Abstract
Background
Aortic valve repair has emerged as a feasible alternative to replacement in the surgical treatment of selected patients with aortic valve (AV) pathology. In order to provide a synopsis of the current literature, we preformed a systematic review with a focus on valve-related events following AV repair.
Methods
Structured keyword searches of Embase and PubMed were performed in January 2012. A study was eligible for inclusion if it reported early mortality, late mortality, or valve-related morbidity in the adult population.
Results
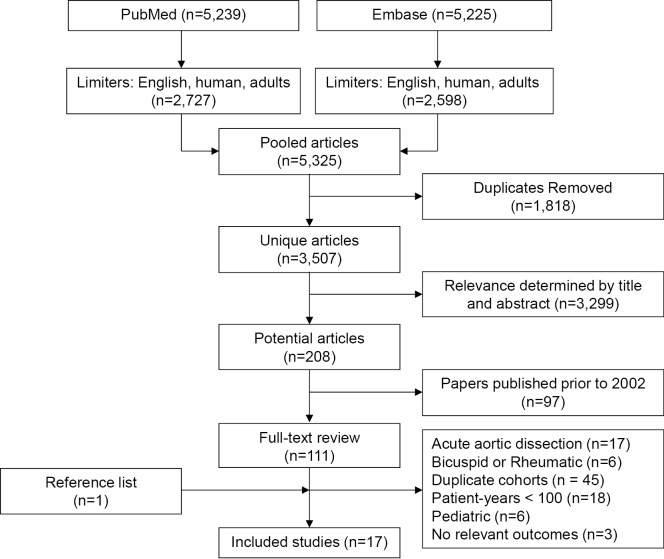
Initial search results identified 3,507 unique studies. After applying inclusion and exclusion criteria, 111 studies remained for full-text review. Of these, 17 studies involving 2,891 patients were included for quantitative assessment. No randomized trials were identified. Tricuspid and bicuspid AV pathologies were present in 65% (range, 21-100%) and 13.5% (range, 5-100%) of the population, respectively. Cusp repair techniques were applied in a median of 46% (range, 5-100%) of patients. The median requirement for early reoperation for post-operative bleeding and early reintervention for primary AV repair failure was 3% (range, 0-10%) and 2% (range, 0-16%), respectively. Pooled early mortality was 2.6% (95% CI: 1.4-4.4%, I2 =0%). Late mortality and valve-related events were linearized [(number of events/number of patient-years) ×100] (%/pt-yr) for each study. Late operated valve endocarditis was reported at median event rate of 0.23%/pt-yr (range, 0-0.78%/pt-yr), while a composite outcome of neurological events and thromboembolism occurred at a median rate of 0.52%/pt-yr (0-0.95%/pt-yr). Late AV re-intervention requiring AV replacement or re-repair occurred at a rate of 2.4%/pt-yr (range, 0-4.2%/pt-yr). The median 5-year freedom from AV re-intervention and late recurrent aortic insufficiency >2+ estimated from survival curves was 92% (range, 87-98%) and 88% (range, 87-100%), respectively. Pooled late mortality produced summary estimate of 1.3%/pt-yr (95% CI: 0.9-2.1%, I2=0%).
Conclusions
The present systematic review confirmed the low operative risk of patients who underwent aortic valve preservation and repair. There is a need for long-term follow-up studies with meticulous reporting of outcomes following AV repair, as well as comparative studies with aortic valve replacement.
Keywords: Aortic valve repair, aortic valve preservation, valve-sparing root replacement, systematic review
Introduction
Aortic valve preservation and repair is emerging as a feasible alternative to aortic valve replacement in the treatment of aortic root pathology with or without aortic insufficiency (Figure 1). Preservation of the native aortic valve leaflets has gained popularity in cardiac centers worldwide due to the success of repair techniques and the potential of avoiding the long-term complications of prosthetic valves. However, unlike the mitral valve, which is associated with a relatively high proportion of repair, aortic valve repair is only performed in less than 2% of all aortic valve surgery (1). This difference is largely due to the fact that the most commonly seen pathology of the aortic valve is calcific aortic stenosis, which is not amenable to repair. In addition, there is a much longer and more established experience with successful mitral valve repair techniques (2).
Figure 1.
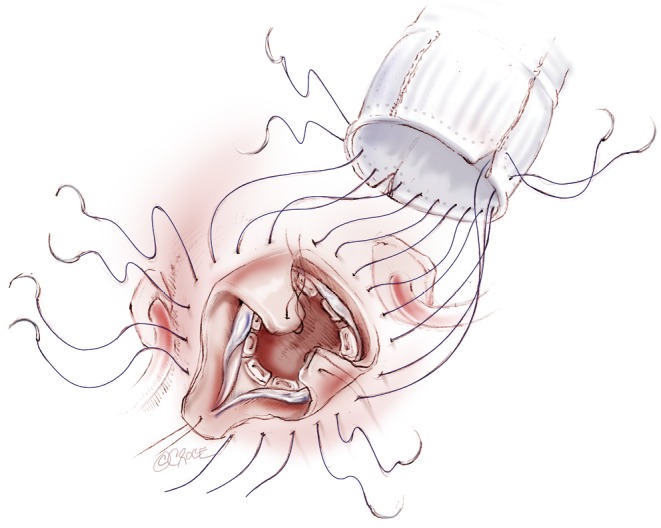
Aortic valve-sparing surgery using the re-implantation technique
Ideally, a prosthetic valve should sustain excellent hemodynamics at rest and exercise, have minimal transaortic pressure gradients, should be durable in the long-term, resist thrombus formation without the need for anticoagulation, and be simple to implant. Unfortunately, the ideal prosthetic valve does not yet exist. Instead, native valve disease is typically replaced by “prosthetic valve disease” (3). Current prosthetic devices are associated with complications including, but not limited to, valve thrombosis and thromboembolic events, bleeding events associated with anticoagulation use, prosthetic valve endocarditis, and structural valve deterioration (4). In effect, successful aortic valve repair has become an attractive alternative in circumventing these potential complications by preservation of the native aortic valve apparatus. The long-term outcome following aortic valve repair, however, remains uncertain.
In an effort to summarize valve repair data from centers worldwide, Savage and Carr have completed an extensive systematic review of 11 major studies from 1990-2002 addressing valve repair for aortic insufficiency in adults (2). Although many studies reported 5- or 10-year outcomes after valve repair, data for long-term outcomes was scarce. Furthermore, a large proportion of patients in these studies undergoing valve repair had rheumatic valve disease, which has been associated with poor long-term outcome. Although the systematic review presented a large proportion of aortic valve repairs in the decade from 1990-2002, valve repair techniques have since evolved. Therefore, we performed a systematic review of studies published from 2002-2011, aiming to assess outcomes and complications associated with reconstructive valve surgery.
Methods
Search strategy
Structured keyword searches of PubMed [1966-2012] and Embase [1980-2012] were performed on January 25, 2012 using (aortic valve repair) OR (aortic valve reconstruction) OR (aortic valve preservation) OR (valve-sparing root replacement) with human, adult and English language limitations. Articles were deemed eligible for inclusion if they were observational studies published after 2002, and reported morbidity or mortality for aortic valve repair or preservation. Articles were excluded if they were case reports, if they had a substantial proportion of acute aortic dissections (>25%), or when only a specific sub-population of AV repair was evaluated (e.g., bicuspid AV, rheumatic, etc.). In order to obtain meaningful data on long-term outcomes, studies were also excluded when there was less than 100 patient-years follow-up. Two reviewers (R.S. and T.M.) screened titles and abstracts to identify articles for full-text review. In the case of multiple publications of overlapping patient populations from the same center, the study with the largest number of patient-years or most recent year of publication was selected. Reference lists of included articles were screened to aid in identification of relevant studies. No authors required contact for clarification of published data.
Data extraction and analysis
Microsoft Excel for Windows was used for data extraction and statistical analysis. One reviewer (R.S.) transcribed data into a standardized collection form. A second reviewer (M.B.) verified extracted data and discrepancies were rectified through consensus. Patient-years (pt-yrs) were calculated for each study by multiplying the number of patients with the mean follow-up time. Outcome events were catalogued according to the definitions described by the Liaison Committee for Standardized Definitions of Prosthetic Heart Valves (5). Studies not reporting a specific outcome measure were excluded from analysis of that outcome. Early mortality was presented as a percent, while late mortality and valve-related outcomes were linearized [(number of events/number of patient-years) × 100] for each study. A composite outcome was used for late thromboembolic and neurological events. Summary effects measures for early and late mortality were obtained by logarithmically pooling data with an inverse-variance weighted fixed effects model. The summary effects measures were presented with a 95% confidence interval (95% CI). When early or late mortality was reported as zero, the value was adjusted to an event rate of 0.5 to permit computation in the fixed effects model. If early or late mortality was not reported in an article, it was excluded from the pooled analysis. Heterogeneity of the summary effects measures were assessed with the I2 test and considered present when I2>50%. Continuous data were presented as a median and range.
Results
Identification of studies
The keyword search results of PubMed and Embase produced 5,325 potentially relevant articles (Figure 2). After removal of duplicate studies present in both search engines, 3,507 unique articles remained. Further screening for relevance by title and abstract removed an additional 3,299 papers. The resulting 208 studies were restricted based on the year of publication (January 2002 - January 2011), which led to the identification of 111 studies. Full-text review of these articles were performed by two reviewers (R.S. and M.B.) and articles were further excluded due to the following reasons: large proportion of acute aortic dissections, studies including only subgroups of AV disease, duplicate cohorts, patient-years <100, paediatric population, lack of relevant outcomes, as summarized in Figure 2. After the selection process, 16 unique studies were identified for inclusion. A reference list review of these articles was undertaken and one additional article was identified for inclusion. In summary, after screening 3,507 unique articles, 17 studies were included for quantitative assessment in the present systematic review (4,6-21).
Figure 2.
Study selection flow chart
Study characteristics
There were no randomized trials comparing aortic valve repair to replacement. The majority of studies reported single institutional data (6,8,10-21). However, two studies reported outcomes from multi-center experiences (7,9). Pooling the populations from the studies produced a total of 2,891 patients that underwent AV repair (4,6-21) (Table 1). The median follow-up time was 3.9 yrs (1.4-15 yrs), which produced a median follow-up period of 387 pt-yrs (116-3,072 pt-yrs). The majority of the patients who underwent AV repair were male (median: 76.4%, range, 64.4-82.6%), with a median age of 53.5 yrs (range, 32.9-61.0 yrs). Tricuspid (4,6,8,13,17) and bicuspid (4,6-10,13,16,19-21) aortic valve pathology were present in 65% (range, 21-100%) and 13.5% (range, 5-100%) of the population, respectively. Preoperative aortic insufficiency (AI) greater than 2+ was present in a median of 52% (range, 0-93%) of patients. Cusp repair techniques were applied in a median of 46% (range, 5-100%) of patients (4,6,7,9-13,15-21).
Table 1. Study characteristics.
| Author (Reference) | Year | Patients (N) | Age (yrs) | F/U (yrs) | F/U (pt-yrs) | Preop. AI (>2+) (%) | Marfan (%) | Bicuspid AV (%) |
|---|---|---|---|---|---|---|---|---|
| Aicher (4) | 2010 | 640 | 56 | 4.8 | 3,072 | NR | NR | 32 |
| Lansac (6) | 2010 | 144 | 54.4 | 2.2 | 317 | NR | 13 | 22.9 |
| Lansac (7) | 2006 | 83 | 49.3 | 1.4 | 116 | 32.9 | 20.5 | 4.8 |
| Boodhwani (8) | 2009 | 264 | 54 | 3.9 | 1,030 | 74.6 | NR | 34.1 |
| DePaulis (9) | 2010 | 278 | 56 | 4.3 | 1,195 | 48.9 | 15.1 | 11.1 |
| Settapani (10) | 2009 | 60 | 60 | 2.4 | 144 | 64 | 5 | 15 |
| Urbanski (11) | 2010 | 106 | 61 | 1.4 | 148 | 60 | NR | NR |
| Doss (12) | 2010 | 66 | 41.2 | 5.1 | 337 | 0 | NR | 100 |
| Badiu (13) | 2010 | 102 | 47 | 2 | 204 | 93.3 | 20.6 | 10.8 |
| Cameron (14) | 2009 | 85 | 33 | 15 | 1,275 | NR | NR | NR |
| Svensson (15) | 2010 | 129 | NR | 3 | 387 | 28.4 | 47.3 | NR |
| David (16) | 2010 | 289 | 48 | 7.3 | 2,110 | 51.2 | 34 | 8.3 |
| Izumoto (17) | 2006 | 40 | 61 | 3.6 | 144 | NR | NR | NR |
| Tanaka (18) | 2011 | 60 | 33 | 4.6 | 276 | 23.3 | 90 | NR |
| Oka (19) | 2011 | 101 | 49.7 | 5 | 505 | 70.3 | 23.8 | 11.9 |
| Kallenbach (20) | 2005 | 284 | 53 | 3.4 | 966 | 50.8 | 19 | 6 |
| Minakata (21) | 2004 | 160 | 55 | 4.2 | 672 | 60.3 | NR | 33.8 |
Preop. AI, preoperative aortic valve insufficiency; F/U, follow up; N, number; NR, not reported; yrs, years; pt-yrs, patient-years; AV, aortic valve
Aortic root reconstruction was required in 12 studies necessitating the use of valve sparing aortic root replacement with a reimplantation (9-16,18-20) or remodelling (7,13,14,16,18,19) technique. The need for aortic root replacement was reported in a median of 93.5% (range, 1-100%) of patients from the 12 studies. This represented 52% of all the patients included in the 17 studies.
The studies reported a median cardiopulmonary bypass time of 143 mins (range, 64-175 mins) (7-9,12,13,16,17,20,21) and a median cross-clamp time of 125 mins (range, 87-138 mins) (6-9,11-13,16,17,20,21). The prevalence of concomitant cardiac procedures at the time of AV repair had a median value of 24.5% (range, 1.9-80.9%) (4,6-12,16,18,20,21). The median rate of redo cardiac surgery at the time of repair was 5.4% (range, 0-11.8%) (6,8,12,13,16,19-21).
Early outcomes
Early mortality rate was provided in all of the included studies (4,6-21) (Table 2). The pooled, fixed effect estimate of early mortality was 2.6% (95% CI: 1.4-4.4%, I2=0%). Eleven studies reported a median perioperative neurological event rate of 1% (range, 0-7%) (4,6-8,10,12,13,15,16,20,21). The requirement for exploratory resternotomy for post-operative bleeding occurred at a median of 3% (range, 0-10%) in ten studies (4,7-10,12,13,16,20,21). A median of 2% (range, 0-16%) of patients required early AV reintervention due to failure of primary AV repair (6-8,16,20,21).
Table 2. Operative approach and outcomes.
| Author (Reference) | Year | Cusp repair (%) | Valve sparing root replacement technique (%) |
Early mort. (%) | OVE (%/pt-yr) | Reop. (%/pt-yr)‡ | Late mort. (%/pt-yr) | |
|---|---|---|---|---|---|---|---|---|
| Reimplantation | Remodelling | |||||||
| Aicher (4) | 2010 | 83 | NR | NR | 3.44 | 0.16 | 1.2 | NR |
| Lansac (6) | 2010 | 58.4 | NR | 100 | 2.8 | 0.32 | 2.5 | 1.6 |
| Lansac (7) | 2006 | 6.2 | NR | 100 | 3.6 | NR | 4.2 | 0 |
| Boodhwani (8) | 2009 | † | NR | NR | 1.14 | 0.10 | 0 | 1.1 |
| DePaulis (9) | 2010 | 9 | 100 | NR | 1.80 | NR | 1.4 | 0.42 |
| Settapani (10) | 2009 | 5 | 100 | NR | 1.67 | NR | 3.5 | 1.4 |
| Urbanski (11) | 2010 | 9.4 | 1 | NR | 0 | NR | 0 | NR |
| Doss (12) | 2010 | 100 | 25.8 | NR | 0 | 0.30 | 0 | 3.0 |
| Badiu (13) | 2010 | 60.8 | 72.5 | 27.5 | 0.98 | NR | 2.4 | 0.40 |
| Cameron (14) | 2009 | NR | 53 | 47 | 0 | NR | 0.47 | 0.16 |
| Svensson (15) | 2010 | 42 | 100 | NR | 0 | 0.78 | 1.0 | 0.26 |
| David (16) | 2010 | 38.1 | 78.9 | 21.1 | 1.73 | 0.14 | 0.33 | 1.2 |
| Izumoto (17) | 2006 | 80 | NR | NR | 2.5 | NR | 3.5 | NR |
| Tanaka (18) | 2011 | 8.4 | 88.3 | 11.7 | 0 | NR | 0 | 1.5 |
| Oka (19) | 2011 | 50.5 | 99 | 1 | 0 | NR | 1.9 | 2.0 |
| Kallenbach (20) | 2005 | 6.3 | 100 | NR | 3.17 | 0.41 | 1.5 | 2.1 |
| Minakata (21) | 2004 | 100 | NR | NR | 0.63 | 0 | 2.4 | 2.4 |
†, tailored to specific etiology; ‡ composite endpoint: late AVR and re-repair; NR, not reported; pt-yr, patient-year; mort., mortality; OVE, operated valve endocarditis
Late outcomes and valve-related events
Data on late bleeding events was only available from two studies, and both of these reported no bleeding complications (7,19). Operated valve endocarditis was reported as an outcome in 8 studies (6,8,12,14-16,20,21), with a median event rate of 0.23%/pt-yr (range, 0-0.78%/pt-yr) (Table 2). The composite outcome measure of late neurological events and thromboembolism reported a rate of 0.52%/pt-yr (0-0.95%/pt-yr) (4,6-9,16,17,20,21). Late AV re-intervention requiring AV replacement or re-repair occurred at a rate of 2.4%/pt-yr (range, 0-4.2%/pt-yr) (4,6-21). The median freedom from AV re-intervention estimated from survival curves was 92% (range, 87-98%) at 5 years (8-10,13,16-19,21). The survival curve estimates of freedom from late recurrent AI >2+ was a median of 88% (range, 87-100%) at 5 years (4,8,12,13,16,19). Late mortality was pooled to produce a fixed effects summary estimate of 1.3%/pt-yr (95% CI: 0.9-2.1%/pt-yr, I2=0%) (6-10,14-16,18-21).
Discussion
Aortic valve preservation and repair is a promising alternative to aortic valve replacement in selected patients with aortic valve disease. Data on outcomes following aortic valve repair is largely limited to single center case series with variable duration of follow-up. In this systematic review, we identified a contemporary series of 17 such observational studies including over 2,800 patients from 16 centers. We found that only one study reported all outcomes as per guidelines for reporting valve related morbidity (2). In-hospital mortality following aortic valve repair was acceptable at 2.6%. The incidence of valve related complications was also low. Recurrent aortic valve insufficiency or stenosis requiring AV re-operation occurred at a median rate of 2.4%/pt-yr.
Systematic review of outcomes following aortic valve repair is clearly needed to inform decision making for patients with aortic valve disease who may be eligible for repair. However, the lack of randomized trials and non-randomized studies comparing aortic valve repair with replacement makes it difficult to define the role of repair in the management of aortic valve disease. While there is a wealth of data that exists on outcomes following aortic valve replacement, data on outcomes following aortic valve repair is limited to selected centers and surgeons. Combining data from these observational studies, although tempting, comes with important challenges. First, the patient population and the surgical techniques for aortic valve repair are continually evolving. Therefore, outcomes from older series may not reflect current practice. Second, the length of follow-up among studies is variable and often limited to a few years. As such, only a handful of studies may provide the much needed long-term data. Third, outcomes are often not reported according to standardized definitions from guidelines for reporting morbidity and mortality following valvular interventions. Fourth, there are occasionally multiple publications from the same center that involved overlapping cohorts of patients undergoing aortic valve repair. Lastly, meta-analytic techniques for failure-time data, which comprises the majority of outcomes following aortic valve repair, are infrequently used and are not well developed. This makes it difficult to quantitatively combine data to derive summary measures of outcome.
Despite these challenges, important messages regarding aortic valve repair can be derived from this systematic review. Data from over 2,800 patients confirms that aortic valve preservation and repair may be performed in experienced centers with a low operative mortality. Early morbidity and re-intervention rates are also low in the published literature despite the complexity of the surgical procedures with many patients requiring associated aortic root replacement and some presenting with acute aortic syndromes. An important reason for the low mortality and morbidity likely relates to the relatively young age and appropriate selection of patients. The median age of the patients included in this review was 54 years and very few patients (~5%) were undergoing re-operative surgery.
The mid- to long-term outcome following aortic valve repair appears to be acceptable. Five-year rates of freedom from reoperation are above 90%. There is, however, limited data on 10-year outcome. Extrapolating from an average linearized rate of re-operation of 2.4%/pt-yr, freedom from reoperation at 10-years is likely around 75-80%. This compares favourably to bio-prosthetic aortic valve replacement, which in this population, carries a median durability of approximately 8 to 10 years (i.e., 50% of bioprosthetic valves fail at approximately 10 years). The risk of re-operation is also higher with aortic valve repair versus mechanical valve replacement where the freedom from reoperation is typically over 90% at 10 years. It is also important to note that the rates of reoperation are highly variable between centers with linearized rates of less than 1% in some centers and close to 4% in others.
In contrast to prosthetic aortic valve replacement, however, the risk of thromboembolic complications was low at 0.5%/pt-yr. The linearized risk of thromboembolic complications following mechanical or biologic aortic valve replacement is estimated at 1-2%. It is reported to be higher following composite replacement of the aortic valve and root with a mechanical prosthesis, being as high as 10%/pt-yr in some series. This translates to a risk of close to 10-20% over 10 years in patients with mechanical valve replacement compared to approximately to 5% over 10 years with aortic valve repair.
Perhaps the most important issue related to outcomes following aortic valve repair is the generalizability of the findings. Aortic valve repair is still performed at certain specialized centers in selected patients and within those centers, only by a handful of surgeons. The published outcomes, thus, may not be generalized to all patients undergoing aortic valve repair. The published studies are also likely to include the learning curve of many surgeons and with improvements in techniques may overestimate the risk of reoperation.
In summary, this systematic review confirms the low operative risk of selected patients undergoing aortic valve preservation and repair in specialized centers. The risk of aortic valve re-intervention is acceptable and better than that expected with a bioprosthetic valve replacement. However, patients undergoing repair do carry a risk of reoperation in the long-term, which may reduce with improving surgical technique and better patient selection. The burden of thromboembolic and bleeding complications is substantially lower in patients undergoing repair compared to mechanical valve replacement. There is a need for long-term follow-up studies with meticulous reporting of outcomes following AV repair as well as comparative studies with aortic valve replacement.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Iung B, Baron G, Butchart EG, et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur Heart J 2003;24:1231-43 [DOI] [PubMed] [Google Scholar]
- 2.Carr JA, Savage EB. Aortic valve repair for aortic insufficiency in adults: a contemporary review and comparison with replacement techniques. Eur J Cardiothorac Surg 2004;25:6-15 [DOI] [PubMed] [Google Scholar]
- 3.Pibarot P, Dumesnil JG. Prosthetic Heart Valves: Selection of the Optimal Prosthesis and Long-Term Management. Circulation 2009;119:1034-48 [DOI] [PubMed] [Google Scholar]
- 4.Aicher D, Fries R, Rodionycheva S, et al. Aortic valve repair leads to a low incidence of valve-related complications. Eur J Cardiothorac Surg 2010;37:127-32 [DOI] [PubMed] [Google Scholar]
- 5.Akins CW, Miller DC, Turina MI, et al. Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;85:1490-5 [DOI] [PubMed] [Google Scholar]
- 6.Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring to standardise aortic valve repair: preliminary results of a prospective multicentric cohort of 144 patients. Eur J Cardiothorac Surg 2010;38:147-54 [DOI] [PubMed] [Google Scholar]
- 7.Lansac E, Di Centa I, Bonnet N, et al. Aortic prosthetic ring annuloplasty: a useful adjunct to a standardized aortic valve-sparing procedure. Eur J Cardiothorac Surg 2006;29:537-44 [DOI] [PubMed] [Google Scholar]
- 8.Boodhwani M, de Kerchove L, Glineur D, et al. Repair-oriented classification of aortic insufficiency: Impact on surgical techniques and clinical outcomes. J Thorac Cardiovasc Surg 2009;137:286-94 [DOI] [PubMed] [Google Scholar]
- 9.De Paulis R, Scaffa R, Nardella S, et al. Use of the Valsalva graft and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S23-7 [DOI] [PubMed] [Google Scholar]
- 10.Settepani F, Bergonzini M, Barbone A, et al. Reimplantation valve-sparing aortic root replacement with the Valsalva graft: what have we learnt after 100 cases? Interact Cardiovasc Thorac Surg 2009;9:113-6 [DOI] [PubMed] [Google Scholar]
- 11.Urbanski PP. Basal cusp enlargement for repair of aortic valve insufficiency. J Thorac Cardiovasc Surg 2010;139:98-102 [DOI] [PubMed] [Google Scholar]
- 12.Doss M, Risteski P, Sirat S, et al. Aortic root stability in bicuspid aortic valve disease: patch augmentation plus reduction aortoplasty versus modified David type repair. Eur J Cardiothorac Surg 2010;38:523-27 [DOI] [PubMed] [Google Scholar]
- 13.Badiu CC, Eichinger W, Bleiziffer S, et al. Should root replacement with aortic valve-sparing be offered to patients with bicuspid valves or severe aortic regurgitation? Eur J Cardiothorac Surg 2010;38:515-22 [DOI] [PubMed] [Google Scholar]
- 14.Cameron DE, Alejo DE, Patel ND, et al. Aortic root replacement in 372 Marfan patients: evolution of operative repair over 30 years. Ann Thorac Surg 2009;87:1344-9; discussion 1349-50 [DOI] [PubMed] [Google Scholar]
- 15.Svensson LG, Cooper M, Batizy LH, et al. Simplified David reimplantation with reduction of anular size and creation of artificial sinuses. Ann Thorac Surg 2010;89:1443-7 [DOI] [PubMed] [Google Scholar]
- 16.David TE, Maganti M, Armstrong S. Aortic root aneurysm: Principles of repair and long-term follow-up. J Thorac Cardiovasc Surg 2010;140:S14-9 [DOI] [PubMed] [Google Scholar]
- 17.Izumoto H, Kawazoe K, Oka T, et al. Aortic valve repair for aortic regurgitation: intermediate-term results in patients with tricuspid morphology. J Heart Valve Dis 2006;15:169-73; discussion 173 [PubMed] [Google Scholar]
- 18.Tanaka H, Ogino H, Matsuda H, et al. Midterm outcome of valve-sparing aortic root replacement in inherited connective tissue disorders. Ann Thorac Surg 2011;92:1646-9; discussion 1649-50 [DOI] [PubMed] [Google Scholar]
- 19.Oka T, Okita Y, Matsumori M, et al. Aortic regurgitation after valve-sparing aortic root replacement: modes of failure. Ann Thorac Surg 2011;92:1639-44 [DOI] [PubMed] [Google Scholar]
- 20.Kallenbach K, Karck M, Pak D, et al. Decade of aortic valve sparing reimplantation are we pushing the limits too far? Circulation 2005;112:I253-9 [DOI] [PubMed] [Google Scholar]
- 21.Minakata K, Schaff HV, Zehr KJ, et al. Is repair of aortic valve regurgitation a safe alternative to valve replacement? J Thorac Cardiovasc Surg 2004;127:645-53 [DOI] [PubMed] [Google Scholar]