Duran CG. Reconstructive techniques for rheumatic aortic valve disease. J Card Surg 1988;3:23-8 [DOI] [PubMed] [Google Scholar]
- 31.Cosgrove DM, Rosenkranz ER, Hendren WG, et al. Valvuloplasty for aortic insufficiency. J Thorac Cardiovasc Surg 1991;102:571-6; discussion 576-7 [PubMed] [Google Scholar]
- 32.David TE. Remodeling the aortic root and preservation of the native aortic valve. Operative Techniques Card Thorac Surg 1996;1:44-56 [Google Scholar]
- 33.Izumoto H, Kawazoe K, Kawase T, et al. Subvalvular circular annuloplasty as a component of aortic valve repair. J Heart Valve Dis 2002;11:383-5 [PubMed] [Google Scholar]
- 34.Lansac E, Di Centa I, Varnous S, et al. External aortic annuloplasty ring for valve-sparing procedures. Ann Thorac Surg 2005;79:356-8 [DOI] [PubMed] [Google Scholar]
- 35.Rankin JS, Conger JL, Tuzun E, et al. In vivo testing of an intra-annular aortic valve annuloplasty ring in a chronic calf model. Eur J Cardiothorac Surg 2012;42:149-54 [DOI] [PubMed] [Google Scholar]
- 36.de Kerchove L, Vismara R, Mangini A, et al. In vitro comparison of three techniques for ventriculo-aortic junction annuloplasty. Eur J Cardiothorac Surg 2012;41:1117-23; discussion 1123-4 [DOI] [PubMed] [Google Scholar]
- 37.Scharfschwerdt M, Pawlik M, Sievers HH, et al. In vitro investigation of aortic valve annuloplasty using prosthetic ring devices. Eur J Cardiothorac Surg 2011;40:1127-30 [DOI] [PubMed] [Google Scholar]