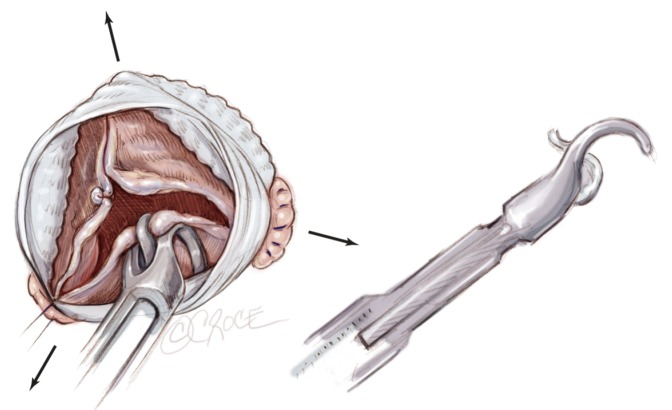
Introduction
A standardized approach to aortic valve repair is described associating a physiological remodeling of the root, with resuspension of cusp effective height and a subvalvular aortic annuloplasty using an external expansible ring. This device achieves a complete calibrated annuloplasty, in order to increase cups coaptation height and protect the repair.
This is a video-atlas describing the surgical steps of a physiological and standardized approach to aortic valve repair, to treat an aortic root aneurysm associated with grade III to IV eccentric aortic insufficiency on a 41-year old patient with a tricuspid aortic valve (Video 1). Ideal valve sparing root replacement procedure should treat dilatation of the aortic annular base, while preserving dynamics of the aortic root with vortices (neosinuses of Valsalva), root expansibility (interleaflet triangles) as well as restoring cusp coaptation (1-5).
Video 1.

Valve sparing root replacement: the remodeling technique with external ring annuloplasty
The two original valve sparing procedures - remodeling of the aortic root and reimplantation of the aortic valve - focused on root reconstruction to reduce the dilated root diameters in order to restore proper valve function (6,7). The reimplantation technique performs external subvalvular aortic annuloplasty but withdraws the sinuses of Valsalva and includes the interleaflet triangles within a graft tube, thus impairing root dynamics (8-11). In contrast, the remodeling technique provides more physiologic movements of the cusps within three reconstructed neo-sinuses, thus preserving root expansibility through the interleaflet triangles, but without addressing annular base dilation (8-13). In vitro and in vivo studies have documented that cusp motion and flow patterns across the reconstructed aortic root are more physiologic (I) after remodeling of the aortic root than after reimplantation of the aortic valve, and (II) after procedures using a prosthetic conduit fashioned with neo- sinuses of Valsalva than without (9-11). Numerous technical variations have aimed to associate preservation of aortic root dynamics with the treatment of dilated native annulus (14,15). This resulted in a lack of standardization and limited their widespread application.
Furthermore, most failures with valve sparing techniques are due to residual cusp prolapse, either as a primary unrecognized lesion or secondary to an induced prolapse after root reconstruction (12,16). Schäfers et al. proposed to address this issue with a dedicated caliper in order to restore cusp effective height up to 8-10 mm (1,4,12). Therefore we suggest a standardized approach of aortic valve repair addressing both the aorta and the valve, associating a physiological reconstruction of the aortic root according to the remodeling technique, with resuspension of cusp effective height and an expansible subvalvular ring annuloplasty (CAVIAAR technique) (Figure 1) (2,17-19).
Figure 1.
Remodeling of the aortic root associated to an external subvalvular aortic annuloplasty (CAVIAAR technique), combining advantages of the original remodeling and reimplantation techniques
To address the need for a dedicated aortic annuloplasty device, we designed a new expansible aortic ring in order to achieve a complete and calibrated annuloplasty in diastole, while maintaining systolic expansibility of the aortic root (Extra-Aortic™, CORONEO, Inc., Montreal, QC, Canada) (19). As such, cusp coaptation height is increased, reducing stress on the cusps and protecting the repair.
Operative techniques
Dissection of the subvalvular plane
The aneurysmal portion of the ascending aorta is resected and sectioned at the level of the sinotubular junction. External dissection of the aortic root is performed down to the base of the aortic annulus. It begins at the non-coronary sinus. Aortic root is then liberated from the pulmonary artery and infundibulum and from the roof of the left atrium, in order to reach the subvalvular plane. The wall of the aortic sinus is totally removed leaving a fringe of aortic wall of approximately 2 mm. The dissection is completed by freeing the sub-valvular plane and the pulmonary infundibulum. The commissures and coronary arteries are individualized.
Choice of expansible ring diameter is based on internal aortic annular base diameter measured with Hegar dilators (Table 1).
Table 1. Criteria for choice of the aortic ring and tube graft diameters.
| Aortic annular base Ø (Hegar dilators, mm) |
||||||
|---|---|---|---|---|---|---|
| 25-27 | 28-30 | 31-33 | 34-39 | >40 | ||
| Tube graft Ø (mm) | 26 | 28 | 30 | 32 | 34 | |
| Subvalvular aortic ring Ø (mm) | 25 | 27 | 29 | 31 | 33 | |
Ø, diameter
Placement of sub-valvular U-anchoring stitches for ring annuloplasty
Five threads of 2.0 coated polyester fiber pledgeted are placed from the inside out as “U” stitches (width of 3 mm) circumferentially in the subvalvular plane, clock-wise beginning from the non-coronary sinus. Three sutures are positioned 2 mm below the nadir of each cusp and two are placed at the base of the interleaflet triangles between the non and left coronary sinuses and between the left and right coronary sinuses (Figure 2). A sixth suture is passed from externally at the level of the interleaflet triangle between the right and non coronary sinus, without pinching inside in order to limit the risk of membranous septum or the bundle of His lesion.
Figure 2.
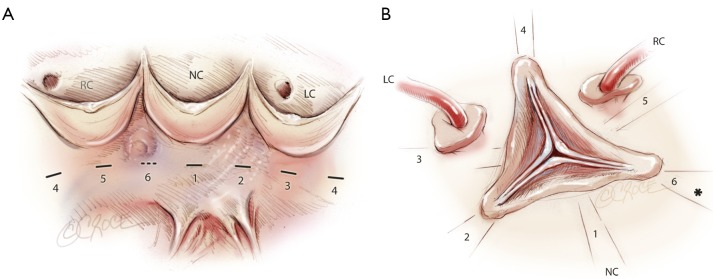
Placement of the five inside-out anchoring subvalvular “U” stitches (-), a sixth stitch is place externally (---). *Membranous septum; NC, non coronary; LC, left coronary; RC, right coronary
First step of aortic cusp repair
The aortic valves is carefully inspected. Pliable non retracted cusps are suitable for valve repair. The presence of an intact fenestration, a bicuspid valve or limited calcification is not a contraindication. As described by Schäfers et al., the geometric height of each cusp is measured with a ruler from the aortic insertion in the nadir of the sinus to the Nodulus of Arantius of the respective cusp. Cusps are considered as retracted if geometric height is below 17 mm in tricuspid aortic valves and below 20 mm in bicuspid valves (20).
A polypropylene 6/0 stay suture is passed through each noduli of Arantius. A grasper pulls outwards on the corresponding commissure while the two stitches at the level of the noduli of Arantia are retracted in the opposite direction. The excess length of free edge is then determined. Same step is performed for each hemi-cusp. Distance between the two stitches determines the area for the central plicating stitches to equalize each hemi-cusp free edge length (polypropylene 5 or 6/0-13) (Figure 3) (21).
Figure 3.
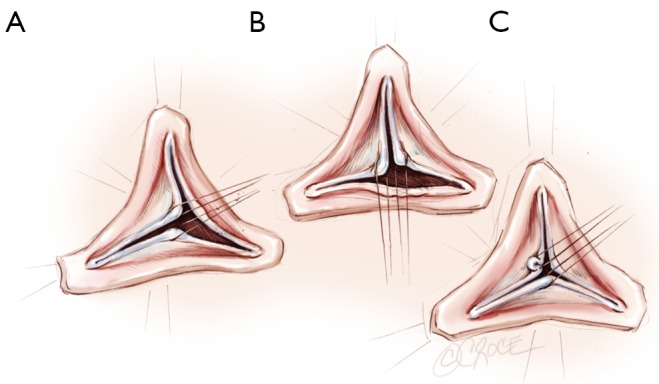
First step of aortic cusp repair: alignment of adjacent cusp free edges. Distance between the two stitches determines the area for the central plicating stitches to equalize each hemi-cusp
Tailoring of the Valsalva graft
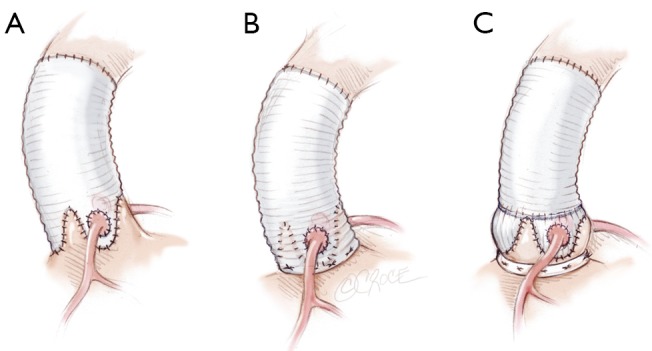
The Remodeling technique is performed using a bulged graft (Gelweave ValsalvaTM graft, Vascutek, Inchinnan, England). The distal collar below the skirt of the graft is resected. The graft is scalloped to have three symmetrical neosinuses using the linear demarcations on the bulging part of the graft. The heights of the scallops to suture the commissures are cut up to the transition point between circumferential and axial folds in the graft. The thickest pre-marked line is resected in order to facilitate the suturing of the prosthesis (Figure 4A).
Figure 4.
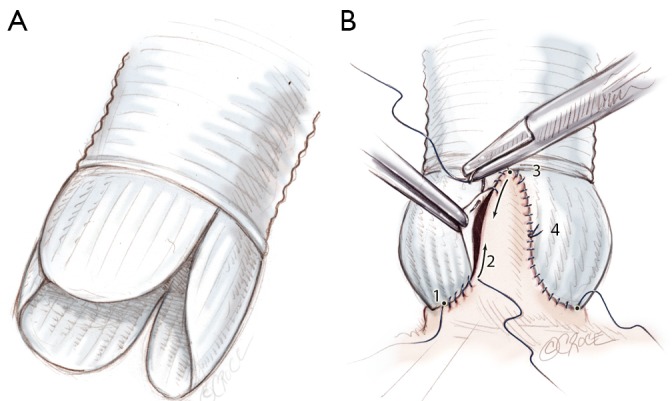
A. Tailoring the Valsalva graft into three symmetrical neosinuses; B. Standardized Remodeling of the aortic root step by step. Suture of the graft starts on the left coronary sinus at the nadir of the sinus (1) towards half of the sinus (2). Another running suture is begun at the tip of the commissure (3) towards corresponding hemi-sinus (4)
Remodeling of the aortic root, and suturing the prosthesis
The prosthesis is sutured following the sigmoid shape of the aortic annulus. Suture starts at the nadir of the left coronary sinus towards the commissure up to half of the sinus (Figure 4B). The first stitch is knotted at the base of the sinus, in order to facilitate suture tension. Stitches are passed very close to the annulus following leaflet insertion. Another running suture is begun at the tip of the commissure towards corresponding hemi-sinus. Right and non coronary sinuses are then sutured (Figure 4B). This technique allows a bulging aspect of the neosinuses of Valsalva and the preservation of the interleaflet triangles.
Second step for cusp repair: effective height resuspension
After remodeling of the aortic root is performed, commissural traction sutures are placed to mimic a pressurized aortic root in order to measure the effective height of each cusp (Figure 5A). A dedicated cusp caliper is used to evaluate any residual or induced cusp prolapse (Fehling Instruments, Karlstein, Germany) (Figure 5B) (1,4). Plicating stitches are added on the free edge of the culprit leaflet until an effective height of 9 mm is obtained.
Figure 5.
Second step for cusp repair: resuspension of cusp effective height using a caliper as described by Schäfers et al. (Fehling Instruments, Karlstein, Germany)
Aortic annuloplasty ring placement
The six anchoring “U” stitches are passed through the inner aspect of the prosthetic expansible aortic ring (Extra aortic, CORONEO Inc., Montreal, QC, Canada) (Figure 6A). The ring is then descended around the remodelled aortic root (Figure 6B). “U” stitches are tied to secure the ring in subvalvular position (Figure 6B).
Figure 6.
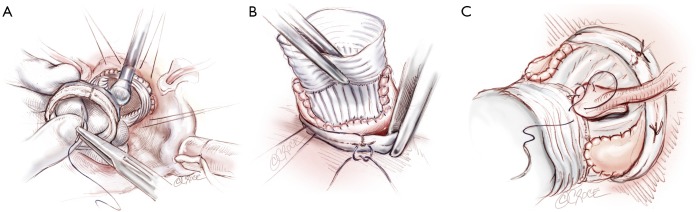
Placement of the subvalvular expansible aortic ring (A-B) and final aspect of the aortic root (C)
Coronary reimplantation and final inspection
The coronary ostia are then reimplanted according to the usual technique. The thicker part of the Gelweave graft can be cut to suture the coronaries ostia if they are ascensionned which is often the case for the right coronary button (Figure 6C). Distal anastomosis is performed using a 4-5/0 Prolene running suture in a standard fashion.
Transoesophageal echocardiography is performed after weaning from cardiopulmonary bypass when mean aortic pressure is above 70 mmHg. Any eccentric jet or aortic regurgitation (> grade 1) prompts further correction (12,16).
Comments
Compared to normal aortic diameters, dystrophic roots are characterized as a diameter disease, with almost a constant dilation of the aortic annular base (>25 mm) and/or the sino-tubular junction (>35 mm) diameters preventing coaptation of otherwise thin and pliable valves (2-3,12). Cusp prolapse is often associated (20). Recent reports evidenced that aortic annular base dilation (>25-28 mm) is a risk factor for long-term aortic valve repair failure, not only in aortic root aneurysms (12-13,22) but also in isolated aortic insufficiency (23).
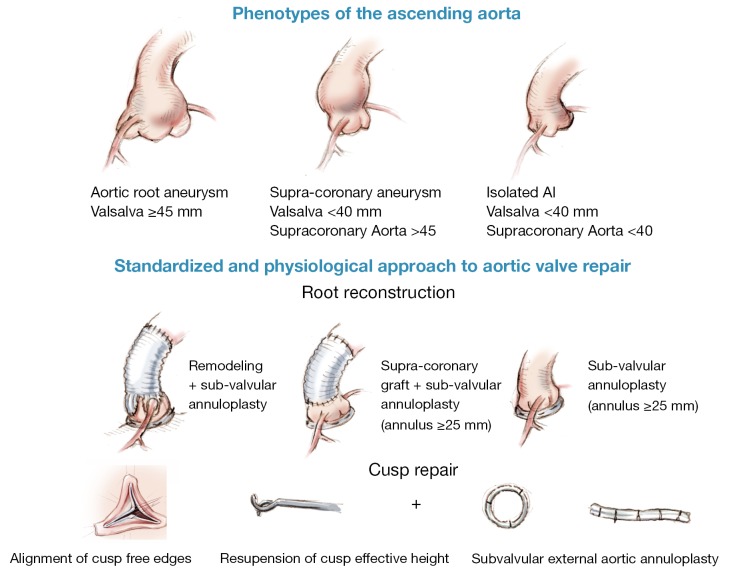
Dystrophic ascending aorta may be classified into three phenotypes: (I) aortic root aneurysms (sinuses of Valsalva >45 mm), (II) supra-coronary aneurysm (sinuses of Valsalva <40 mm); (III) isolated AI (all diameters <40 mm) (2). Depending on the phenotype of the ascending aorta, we propose a physiological and standardized approach to aortic valve repair. Reduction of the sinotubular junction diameter is achieved through a physiological reconstruction of the root according to the Remodeling technique (root aneurysm), or a supracoronary graft (supracoronary aneurysm). Effective height of each cusp is systematically assessed, free edge of the cusp is resuspended up to 9 mm. Expansible subvalvular annuloplasty is added when aortic annular base diameter is ≥25 mm using an external expansible aortic ring (2,17-19,24) (Figure 7).
Figure 7.
Standardized and physiological approach to aortic valve repair according to each phenotype of ascending aorta
As for mitral valve repair, aortic annuloplasty is gaining wider acceptance for aortic valve repair (19,25-29). Ideal aortic annuloplasty should ensure good haemodynamic function, biocompatibility, stability of aortic annular base diameter reduction and be easy to implant without interference with the conduction system or cusp tissue. Although plicating stitches are fast and easy to perform they impair valve dynamics and induce a low amount of annular reduction. It produces a partial annuloplasty and results in only a minimal reduction in diameter of the aortic annular base (23-26,30).
Controversy remains between external and internal annuloplasty rings (24-26). Although subvalvular plane in the right coronary sinus is easier to reach with internal rings, endovascular placement may interfere with cusp mobility and increase risks for hemolytic or thromboembolic events. Advantage of a ring placed externally is to prevent these complications and avoids placing tension on the device’s fixation stitches by the expanding aorta.
Aortic annuloplasty combined with resuspension of cusp effective height are key steps for a reproducible aortic valve repair. Further clinical evaluation of current devices will help to define the most standardized and safe way to perform an aortic annuloplasty.
Acknowledgements
Disclosure: Emmanuel Lansac discloses Consultancy Agreements with the company CORONEO, Inc.
References
- 1.Bierbach BO, Aicher D, Issa OA, et al. Aortic root and cusp configuration determine aortic valve function. Eur J Cardiothorac Surg 2010;38:400-6 [DOI] [PubMed] [Google Scholar]
- 2.Lansac E, Di Centa I, Raoux F, et al. A lesional classification to standardize surgical management of aortic insufficiency towards valve repair. Eur J Cardiothorac Surg 2008;33:872-8; discussion 878-80 [DOI] [PubMed] [Google Scholar]
- 3.El Khoury G, Glineur D, Rubay J, et al. Functional classification of aortic root/valve abnormalities and their correlation with etiologies and surgical procedures. Curr Opin Cardiol 2005;20:115-21 [DOI] [PubMed] [Google Scholar]
- 4.Schäfers HJ, Bierbach B, Aicher D. A new approach to the assessment of aortic cusp geometry. J Thorac Cardiovasc Surg 2006;132:436-8 [DOI] [PubMed] [Google Scholar]
- 5.Sutton JP, 3rd, Ho SY, Anderson RH. The forgotten interleaflet triangles: a review of the surgical anatomy of the aortic valve. Ann Thorac Surg 1995;59:419-27 [DOI] [PubMed] [Google Scholar]
- 6.Yacoub MH, Gehle P, Chandrasekaran V, et al. Late results of a valve-preserving operation in patients with aneurysms of the ascending aorta and root. J Thorac Cardiovasc Surg 1998;115:1080-90 [DOI] [PubMed] [Google Scholar]
- 7.David TE, Armstrong S, Maganti M, et al. Long-term results of aortic valve-sparing operations in patients with Marfan syndrome. J Thorac Cardiovasc Surg 2009;138:859-64; discussion 863-4 [DOI] [PubMed] [Google Scholar]
- 8.De Paulis R, De Matteis GM, Nardi P, et al. Opening and closing characteristics of the aortic valve after valve-sparing procedures using a new aortic root conduit. Ann Thorac Surg 2001;72:487-94 [DOI] [PubMed] [Google Scholar]
- 9.Fries R, Graeter T, Aicher D, et al. In vitro comparison of aortic valve movement after valve-preserving aortic replacement. J Thorac Cardiovasc Surg 2006;132:32-7 [DOI] [PubMed] [Google Scholar]
- 10.Soncini M, Votta E, Zinicchino S, et al. Aortic root performance after valve sparing procedure: a comparative finite element analysis. Med Eng Phys 2009;31:234-43 [DOI] [PubMed] [Google Scholar]
- 11.Hanke T, Charitos EI, Stierle U, et al. Factors associated with the development of aortic valve regurgitation over time after two different techniques of valve-sparing aortic root surgery. J Thorac Cardiovasc Surg 2009;137:314-9 [DOI] [PubMed] [Google Scholar]
- 12.Kunihara T, Aicher D, Rodionycheva S, et al. Preoperative aortic root geometry and postoperative cusp configuration primarily determine long-term outcome after valve-preserving aortic root repair. J Thorac Cardiovasc Surg 2012;143:1389-95 [DOI] [PubMed] [Google Scholar]
- 13.Luciani GB, Casali G, Tomezzoli A, et al. Recurrence of aortic insufficiency after aortic root remodeling with valve preservation. Ann Thorac Surg 1999;67:1849-52; discussion 1853-6. [DOI] [PubMed]
- 14.Svensson LG, Cooper M, Batizy LH, et al. Simplified david reimplantation with reduction of anular size and creation of artificial sinuses. Ann Thorac Surg 2010;89:1443-7 [DOI] [PubMed] [Google Scholar]
- 15.Bakhtiary F, Monsefi N, Trendafilow M, et al. Modification of the David procedure for reconstruction of incompetent bicuspid aortic valves. Ann Thorac Surg 2009;88:2047-9 [DOI] [PubMed] [Google Scholar]
- 16.le Polain de Waroux JB, Pouleur AC, Robert A, et al. Mechanisms of recurrent aortic regurgitation after aortic valve repair: predictive value of intraoperative transesophageal echocardiography. JACC Cardiovasc Imaging 2009;2:931-9 [DOI] [PubMed] [Google Scholar]
- 17.Lansac E, Di Centa I, Bonnet N, et al. Aortic prosthetic ring annuloplasty: a useful adjunct to a standardized aortic valve-sparing procedure? Eur J Cardiothorac Surg 2006;29:537-44 [DOI] [PubMed] [Google Scholar]
- 18.Lansac E, Di Centa I, Sleilaty G, et al. An aortic ring: from physiologic reconstruction of the root to a standardized approach for aortic valve repair. J Thorac Cardiovasc Surg 2010;140:S28-35; discussion S45-51. [DOI] [PubMed]
- 19.Lansac E, Di Centa I, Raoux F, et al. An expansible aortic ring for a physiological approach to conservative aortic valve surgery. J Thorac Cardiovasc Surg 2009;138:718-24 [DOI] [PubMed] [Google Scholar]
- 20.Schäfers HJ, Schmied W, Marom G, et al. Cusp height in aortic valves. J Thorac Cardiovasc Surg. 2012 doi: 10.1016/j.jtcvs.2012.06.053. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Boodhwani M, de Kerchove L, Watremez C, et al. Assessment and repair of aortic valve cusp prolapse: implications for valve-sparing procedures. J Thorac Cardiovasc Surg 2011;141:917-25 [DOI] [PubMed] [Google Scholar]
- 22.Marom G, Haj-Ali R, Rosenfeld M, et al. Aortic root numeric model: Annulus diameter prediction of effective height and coaptation in post-aortic valve repair. J Thorac Cardiovasc Surg 2013;145:406-411.e1. [DOI] [PubMed]
- 23.Aicher D, Kunihara T, Abou Issa O, et al. Valve configuration determines long-term results after repair of the bicuspid aortic valve. Circulation 2011;123:178-85 [DOI] [PubMed] [Google Scholar]
- 24.Lansac E, Di Centa I. From dynamic anatomy to aortic annuloplasty: the tale of the ring. In: Yankah CA, Weng Y, Hetzer R. eds. Aortic Root Surgery: The biological Solution. Berlin Heigdelberg: Springer-Verlag, 2010:102-32. [Google Scholar]
- 25.Schäfers HJ. Aortic annuloplasty: a new aspect of aortic valve repair. Eur J Cardiothorac Surg 2012;41:1124-5 [DOI] [PubMed] [Google Scholar]
- 26.de Kerchove L, Vismara R, Mangini A, et al. In vitro comparison of three techniques for ventriculo-aortic junction annuloplasty. Eur J Cardiothorac Surg 2012;41:1117-23; discussion 1123-4 [DOI] [PubMed] [Google Scholar]
- 27.Rankin JS, Beavan LA, Cohn WE. Technique for aortic valve annuloplasty using an intra-annular “hemispherical” frame. J Thorac Cardiovasc Surg 2011;142:933-6 [DOI] [PubMed] [Google Scholar]
- 28.Fattouch K, Sampognaro R, Speziale G, et al. New technique for aortic valve functional annulus reshaping using a handmade prosthetic ring. Ann Thorac Surg 2011;91:1154-8 [DOI] [PubMed] [Google Scholar]
- 29.Hahm SY, Choo SJ, Lee JW, et al. Novel technique of aortic valvuloplasty. Eur J Cardiothorac Surg 2006;29:530-6 [DOI] [PubMed] [Google Scholar]
- 30.Mangini A, Lemma MG, Soncini M, et al. The aortic interleaflet triangles annuloplasty: a multidisciplinary appraisal. Eur J Cardiothorac Surg 2011;40:851-7 [DOI] [PubMed] [Google Scholar]