Abstract
Objective
Generation of negative intrathoracic pressure during the decompression phase of cardiopulmonary resuscitation (CPR) enhances the refilling of the heart. We tested the hypothesis that when compared with closed chest manual chest compressions (CC) at 80 CC per minute (CPR at 80CC/min), treatment with active compression decompression (ACD) CPR at 80 CC/min combined with augmentation of negative intrathoracic pressure would lower intracranial pressure (ICP) and increase cerebral perfusion thereby improving neurologically intact survival rates following prolonged untreated cardiac arrest.
Design
Prospective, randomized animal study. Setting: Animal laboratory facilities.
Subjects
A total of 26 female farm pigs in two different protocols (n=17 and n=9).
Interventions, Measurements and Main Results
17 pigs were subjected to 8.5 minutes of untreated ventricular fibrillation and prospectively randomized to CPR at 80 CC/min or ACD CPR at 80 CC/min plus an impedance threshold device (ITD). Coronary perfusion pressures (29.5 ± 2.7 versus 22.4 ± 1.6 mmHg, p=0.03), carotid blood flow (44.0 ± 12.2 versus 30.9 ± 10.4, p=0.03), and 24-hour neurological survival (88% versus 22%, p=0.015) were higher with ACD CPR + ITD. Cerebral perfusion pressures, measured in 9 additional pigs, were improved with ACD CPR + ITD (21.9 ± 1.2 versus 8.9 ± 0.8 mmHg, p<0.0001). With ACD CPR + ITD, mean diastolic ICP during decompression was lower (12.2 ± 0.2 versus 16.6 ± 1.2 mmHg, p=0.02) and the downward slope of the decompression phase ICP curve was steeper (-60.3 ± 12.9 versus -46.7 ± 11.1 mmHg/sec, p<0.001).
Conclusions
ACD CPR + ITD increased cerebral perfusion pressures and lowered diastolic ICP and ICP rate during the decompression phase. These mechanisms may underlie the observed increase in cerebral perfusion pressure, carotid blood flow and survival rates with favorable neurologic outcomes in this pig model of cardiac arrest.
Keywords: cardiac arrest, cardiopulmonary resuscitation, cerebrovascular circulation, intracranial pressure, perfusion, intrathoracic pressure
Introduction
Multiple approaches have been developed in an effort to optimize the bellows-like action of the chest during CPR to improve upon the currently dismal results with manual closed-chest CPR (1-4). Two non-invasive technologies consisting of active compression decompression (ACD) CPR and an impedance threshold device (ITD), when combined, provide near normal blood flow to the brain and greater than 70% of normal blood flow to the heart (5, 6). These devices were recently shown to increase survival rates with favorable neurological outcomes in patients in cardiac arrest when compared with standard (STD) CPR (7). The ACD CPR device is used to compress and actively decompress the chest, and serves as the external motor to power the bellows-like movement of the thorax. The ITD selectively prevents inflow of respiratory gases during the decompression phase, thus capturing the kinetic recoil energy of the chest and generating a greater and more prolonged negative intrathoracic pressure. By augmenting negative intrathoracic pressure during the decompression phase of CPR, this device combination provides a means to enhance venous blood flow back to the chest to refill the heart and simultaneously lowers intracranial pressures. These two physiological mechanisms may contribute to the beneficial effects of these two technologies, which include increased coronary and cerebral perfusion in animals, and improved survival rates in humans (5, 7-12). Changes in intracranial pressure (ICP) have not been measured previously during ACD CPR + ITD. Thus, the objectives of this study were to demonstrate that this device combination can increase 24-hr survival rates with favorable neurological function in an animal model of prolonged cardiac arrest and to elucidate a possible mechanism for the survival benefit.
Materials and Methods
Animal Studies
All animal studies were approved by the Institutional Animal Care Committee of the Minneapolis Medical Research Foundation at Hennepin County Medical Center. All animals received treatment and care in compliance with the 1996 Guide for the Care and Use of Laboratory Animals by the National Research Council in accordance with the USDA Animal Welfare Act, PHS Policy, and the American Association for Accreditation of Laboratory Animal Care.
Device Description
A prototype manual device was developed to perform ACD CPR and CPR at 80 CC/min that provided accurate feedback to the user related to compression and decompression forces. It was secured to the chest with an adhesive engineered to withstand up to 30 lbs of decompressive force. The device consisted of an ergonomic handle, electronics to measure compression and decompression forces, a compression pad, and an adhesive pad. An ITD (ResQPOD®, Advanced Circulatory Systems, Inc., Roseville, Minnesota) provided inspiratory resistance of up to 10 cmH2O to augment negative intrathoracic pressure during the chest recoil phase, as has been described previously (5, 6, 13-17).
Preparatory phase
The surgical preparation and monitoring techniques have been previously described (6, 17). Briefly, initial sedation was achieved with 7 ml (100 mg/ml) of intramuscular ketamine HCl (Ketaset®, Fort Dodge Animal Health, Fort Dodge, IA) followed by an intravenous bolus of propofol (PropoFlo®, Abbott Laboratories, North Chicago, IL), (2.3 mg/kg), via the lateral ear vein. The trachea was intubated with a 7.5 mm cuffed endotracheal tube inflated to prevent air leaks.
A Millar catheter (Mikro-Tip® Transducer, Millar Instruments, Inc., Houston, TX) was used to continuously measure central aortic pressure and acquire arterial blood gases. Carotid artery flow was measured using an ultrasound flow probe attached to the internal carotid artery (Transonic Systems, Indiana). Endotracheal pressures were measured as a surrogate for intrathoracic pressures, with a micromanometer-tipped Millar catheter positioned 2 cm below the tip of the endotracheal tube. These pressures, correlate closely with intrathoracic pressures and right atrial pressures during the chest wall decompression phase of CPR (18).
All data were digitized using a computerized data analysis program (Superscope II vl.295, GW Instruments, Somerville, MA) and a Power Macintosh G3® computer (Apple Computer, Inc., Cupertino, CA). End tidal (ET) CO2, tidal volume, and arterial oxygen saturation were recorded with a CO2SMO Plus®, (Novametrix Medical Systems, Wallingford, CT).
Protocols
Protocol 1: Survival Study
The survival study was performed according to Utstein-style guidelines on 17 female farm pigs (28 ± 0.6 kg) (19). Ventricular fibrillation (VF) was induced in the anesthetized animal with a 50 Hz, 7.5 V AC electrical current through a right ventricular endocardial electrode. After 8.5 minutes of untreated cardiac arrest, animals were randomized to 6 minutes of CPR @ 80 CC/min or ACD CPR + ITD @ 80 CC/min. This model did not include bystander CPR, a common clinical scenario. The model represents a clinically relevant VF time if no bystander CPR is performed and either Basic or Advanced Life Support crews provide CPR. In contrast to many prior animal studies with ACD CPR + ITD, in this study both methods of CPR were performed manually by a single EMT-trained individual to assure uniformity of CPR delivery throughout the protocol. To assure that the compression force was matched between groups, the rescuer was able to observe the amount of force applied to the chest on a beat-to-beat real-time basis. Further, an audible metronome was used to guide the compression rate. This afforded the operator instantaneous feedback so that the compression rate and force were indistinguishable between groups. In this study ACD CPR was performed with the device secured to the chest by an adhesive pad. CPR at 80 CC/min was performed with the same device but without being secured to the chest by the adhesive, thus allowing for the same target compression forces and preventing active chest decompression.
All animals were ventilated during CPR with supplemental oxygen (2 LPM) with a bag-valve resuscitator (Smart Bag, O2 Systems, Canada), with continuous uninterrupted chest compressions, a compression to ventilation ratio of 10:1, asynchronous ventilations, and a tidal volume of approximately 400 ml. All compressions were performed at a rate of 80 CC/min. After six minutes of CPR, each animal was immediately defibrillated with up to 3 sequential 200 J transthoracic biphasic (Zoll M-Series) shocks. Animals that remained in cardiac arrest received a single dose of intravenous epinephrine (0.045 mg/kg) and an additional 2 minutes of CPR. Each animal then received up to 3 additional sequential 200 J transthoracic shocks. All animals with successful restoration of spontaneous circulation (ROSC) were reconnected to the ventilator. Pigs were weaned from the ventilator over the subsequent 2 hours and placed back in their pens overnight. No additional post-resuscitation care was provided. Survival rates, complication rates, and neurological status were evaluated 24 hours after resuscitation. The Swine Neurologic Deficit Score was used to evaluate level of consciousness, respiratory pattern, cranial nerve function, motor and sensory function, and behavior evaluation, including ability to drink, chew, stand, and walk (20). The Cerebral Performance Score (CPC), a neurological assessment based on a 5-point evaluation of the level of consciousness was used to determine neurological function. Twenty-four hours after resuscitation, euthanasia was performed by an injection of 10 ml of 10M potassium chloride solution and necropsy was performed.
Protocol 2: Acute Study
The same surgical preparation, measurement and recording techniques that were used in the survival study described above were used in the acute protocol with the exception of the insertion of an intracranial bolt. In the acute, mechanism study (n=9), an intracranial bolt was inserted into the animals and intracranial pressures were measured with a 3.5 French micromanometer pressure transducer (Mikro-Tip® Transducer, Millar Instruments, Inc., Houston, TX) as previously described (21). ICP was not measured in the survival protocol to ensure that neurological assessments were not influenced by the presence of an intracranial bolt. Once the animals were stable and all monitoring devices were in place, VF was induced. Following 8.5 minutes of VF, five minutes of ACD CPR + ITD or CPR @ 80 CC/min was initiated and followed by another five minutes of the alternative method of CPR. The order of the type of CPR was randomized before the start of the study.
Hemodynamic measurements were averaged over three compression cycles at the end of each minute of CPR. Coronary perfusion pressures were calculated as mean diastolic (decompression) aortic pressure minus mean diastolic (decompression) right atrial pressure. Cerebral perfusion pressures (CerPP) were calculated as mean arterial pressure (MAP) minus mean ICP. Mean carotid flow measurements were averaged over a minute of continuous measurement.
Statistical analysis
The primary endpoint of the survival protocol was 24-hour survival with favorable neurological function, as defined by a cerebral performance category score of 1 or 2 (22). The primary endpoint for the acute protocol was CerPP. The Fisher's exact test was used for survival rate analysis. Hemodynamic and perfusion parameters were analyzed by a Student's t-test. All data are expressed as mean ± SEM unless otherwise indicated. A p-value of <0.05 was considered statistically significant. The sample size, for all protocols, was calculated in order to provide a power of 80%.
Results
Survival Study
The physiological and survival benefits of the combination of ACD CPR + ITD were compared with closed chest manual CPR at 80 CC/min. The physiological data, including the measurement of airway pressures, atrial and aortic pressures, the calculated coronary perfusion pressure, and end tidal CO2 values are shown in Table 1. As hypothesized, airway pressures during the decompression phase of CPR were significantly lower in the device group (-8.8 ± 0.7 mmHg) compared with controls (-2.2 ± 0.2 mmHg, p<0.001). Right atrial pressures during the recoil phase were lower with device combination (-1.2 ± 0.9 mmHg) versus controls (3.3 ± 0.2 mmHg, p=0.01), coronary perfusion pressures (29.5 ± 2.7 mmHg versus 22.4 ± 1.6, p=0.03) were significantly higher, and carotid blood flows were significantly higher with the device combination (43 ± 4.0 ml/min versus 31 ± 3.5 ml/min, p=0.04). Peak ETCO2 levels, an indirect indicator of circulation during CPR, were significantly higher in the ACD CPR + ITD group (34 ± 0.6 vs. 22 ± 0.6 mmHg, p<0.001). Arterial blood gas measurements were measured during the survival study and are summarized in Table 2. The arterial pH levels were lower in the ACD CPR + ITD group during CPR, presumably a result of the increased circulation causing an increase in the clearance of lactate. Base excess (measured after 5.5 minutes of CPR) was -6.3 ± 1.3 mmol/L for the ACD CPR + ITD group and -7.3 ± 1.1 mmol/L for the CPR at 80 CC/min group (p=NS). Arterial oxygen content was similar between groups during CPR.
Table 1.
Hemodynamic measurements (mean ± SEM) at baseline and minute six of either ACD CPR + ITD or CPR at 80 CC/min for the Survival Protocol.
| CPR at 80 CC/min (n=9) | ACD CPR + ITD (n=8) | p-value* | |||
|---|---|---|---|---|---|
| Baseline | 6 min CPR | Baseline | 6 min CPR | ||
| Airway Pressures (mmHg) | 0.4 ± 0.1 | -2.2 ± 0.2 | -0.2 ± 0.6 | -8.8 ± 0.7 | < 0.001 |
| Right Atrial Diastolic Pressure (mmHg) | 1.8 ± 0.2 | 3.3 ±0.3 | 0.8 ± 0.3 | -1.2 ± 0.9 | 0.01 |
| Coronary Perfusion Pressure (mmHg) | 68.1 ±5.7 | 22.4 ± 1.6 | 72.9 ± 2.1 | 29.5 ±2.3 | 0.03 |
| Carotid Blood Flow (ml/min) | 176 ± 28 | 32.2 ± 3.5 | 191 ±21 | 47.0 ± 4.2 | 0.02 |
| End tidal CO2 (mmHg) | 39 ± 1 | 22 ± 1 | 38 ± 1 | 34 ± 1 | < 0.001 |
| Aortic Systolic Pressure (mmHg) | 96.5 ± 6.6 | 67.2 ± 1.4 | 97.9 ± 3.4 | 76.1 ± 1.4 | NS |
| Aortic Diastolic Pressure (mmHg) | 71.7 ± 5.2 | 25.8 ± 0.6 | 76.3 ±2.1 | 26.3 ± 1.0 | NS |
| Right Atrial Systolic Pressure (mmHg) | 1.0 ± 0.3 | 71.6 ± 3.4 | 0.9 ± 0.3 | 78.5 ± 2.3 | NS |
P values compare CPR at 80 CC/min vs ACD CPR + ITD values during the 6th minute of CPR.
There were no statistically significant differences in baseline values between groups.
NS means no significant difference between parameters.
Table 2.
Arterial blood gas measurements following 8.5 minutes of VF and 6 minutes of either ACD CPR + ITD or CPR at 80 CC/min for the Survival Protocol.
| CPR at 80 CC/min (n=9) | ACD CPR + ITD (n=8) | |||
|---|---|---|---|---|
| Baseline | 6 min CPR | Baseline | 6 min CPR | |
| pH, mean ± SEM | 7.45 ±0.02 | 7.40 ± 0.03 | 7.47 ±0.01 | 7.27 ± 0.01 |
| pCO2, mean ± SEM | 38 ± 1 | 29 ± 2 | 39 ± 0 | 44 ± 2 |
| pO2, mean ± SEM | 141 ± 10 | 148 ± 26 | 171 ± 16 | 148 ± 34 |
The overall increase in vital organ perfusion pressures was associated with a significant survival benefit. The initial return of spontaneous circulation (ROSC) rate was 44% in the CPR at 80 CC/min group and 100% in the ACD CPR + ITD group (p<0.05). Seven of eight survivors in the ACD CPR + ITD group required one shock for ROSC and three of four survivors in the CPR at 80 CC/min group required one shock. Once ROSC was achieved, all animals survived 24 hours. Five of nine CPR at 80 CC/min animals received a total of 6 shocks plus epinephrine, but ROSC did not occur.
The main study endpoint, defined a priori, was 24-hour survival with favorable neurological function. The results are summarized in Table 3. Following 8.5 minutes of ventricular fibrillation, the 9 animals treated with CPR at 80 CC/min had significantly poorer outcomes compared with 8 pigs treated with the ACD CPR + ITD. One animal in the ACD CPR + ITD group was excluded from analysis when it was determined that the load cell to measure compression forces was incorrectly calibrated even though the animal survived 24-hours with intact neurological function. Twenty-four hour survival was 44% in the CPR at 80 CC/min group and 100% in the ACD CPR + ITD group (p<0.05). The cerebral performance score, based on a 5-point scoring system (1=normal, 5=brain death), was used to evaluate neurological function at 24 hours. Twenty-four hour survival with good neurological function, defined by a cerebral performance score of 1 or 2, was significantly better in the animals that received treatment with ACD CPR + ITD (88% vs. 22%, p<0.02). With only 4 survivors in the CPR at 80 CC/min group, there was insufficient data to demonstrate a statistically significant improvement in the Swine Neurological Deficit Score between groups as only two of the four CPR at 80 CC/min 24-hour survival animals had abnormal neurological function after 24 hours. When the 24-hour survival animals were compared, total deficit score in the CPR at 80 CC/min group was 124 ± 72 versus 33 ± 17 in the ACD CPR + ITD group (p=0.15).
Table 3. Mean ± SEM. Survival Protocol twenty-four hour survival and neurological assessment score following 8.5 minutes of VF and six minutes of either ACD CPR + ITD or CPR at 80 CC/min.
| CPR @ 80 CC/min (n=9) | ACD CPR + ITD (n=8) | |
|---|---|---|
| 24 Hour Survival, n (%) | 4 (44%)* | 8 (100%)* |
| 24 Hour Survival with CPC 1 or 2, n | (%) 2 (22%)† | 7 (88%)† |
| Neurological Assessment | ||
| Consciousness | 30.0 ± 17.3 | 15.0 ± 5.7 |
| Respiratory Pattern | 0.0 ± 0.0 | 0.0 ± 0.0 |
| Painful Stimulus | 18.8 ± 12.0 | 4.4 ± 3.2 |
| Muscle Tone | 25.0 ± 14.4 | 3.1 ± 3.1 |
| Standing | 10.0 ± 5.8 | 2.5 ± 2.5 |
| Walking | 15.0 ± 8.7 | 5.0 ± 3.8 |
| Restraint | 25.0 ± 14.4 | 7.5 ± 5.3 |
| Total deficit score | 124 ± 72 | 33 ± 17 |
p < 0.05,
p < 0.02.
Acute Study
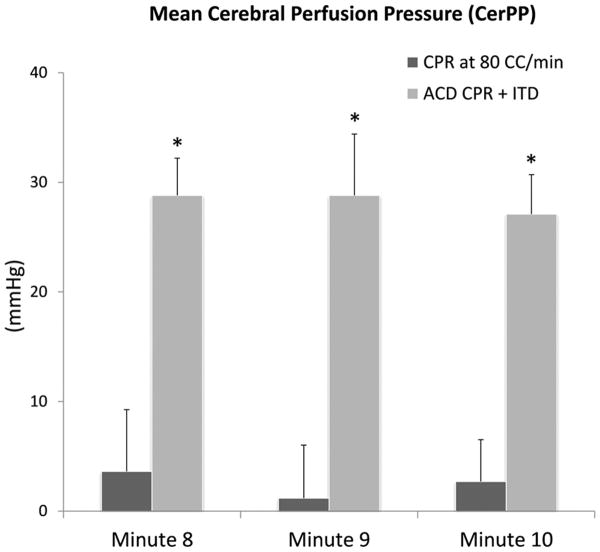
A separate, acute (non-survival) study was performed to investigate the mechanism of increased neurological survival demonstrated in the survival study. Mean CerPP in the mechanism study (n=9) was significantly higher in the ACD CPR + ITD group, especially after prolonged CPR, as shown in minutes 8-10 in Figure 1. The mean CerPP during compression was 21.9 mmHg ± 1.2 for ACD CPR + ITD versus 8.9 ± 0.8 mmHg for CPR at 80 CC/min (p<0.0001) for the entire ten minutes of CPR; the mean CerPP during decompression was 2.3 ± 0.7 mmHg for ACD CPR + ITD versus -5.2 ± 1.1 mmHg (p=0.01). Mean carotid blood flow for ACD CPR + ITD for the entire ten minutes of CPR was also significantly higher (12.0 ± 2.2 ml/min versus 8.0 ± 1.5 ml/min, p=0.008). Notably, regardless of order, carotid blood flow always increased during ACD CPR + ITD use. One animal experienced a broken rib mid-way through the ACD CPR + ITD cycle detectable by a significant spike in aortic pressures during compressions and verified at autopsy; the ACD CPR + ITD data for this animal was excluded. This was the only animal in the Acute Study that experienced a broken rib. Inclusion of this animal in an intention to treat analysis resulted in even higher CerPPs in the ACD CPR + ITD group. Taken together, the combination of the ACD CPR device + ITD provided significantly greater brain perfusion than CPR at 80 CC/min alone.
Figure 1.
Mean ± SEM. The mean cerebral perfusion pressures in the Acute Protocol were significantly higher with ACD + ITD in the last three minutes of the intervention, *p<0.05.
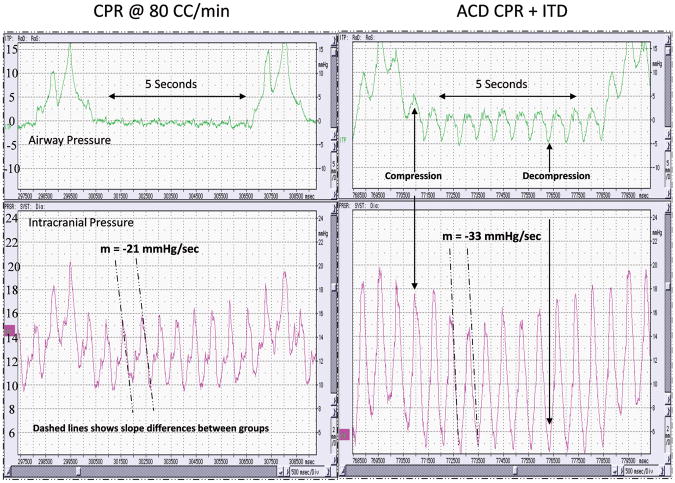
The effects of the CPR interventions on the absolute ICP values and the rate of ICP change during the decompression phase were also assessed. As shown in Table 4, in the animals where ACD CPR + ITD was performed after CPR at 80 CC/min, the rate of ICP reduction, as measured by the slope of the ICP during the first ten milliseconds of decompression, was 1.5 times higher with ACD CPR + ITD (-60.3 ± 12.9 mmHg/sec) versus CPR at 80 CC/min (-46.7 ± 11.1 mmHg/sec, p<0.001). Furthermore, the changes in the rate of ICP reduction and the changes in absolute ICP were highly dependent on the order of CPR performed. The difference in the rate of ICP reduction is illustrated in a representative study in Figure 2. Overall, the ICP was reduced more rapidly with ACD CPR + ITD and the diastolic ICP values were significantly lower with ACD CPR + ITD.
Table 4.
Acute Protocol mean ± SEM measurements of the changes in the rate of ICP reduction during the decompression phase of CPR and the absolute ICP values (systolic, diastolic, mean).
| ACD CPR + ITD to 80 CCM CPR | 80 CCM CPR to ACD CPR + ITD | ACD CPR + ITD vs. 80 CCM CPR | |
|---|---|---|---|
| (n=5) | (n=4) | (n=9) | |
| Rate of ICP Change (mmHg/sec) | -74.5 ± 17.0 to -68.0 ± 14.6 | -22.6 ± 3.9 to -32.4 ± 7.7 | -60.3 ± 13 vs -46.7 ± 11 ** |
| ICP (Systolic, mmHg) | 25.6 ± 1.0 to 29.0 ± 1.6 | 21.4 ± 0.1 to 22.1 +0.2 | 23.8 ± 0.9 vs 25.2 ± 1.8 |
| ICP (Diastolic, mmHg) | 12.7 ± 0.1 to 18.0 ± 2.2 | 15.3 ± 0.3 to 11.6 ± 0.0 * | 12.2 ± 0.2 vs 16.6 ± 1.2 ** |
| ICP (Mean, mmHg) | 18.8 ± 0.5 to 23.2 ± 0.5 † | 18.0 ± 0.2 to 16.9 ± 0.1 † | 17.8 ± 0.5 vs 20.6 ± 1.2 |
The values are reported are the average values for the last three minutes of the type of CPR performed and are also categorized depending on the order of treatment. 80 CCM means CPR at 80 chest compressions/minute,
p < 0.02,
p < 0.005,
p < 0.001.
Figure 2.
Airway pressure, intracranial pressure, and right atrial pressures are simultaneously lowered during ACD CPR + ITD. Note the greater rate of ICP reduction in the ACD CPR + ITD figure in comparison to the ICP reduction during CPR at 80 CC/min. Both figures are from the same animal. ACD CPR + ITD was performed after 5 minutes of CPR at 80 CC/min.
The prototype ACD CPR device functioned as intended during the studies. The adhesive pad stayed in place during compression and decompression and the ergonomic handle was associated with no discomfort during use.
Discussion
The two main objectives of this study were to determine whether ACD CPR with the additional augmentation of negative intrathoracic pressure would increase 24-hr survival rates with favorable neurological function in pigs and to investigate a potential mechanism for this. Building upon prior animal and human studies (5,6,15-17,19) results from the current study demonstrated that when compared with CPR at 80 CC/min, use of ACD CPR + ITD resulted in significantly higher carotid artery blood flow and CerPP during the decompression phase of CPR. These favorable hemodynamic findings were associated with significantly more pigs with favorable neurological function 24 hours after cardiac arrest. This is the first time that ACD CPR + ITD has been shown to increase 24-hour survival and favorable neurological function in pigs compared to CPR at 80 CC/min. A large clinical trial recently reported the same beneficial effect of this approach in humans (7).
The potential link between improved neurological function and changes in ICP during CPR was explored in this investigation. CerPP, indicative of the amount of blood that perfuses the brain with each compression/decompression cycle, was significantly higher with ACD CPR + ITD. Cerebral blood flow measured via radiolabeled microspheres has been measured previously with ACD CPR + ITD and found to be even greater than baseline levels after prolonged arrest (5). In contrast, performance of CPR at 100 CC/min results in only 25% of normal blood flow to the brain (6). In this study we observed that diastolic ICP values were significantly reduced during ACD CPR + ITD; this tracked concurrently and instantaneously with changes in airway pressures as shown in Figure 2. The lower the ICP, the lower the resistance to forward blood flow to the brain. This is the first time that intracranial pressures have been shown to be decreased with ACD CPR + ITD. These findings provide a hemodynamic basis and mechanism for the increase in circulation observed in prior animal studies with ACD CPR + ITD. The reduction in ICP over time was consistently and significantly greater and faster with ACD + ITD in this study. This is reflected by the 1.5-fold higher negative slopes in the ACD CPR + ITD group. The steeper negative ICP slope during ACD CPR + ITD results in more time during each decompression phase for greater cerebral perfusion thus increasing the time for delivery of blood and oxygen to cerebral tissues, as cerebral blood flow resistance to forward flow generated by ICP is lower for a longer period of time. This is a new observation and one which may help to further explain, at a mechanistic level, the beneficial effects of ACD CPR + ITD. Optimization of this important thoraco-cerebral pressure relationship, which we and others have described (23, 24), may be fundamental to optimizing neurological outcomes in cardiac arrest (8, 9, 11).
Another important observation from this investigation is that the coronary perfusion pressures were higher in the control (CPR at 80 CC/min) group than what has previously been observed in other CPR studies (17). We speculate that these differences are due to the larger footprint of the CPR device utilized in these studies which is about 1.5 times larger than a “standard” size human palm. The larger surface area of the compression surface presumably confers a hemodynamic benefit on its own, by spreading the same force out over a larger compressible area.
There are limitations in this study. First, though the study was prospective and animals were randomized to the method of CPR after induction of ventricular fibrillation, the study could not be blinded. However, as noted above, compression rate and force were closely monitored and controlled. Second, although the results of this novel study demonstrate a significant increase in CerPP and carotid blood flow with ACD + ITD, we did not measure actual vital organ blood flow. Nonetheless, the effects on perfusion pressures, and the survival and neurological benefit observed in this study with the use of ACD CPR + ITD suggest that blood flow was significantly higher with ACD CPR + ITD. Thirdly, CPR was performed at 80 CC/min instead of the AHA recommended 100 CC/min so that both groups were matched for CC rate. CPR results may have improved at 100 CC/min. Finally, in contrast to standard clinical post cardiac arrest care, no substantial post-ROSC care was provided to animals in either group. It is possible additional post-ROSC care may have changed the study outcomes.
Conclusion
When compared with CPR at 80 CC/min, application of the ACD CPR device combined with the ITD during pig studies resulted in: 1) a significant improvement in coronary and cerebral perfusion pressures, end tidal CO2, and carotid artery blood flow, 2) a significant improvement in 24-hour survival with favorable neurological function, 3) a significant reduction in ICP during the decompression phase of CPR, and 4) a more rapid decline in ICP hypothesized to result in an increase in the amount and duration of perfusion to the brain.
Acknowledgments
Financial Support: This project was supported by NIH SBIR Grant # R43 HL078011-01 and the Dwight Opperman Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Anja K Metzger, Department of Emergency Medicine University of Minnesota, Minneapolis, MN.
Margot Herman, Mayo Clinic College of Medicine, Rochester, MN.
Scott McKnite, Minnesota Medical Research Foundation, Hennepin County Medical Center, Minneapolis, MN.
Wanchun Tang, Weil Institute of Critical Care Medicine, Rancho Mirage, California.
Demetris Yannopoulos, Division of Cardiovascular Medicine, University of Minnesota, Minneapolis, MN.
References
- 1.Becker LB, Ostrander MP, Barrett J, Kondos GT. Outcome of CPR in a large metropolitan area--where are the survivors? Ann Emerg Med. 1991;20(4):355–361. doi: 10.1016/s0196-0644(05)81654-3. [DOI] [PubMed] [Google Scholar]
- 2.Eisenberg MS, Horwood BT, Cummins RO, Reynolds-Haertle R, Hearne TR. Cardiac arrest and resuscitation: a tale of 29 cities. Ann Emerg Med. 1990;19(2):179–186. doi: 10.1016/s0196-0644(05)81805-0. [DOI] [PubMed] [Google Scholar]
- 3.Lurie KG, Lindo C, Chin J. CPR: the P stands for plumber's helper. Jama. 1990;264(13):1661. doi: 10.1001/jama.1990.03450130031020. [DOI] [PubMed] [Google Scholar]
- 4.Niemann JT. Cardiopulmonary resuscitation. N Engl J Med. 1992;327(15):1075–1080. doi: 10.1056/NEJM199210083271507. [DOI] [PubMed] [Google Scholar]
- 5.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 6.Lurie KG, Voelckel WG, Zielinski T, McKnite S, Lindstrom P, Peterson C, Wenzel V, Lindner KH, Samniah N, Benditt D. Improving standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve in a porcine model of cardiac arrest. Anesth Analg. 2001;93(3):649–655. doi: 10.1097/00000539-200109000-00024. [DOI] [PubMed] [Google Scholar]
- 7.Aufderheide TP, Frascone RJ, Wayne MA, Mahoney BD, Swor RA, Domeier RM, Olinger ML, Holcomb RG, Tupper DE, Yannopoulos D, et al. Standard cardiopulmonary resuscitation versus active compression-decompression cardiopulmonary resuscitation with augmentation of negative intrathoracic pressure for out-of-hospital cardiac arrest: a randomised trial. Lancet. 2011;377(9762):301–311. doi: 10.1016/S0140-6736(10)62103-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plaisance P, Lurie KG, Payen D. Inspiratory impedance during active compression-decompression cardiopulmonary resuscitation: a randomized evaluation in patients in cardiac arrest. Circulation. 2000;101(9):989–994. doi: 10.1161/01.cir.101.9.989. [DOI] [PubMed] [Google Scholar]
- 9.Plaisance P, Lurie KG, Vicaut E, Martin D, Gueugniaud PY, Petit JL, Payen D. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265–271. doi: 10.1016/j.resuscitation.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 10.Voelckel WG, Lurie KG, Sweeney M, McKnite S, Zielinski T, Lindstrom P, Peterson C, Wenzel V, Lindner KH. Effects of active compression-decompression cardiopulmonary resuscitation with the inspiratory threshold valve in a young porcine model of cardiac arrest. Pediatr Res. 2002;51(4):523–527. doi: 10.1203/00006450-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Wolcke BB, Mauer DK, Schoefmann MF, Teichmann H, Provo TA, Lindner KH, Dick WF, Aeppli D, Lurie KG. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 12.Aufderheide T. A tale of seven EMS systems: an impedance threshold device and improved CPR techniques double survival rates after out-of-hospital cardiac arrest. Circulation. 2007;116(16):II–936. [Google Scholar]
- 13.Lurie K, Voelckel W, Plaisance P, Zielinski T, McKnite S, Kor D, Sugiyama A, Sukhum P. Use of an inspiratory impedance threshold valve during cardiopulmonary resuscitation: a progress report. Resuscitation. 2000;44(3):219–230. doi: 10.1016/s0300-9572(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 14.Lurie K, Zielinski T, McKnite S, Sukhum P. Improving the efficiency of cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Crit Care Med. 2000;28(11 Suppl):N207–209. doi: 10.1097/00003246-200011001-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lurie KG, Barnes TA, Zielinski TM, McKnite SH. Evaluation of a prototypic inspiratory impedance threshold valve designed to enhance the efficiency of cardiopulmonary resuscitation. Respir Care. 2003;48(1):52–57. [PubMed] [Google Scholar]
- 16.Lurie KG, Mulligan KA, McKnite S, Detloff B, Lindstrom P, Lindner KH. Optimizing standard cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Chest. 1998;113(4):1084–1090. doi: 10.1378/chest.113.4.1084. [DOI] [PubMed] [Google Scholar]
- 17.Lurie KG, Zielinski T, McKnite S, Aufderheide T, Voelckel W. Use of an inspiratory impedance valve improves neurologically intact survival in a porcine model of ventricular fibrillation. Circulation. 2002;105(1):124–129. doi: 10.1161/hc0102.101391. [DOI] [PubMed] [Google Scholar]
- 18.Chandra N, Guerci A, Weisfeldt ML, Tsitlik J, Lepor N. Contrasts between intrathoracic pressures during external chest compression and cardiac massage. Crit Care Med. 1981;9(11):789–792. doi: 10.1097/00003246-198111000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Idris AH, Becker LB, Ornato JP, Hedges JR, Bircher NG, Chandra NC, Cummins RO, Dick W, Ebmeyer U, Halperin HR, et al. Utstein-style guidelines for uniform reporting of laboratory CPR research. A statement for healthcare professionals from a Task Force of the American Heart Association, the American College of Emergency Physicians, the American College of Cardiology, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, the Institute of Critical Care Medicine, the Safar Center for Resuscitation Research, and the Society for Academic Emergency Medicine. Resuscitation. 1996;33(1):69–84. doi: 10.1016/s0300-9572(96)01055-6. [DOI] [PubMed] [Google Scholar]
- 20.Bircher N, Safar P. Cerebral preservation during cardiopulmonary resuscitation. Crit Care Med. 1985;13(3):185–190. doi: 10.1097/00003246-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Yannopoulos D, McKnite SH, Metzger A, Lurie KG. Intrathoracic pressure regulation for intracranial pressure management in normovolemic and hypovolemic pigs. Crit Care Med. 2006;34(12 Suppl):S495–500. doi: 10.1097/01.CCM.0000246082.10422.7E. [DOI] [PubMed] [Google Scholar]
- 22.Vaagenes P, Cantadore R, Safar P, Moossy J, Rao G, Diven W, Alexander H, Stezoski W. Amelioration of brain damage by lidoflazine after prolonged ventricular fibrillation cardiac arrest in dogs. Crit Care Med. 1984;12(10):846–855. doi: 10.1097/00003246-198410000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Guerci AD, Shi AY, Levin H, Tsitlik J, Weisfeldt ML, Chandra N. Transmission of intrathoracic pressure to the intracranial space during cardiopulmonary resuscitation in dogs. Circ Res. 1985;56(1):20–30. doi: 10.1161/01.res.56.1.20. [DOI] [PubMed] [Google Scholar]
- 24.Yannopoulos D, Aufderheide TP, McKnite S, Kotsifas K, Charris R, Nadkarni V, Lurie KG. Hemodynamic and respiratory effects of negative tracheal pressure during CPR in pigs. Resuscitation. 2006;69(3):487–494. doi: 10.1016/j.resuscitation.2005.11.005. [DOI] [PubMed] [Google Scholar]