Abstract
A newly identified human locus on chromosome 15 was recently associated with zinc accumulation. Based on a prior report of a threefold difference in zinc accumulation between fumble1 heterozygous mutants and control fly strains, it was suggested that phosphopantothenoylcysteine decarboxylase might affect zinc status through its effects on vitamin B5 (pantothenate) metabolism. We report here that outcrossed fumble1 heterozygous mutant flies with low zinc content have been recovered, suggesting that pantothenate metabolism did not alter zinc homeostasis in fumble1 heterozygous flies. We show instead that the Drosophila condition of low body zinc accumulation is an X-chromosome-linked recessive trait.
Keywords: Zinc deficiency, Genome wide association study, Sex-linked mutation, Vitamin B5, Pantothenate kinase, PKAN
Highlights
-
•
Flies with a threefold reduction in zinc accumulation remain viable and fertile.
-
•
There is no causal association between zinc accumulation and fly pantothenate kinase mutants.
-
•
A widespread X-linked mutation causes a threefold reduction in zinc accumulation in Drosophila.
1. Introduction
Zinc serves as a co-factor for many protein families with diverse functions; notable examples are hydrolases and zinc-finger transcription factors [1]. A role for zinc in enzyme catalysis was first shown for carbonic anhydrase [2,3] and in DNA binding for transcription factor IIIA of Xenopus [4–6]. Zinc accumulates in pancreatic granules [7–9] and mossy fibers of the hippocampus [10–12], where its function remains to be established [13–16]. Variations in intracellular concentrations of free zinc are thought to contribute to cell signaling [17,18]. Zinc deficiency is widespread in humans and can lead to growth retardation, hypogonadism in males, rough skin, impaired immunity and neurological defects [19]. Therefore, it was surprising to discover Drosophila melanogaster strains raised under the same (zinc replete) dietary conditions and bearing a threefold difference in their total body zinc content had no obvious phenotypic defects [20].
A few aspects of zinc homeostasis have been studied in Drosophila, including functional studies of zinc transporters [21–25], of the zinc-responsive metal transcription factor-1 (MTF1) [26–29] and of metallothioneins [30–33], which are under MTF1 control. In addition, the neurotoxic properties of zinc in neurodegenerative disease have been demonstrated in models of Alzheimer's disease [34,35], Parkinson's disease [36,37], as well as in activating apoptosis in Drosophila hemocytes [38]. Moreover, a study of the global transcriptional response to dietary zinc has been published [39].
In our studies of metal determinations on laboratory strains from diverse genetic backgrounds we consistently found total body zinc values below a threshold of 100 mg Zn/g dry body weight [40–42]. In contrast, two loss-of-function fumble (fbl) mutant strains (a P-element insertion and a deficiency) had zinc accumulated at 200 mg/g dry body weight [20]. As fbl encodes for pantothenate kinase [43], the first enzyme involved in the metabolism of Vitamin B5, which is a precursor of coenzyme A, our initial hypothesis was that intermediary metabolism might affect zinc homeostasis in some way. This idea was recently noted by investigators who performed a genome wide association study in humans for loci affecting, amongst other elements, zinc accumulation in the blood [44]. A gene encoding phosphopantothenoylcysteine decarboxylase was present at a chromosome 15 locus associated with changes in zinc accumulation [44]. The human study prompted us to expedite the present report, because we have in the meantime refuted our original hypothesis associating fbl heterozygosity with zinc accumulation. Indeed, when we measured metal composition of the different Drosophila species the values determined for zinc were on the range of 200 mg/g dry body weight [45], which we now show to be the range of zinc accumulation also for wild type D. melanogaster. We report that a recessive X-linked mutation causes a threefold reduction of total body zinc accumulation in many D. melanogaster laboratory strains. Our results are not only pertinent for the community of metal biologists that use Drosophila, but could be significant in experiments involving zinc transcription factors and other zinc-containing proteins in the fruit fly.
2. Materials and methods
2.1. Fly maintenance
D. melanogaster were reared at 25 °C on a standard diet containing: agar (6.5%), sucrose (9.7%), glucose (21.3%), yeast (22.6%), maize (9.7%), treacle (19.3%), soya flour (4.6%), propionic acid (0.5%) and nipagin (0.01%). The fbl1 stock was obtained from Bloomington Drosophila Stock Center at Indiana University (#11777). The wild type strain we used was collected by Rudi Costa from Tannes, Italy and termed Tan3 [45]. Balancer strains and white mutants were from our core lab stocks.
2.2. Flame atomic absorption spectrometry
The metal concentration of zinc in flies was determined by flame atomic absorption spectrometry. With a single exception mentioned in the text, male and females flies were used in combination and 100 mg dry mass was typically collected for each biological replicate. For all experiments shown we used 5 biological replicates. 4- to 7-day old flies were collected, fast-frozen in liquid nitrogen and stored at −80 °C. Samples were freeze-dried for 24 h and their dry mass was measured. Dried flies (100 mg) were acid digested by adding 1.5 ml of 69% nitric acid (HNO3) at 50 °C for 4 h, then at 100 °C for another 4 h, followed by overnight cooling down. Acid-digested samples were diluted with distilled water and the metal content was determined by using an AAnalyst 200 Flame Atomic Absorption Spectrophotometer (Varian Ltd., Yarnton, Oxfordshire, UK). Standards of each metal were used to calibrate the spectrophotometer and calculate metal concentrations in all samples.
3. Results and discussion
In a survey of elemental composition of some of our stocks, we were surprised to discover that a rebalanced fbl1/TM6, Tb stock – generated during our previous study of this mutant's survival rate to the pupal stage of development [20] – accumulated a low amount of zinc (Fig. 1A). This observation suggested that fbl1 was not involved in zinc accumulation. Low zinc was a feature of all other lab stocks we tested, but a wild type reference stock termed Tan3 [45] accumulated threefold more zinc, similar to the original fbl1/TM3 (Fig. 1A). Zinc accumulation in wild type Tan3 flies was consistent with values observed in many other Drosophila species [45]. Our first attempt to explain the new findings was to test for the presence of maternal factors that could influence metal homeostasis, such as the presence of Wolbachia endosymbionts [46–48]. However, crossing of low zinc female fbl1/TM6 to high/normal zinc male fbl1/TM3 flies and exchanging the balancers resulted in new stocks with high/normal zinc accumulation (data not shown). This result meant that (i) the trait of “low zinc accumulation” was not due to a maternal factor, as low zinc fbl1/TM6 females did not transmit it to their progeny, (ii) the 3rd chromosome was not determining zinc concentration in the flies, as the same combination of 3rd chromosomes could result in different zinc accumulations and (iii) the trait of low zinc was recessive, as it disappeared when the two phenotypes were crossed against each other; this was confirmed in the reverse cross of high/normal zinc female fbl1/TM3 to low zinc male fbl1/TM6 flies.
Fig. 1.
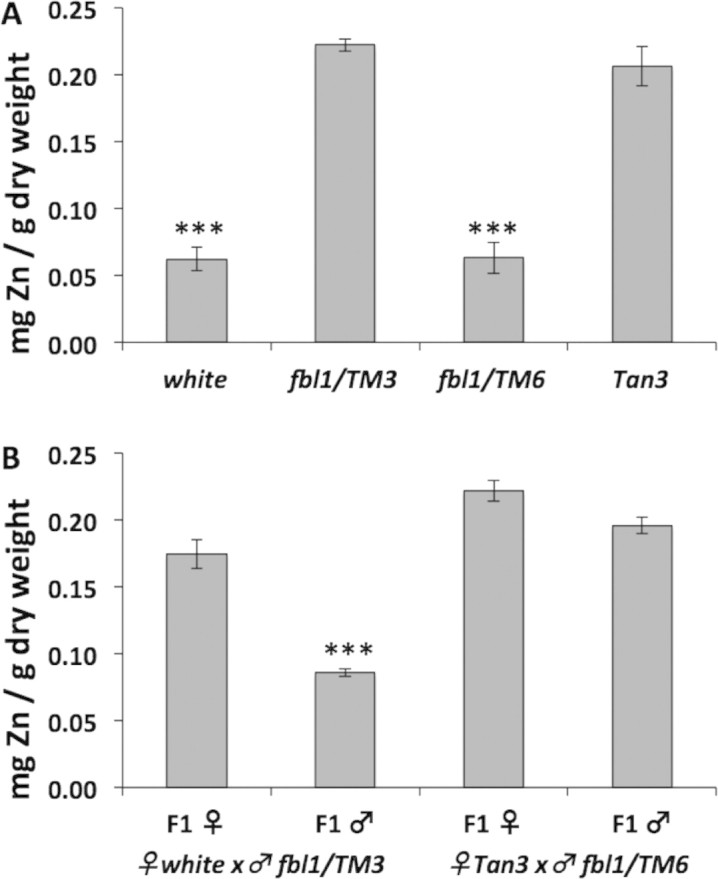
Zinc content in different genotypes of Drosophila melanogaster. (A) Zinc content was measured by atomic absorption spectrometry. white and fbl1, ry506/TM3, ryRK, Sb, Ser (abbreviated fbl1/TM3 throughout the text) were the same stocks as in [20], whereas metal content of fbl1, ry506/TM6, Tb (abbreviated fbl1/TM6, Tb throughout the text) flies was assessed for the first time here. Note that fbl1 heterozygosity does not correlate with total body zinc accumulation. Analysis of Variance indicated significant differences between the genotypes with p < 0.001. (B) Zinc content measured in female and male progeny derived from indicated crosses. Note that male progeny derived from low zinc mothers is also low in zinc, suggesting that the maternal X-chromosome is responsible for the low zinc phenotype. Analysis of Variance indicated significant differences between samples with p < 0.001.
After excluding, by use of appropriate balancers, a role for the 2nd chromosome, we tested if a recessive mutation on the X-chromosome could be causing the low zinc phenotype. For this experiment, we separated the flies by sex prior to determining the metal concentration, because females would serve as heterozygous controls, whereas males would inherit the X-chromosome from their mothers. Indeed, all male progeny collected from low zinc white mothers crossed to high/normal zinc fbl1/TM3 fathers were low in zinc (Fig. 1B). As expected from the recessive nature of the mutation we were following, all female progeny from the same cross were high/normal in zinc accumulation. Finally, when male progeny was derived from high/normal zinc Tan3 females crossed to low zinc fbl1/TM6, Tb males they were high/normal in zinc themselves (Fig. 1B). Therefore, we can conclude that a recessive X-linked mutation causes a threefold reduction of total body zinc accumulation in many D. melanogaster laboratory strains.
Although efforts in mapping the mutation are ongoing, we felt that raising awareness of the presence of this mutation was pertinent in order (i) to correct the assumption we previously published that pantothenate kinase was associated with zinc homeostasis [20], (ii) to avoid misleading efforts to map human genes involved in zinc homeostasis [44] (it is interesting to note that an X-chromosome linked locus affecting zinc homeostasis in humans was also detected in this study), (iii) to inform on the choice of control flies, as the previously unrecognized variation may have influenced other studies, including the suggestion that TRPM channels transport zinc [49,50] and (iv) to highlight the ability of fruit flies to survive in the lab with threefold less zinc, a finding that begs an answer on where the excess zinc may be stored and how zinc is distributed (preferentially or not) to target destinations in wild type flies.
Acknowledgments
We thank Anuja Mehta for critical reading of the manuscript and Helge Uhrigshardt for the on-line graphic to highlight this paper. This work was supported financially by the Marie Curie International Reintegration Grant “DrosoFela” (MIRG-CT-2007-204832) and the Consejo Nacional de Ciencia y Tecnologia project grant (#179835). L.B. received a Leonardo da Vinci training placement at Queen Mary University of London from the Universitá degli Studi di Ferrara, Italy. Funding bodies were not involved in the writing of this article and did not interfere with any scientific aspect of this work.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Maret W. Zinc and the zinc proteome. Met. Ions Life Sci. 2013;12:479–501. doi: 10.1007/978-94-007-5561-1_14. [DOI] [PubMed] [Google Scholar]
- 2.Hove E., Elvehjem C.A., Hart E.B. The relation of zinc to carbonic anhydrase. J. Biol. Chem. 1940;136:425–434. [Google Scholar]
- 3.Keilin D., Mann T. Carbonic anhydrase. Purification and nature of the enzyme. Biochem. J. 1940;34:1163–1176. doi: 10.1042/bj0341163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hanas J.S., Hazuda D.J., Bogenhagen D.F., Wu F.Y., Wu C.W. Xenopus transcription factor A requires zinc for binding to the 5 S RNA gene. J. Biol. Chem. 1983;258:14120–14125. [PubMed] [Google Scholar]
- 5.Brown R.S., Sander C., Argos P. The primary structure of transcription factor TFIIIA has 12 consecutive repeats. FEBS Lett. 1985;186:271–274. doi: 10.1016/0014-5793(85)80723-7. [DOI] [PubMed] [Google Scholar]
- 6.Miller J., McLachlan A.D., Klug A. Repetitive zinc-binding domains in the protein transcription factor IIIA from Xenopus oocytes. EMBO J. 1985;4:1609–1614. doi: 10.1002/j.1460-2075.1985.tb03825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okamoto K., Kawanishi H. Submicroscopic histochemical demonstration of intracellular reactive zinc in beta cells of pancreatic islets. Endocrinol. Jpn. 1966;13:305–318. doi: 10.1507/endocrj1954.13.305. [DOI] [PubMed] [Google Scholar]
- 8.Pihl E. An ultrastructural study of the distribution of heavy metals in the pancreatic islets as revealed by the sulfide silver method. Acta Pathol. Microbiol. Scand. 1968;74:145–160. doi: 10.1111/j.1699-0463.1968.tb03466.x. [DOI] [PubMed] [Google Scholar]
- 9.Westmoreland N., Hoekstra W.G. Ultrastructural localization of zinc in rat exocrine pancreas by autoradiography. Histochemie. 1969;18:261–266. doi: 10.1007/BF00306172. [DOI] [PubMed] [Google Scholar]
- 10.McLardy T. Pathological zinc-rich synapses. Nature. 1962;195:1315–1316. doi: 10.1038/1951315a0. [DOI] [PubMed] [Google Scholar]
- 11.Haug F.M. Electron microscopical localization of the zinc in hippocampal mossy fibre synapses by a modified sulfide silver procedure. Histochemie. 1967;8:355–368. doi: 10.1007/BF00401978. [DOI] [PubMed] [Google Scholar]
- 12.Ibata Y., Otsuka N. Electron microscopic demonstration of zinc in the hippocampal formation using Timm's sulfide silver technique. J. Histochem. Cytochem. 1969;17:171–175. doi: 10.1177/17.3.171. [DOI] [PubMed] [Google Scholar]
- 13.Maret W. Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv. Nutr. 2013;4:82–91. doi: 10.3945/an.112.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hennigar S.R., Kelleher S.L. Zinc networks: the cell-specific compartmentalization of zinc for specialized functions. Biol. Chem. 2012;393:565–578. doi: 10.1515/hsz-2012-0128. [DOI] [PubMed] [Google Scholar]
- 15.Takeda A., Nakamura M., Fujii H., Tamano H. Synaptic Zn(2+) homeostasis and its significance. Metallomics. 2013;5:417–423. doi: 10.1039/c3mt20269k. [DOI] [PubMed] [Google Scholar]
- 16.Sekler I., Silverman W.F. Zinc homeostasis and signaling in glia. Glia. 2012;60:843–850. doi: 10.1002/glia.22286. [DOI] [PubMed] [Google Scholar]
- 17.Taylor K.M., Hiscox S., Nicholson R.I., Hogstrand C., Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 2012;5 doi: 10.1126/scisignal.2002585. ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson M., Hogstrand C., Maret W. Picomolar concentrations of free zinc(II) ions regulate receptor protein–tyrosine phosphatase beta activity. J. Biol. Chem. 2012;287:9322–9326. doi: 10.1074/jbc.C111.320796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012;26:66–69. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez L., Sabaratnam N., Aktar R., Bettedi L., Mandilaras K., Missirlis F. Zinc accumulation in heterozygous mutants of fumble, the pantothenate kinase homologue of Drosophila. FEBS Lett. 2010;584:2942–2946. doi: 10.1016/j.febslet.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 21.Lye J.C., Richards C.D., Dechen K., Warr C.G., Burke R. In vivo zinc toxicity phenotypes provide a sensitized background that suggests zinc transport activities for most of the Drosophila Zip and ZnT genes. J. Biol. Inorg. Chem. 2013;18:323–332. doi: 10.1007/s00775-013-0976-6. [DOI] [PubMed] [Google Scholar]
- 22.Lye J.C., Richards C.D., Dechen K., Paterson D., de Jonge M.D., Howard D.L., Warr C.G., Burke R. Systematic functional characterization of putative zinc transport genes and identification of zinc toxicosis phenotypes in Drosophila melanogaster. J. Exp. Biol. 2012;215:3254–3265. doi: 10.1242/jeb.069260. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Zhou B. Dietary zinc absorption: a play of Zips and ZnTs in the gut. IUBMB Life. 2010;62:176–182. doi: 10.1002/iub.291. [DOI] [PubMed] [Google Scholar]
- 24.Wang X., Wu Y., Zhou B. Dietary zinc absorption is mediated by ZnT1 in Drosophila melanogaster. FASEB J. 2009;23:2650–2661. doi: 10.1096/fj.08-126649. [DOI] [PubMed] [Google Scholar]
- 25.Groth C., Sasamura T., Khanna M.R., Whitley M., Fortini M.E. Protein trafficking abnormalities in Drosophila tissues with impaired activity of the ZIP7 zinc transporter Catsup. Development. 2013;149:3018–3027. doi: 10.1242/dev.088336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egli D., Selvaraj A., Yepiskoposyan H., Zhang B., Hafen E., Georgiev O., Schaffner W. Knockout of ‘metal-responsive transcription factor’ MTF-1 in Drosophila by homologous recombination reveals its central role in heavy metal homeostasis. EMBO J. 2003;22:100–108. doi: 10.1093/emboj/cdg012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sims H.I., Chirn G.W., Marr M.T., 2nd Single nucleotide in the MTF-1 binding site can determine metal-specific transcription activation. Proc. Natl. Acad. Sci. U.S.A. 2012;109:16516–16521. doi: 10.1073/pnas.1207737109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahadorani S., Mukai S., Egli D., Hilliker A.J. Overexpression of metal-responsive transcription factor (MTF-1) in Drosophila melanogaster ameliorates life-span reductions associated with oxidative stress and metal toxicity. Neurobiol. Aging. 2010;31:1215–1226. doi: 10.1016/j.neurobiolaging.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Gunther V., Lindert U., Schaffner W. The taste of heavy metals: gene regulation by MTF-1. Biochim. Biophys. Acta. 2012;1823:1416–1425. doi: 10.1016/j.bbamcr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Egli D., Yepiskoposyan H., Selvaraj A., Balamurugan K., Rajaram R., Simons A., Multhaup G., Mettler S., Vardanyan A., Georgiev O., Schaffner W. A family knockout of all four Drosophila metallothioneins reveals a central role in copper homeostasis and detoxification. Mol. Cell. Biol. 2006;26:2286–2296. doi: 10.1128/MCB.26.6.2286-2296.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Egli D., Domenech J., Selvaraj A., Balamurugan K., Hua H., Capdevila M., Georgiev O., Schaffner W., Atrian S. The four members of the Drosophila metallothionein family exhibit distinct yet overlapping roles in heavy metal homeostasis and detoxification. Genes Cells. 2006;11:647–658. doi: 10.1111/j.1365-2443.2006.00971.x. [DOI] [PubMed] [Google Scholar]
- 32.Atanesyan L., Gunther V., Celniker S.E., Georgiev O., Schaffner W. Characterization of MtnE, the fifth metallothionein member in Drosophila. J. Biol. Inorg. Chem. 2011;16:1047–1056. doi: 10.1007/s00775-011-0825-4. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Rafael S., Kurz A., Guirola M., Capdevila M., Palacios O., Atrian S. Is MtnE, the fifth Drosophila metallothionein, functionally distinct from the other members of this polymorphic protein family? Metallomics. 2012;4:342–349. doi: 10.1039/c2mt00182a. [DOI] [PubMed] [Google Scholar]
- 34.Lang M., Wang L., Fan Q., Xiao G., Wang X., Zhong Y., Zhou B. Genetic inhibition of solute-linked carrier 39 family transporter 1 ameliorates abeta pathology in a Drosophila model of Alzheimer's disease. PLoS Genet. 2012;8:e1002683. doi: 10.1371/journal.pgen.1002683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hua H., Munter L., Harmeier A., Georgiev O., Multhaup G., Schaffner W. Toxicity of Alzheimer's disease-associated Abeta peptide is ameliorated in a Drosophila model by tight control of zinc and copper availability. Biol. Chem. 2011;392:919–926. doi: 10.1515/BC.2011.084. [DOI] [PubMed] [Google Scholar]
- 36.Saini N., Georgiev O., Schaffner W. The parkin mutant phenotype in the fly is largely rescued by metal-responsive transcription factor (MTF-1) Mol. Cell. Biol. 2011;31:2151–2161. doi: 10.1128/MCB.05207-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saini N., Schaffner W. Zinc supplement greatly improves the condition of parkin mutant Drosophila. Biol. Chem. 2010;391:513–518. doi: 10.1515/BC.2010.052. [DOI] [PubMed] [Google Scholar]
- 38.Filipiak M., Tylko G., Pyza E. Zinc induces caspase-dependent mitochondrial pathway of the programmed cell death in haemocytes of Drosophila melanogaster. Biometals. 2012;25:507–516. doi: 10.1007/s10534-012-9530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yepiskoposyan H., Egli D., Fergestad T., Selvaraj A., Treiber C., Multhaup G., Georgiev O., Schaffner W. Transcriptome response to heavy metal stress in Drosophila reveals a new zinc transporter that confers resistance to zinc. Nucleic Acids Res. 2006;34:4866–4877. doi: 10.1093/nar/gkl606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bettedi L., Aslam M.F., Szular J., Mandilaras K., Missirlis F. Iron depletion in the intestines of Malvolio mutant flies does not occur in the absence of a multicopper oxidase. J. Exp. Biol. 2011;214:971–978. doi: 10.1242/jeb.051664. [DOI] [PubMed] [Google Scholar]
- 41.Freeman A.A., Mandilaras K., Missirlis F., Sanyal S. An emerging role for Cullin-3 mediated ubiquitination in sleep and circadian rhythm: insights from Drosophila. Fly. 2013;7:39–43. doi: 10.4161/fly.23506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez L., Zubow K., Nield J., Gambis A., Mollereau B., Lazaro F.J., Missirlis F. Biophysical and genetic analysis of iron partitioning and ferritin function in Drosophila melanogaster. Metallomics. 2013;;5:997–1005. doi: 10.1039/c3mt00118k. [DOI] [PubMed] [Google Scholar]
- 43.Afshar K., Gonczy P., DiNardo S., Wasserman S.A. fumble encodes a pantothenate kinase homolog required for proper mitosis and meiosis in Drosophila melanogaster. Genetics. 2001;157:1267–1276. doi: 10.1093/genetics/157.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Evans D.M., Zhu G., Dy V., Heath A.C., Madden P.A., Kemp J.P., McMahon G., St Pourcain B., Timpson N.J., Golding J., Lawlor D.A., Steer C., Montgomery G.W., Martin N.G., Smith G.D., Whitfield J.B. Genome-Wide Association Study identifies loci affecting blood copper, selenium and zinc. Hum. Mol. Genet. 2013 doi: 10.1093/hmg/ddt239. http://dx.doi.org/10.1093/hmg/ddt239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sadraie M., Missirlis F. Evidence for evolutionary constraints in Drosophila metal biology. Biometals. 2011;24:679–686. doi: 10.1007/s10534-011-9420-y. [DOI] [PubMed] [Google Scholar]
- 46.Kremer N., Voronin D., Charif D., Mavingui P., Mollereau B., Vavre F. Wolbachia interferes with ferritin expression and iron metabolism in insects. PLoS Pathog. 2009;5:e1000630. doi: 10.1371/journal.ppat.1000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brownlie J.C., Cass B.N., Riegler M., Witsenburg J.J., Iturbe-Ormaetxe I., McGraw E.A., O’Neill S.L. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathog. 2009;5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang L., Zhou C., He Z., Wang Z.G., Wang J.L., Wang Y.F. Wolbachia infection decreased the resistance of Drosophila to lead. PloS One. 2012;7:e32643. doi: 10.1371/journal.pone.0032643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Georgiev P., Okkenhaug H., Drews A., Wright D., Lambert S., Flick M., Carta V., Martel C., Oberwinkler J., Raghu P. TRPM channels mediate zinc homeostasis and cellular growth during Drosophila larval development. Cell Metab. 2010;12:386–397. doi: 10.1016/j.cmet.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Hofmann T., Chubanov V., Chen X., Dietz A.S., Gudermann T., Montell C. Drosophila TRPM channel is essential for the control of extracellular magnesium levels. PloS One. 2010;5:e10519. doi: 10.1371/journal.pone.0010519. [DOI] [PMC free article] [PubMed] [Google Scholar]


