Abstract
This study was carried out to evaluate the efficacy and safety of doxercalciferol as therapy for secondary hyperparathyroidism (SHPT) in patients with chronic kidney disease (CKD) stage 4 in a prospective clinical trial. A total of 35 CKD-4 patients who had a baseline parathyroid hormone (iPTH) >150 pg/mL and had not received any vitamin D analog in the preceding 8 weeks were followed up at intervals of 6 weeks for 18 weeks on oral therapy with doxercalciferol. The starting dose was 1.5 μg/day, and the dose was increased in steps of 1 μg/day if iPTH did not decrease by at least 30% on the subsequent visit. Doxercalciferol was stopped temporarily if low iPTH (<70 pg/mL), hypercalcemia (>10.7 mg/dL), or severe hyperphosphatemia (>8.0 mg/dL) occurred, and was restarted at a lower dose on reversal of these abnormalities. Calcium acetate was the only phosphate binder used. Mean iPTH decreased by 35.4 ± 4.4% from 381.7 ± 31.3 pg/mL to 237.9 ± 25.7 pg/mL (P < 0.001). The proportion of patients who achieved 30% and 50% suppression of iPTH levels was 83% and 72%, respectively. Mean serum calcium, phosphorus, and calcium-phosphorus product values did not differ significantly from the baseline values. Four, two, and nine patients developed hypercalcemia, severe hyperphosphatemia, and high CaxP (>55), respectively. Almost all patients recovered to an acceptable level within 2 weeks of stopping doxercalciferol and adjusting the phosphate binder dose. In all, 21 patients required temporary stoppage of therapy. Most of them were restarted on therapy at a reduced dose during the study. It can, therefore, be concluded that doxercalciferol is effective in controlling SHPT in CKD-4 patients with an acceptable risk of hyperphosphatemia and hypercalcemia.
Keywords: Chronic kidney disease, doxercalciferol, parathyroid hormone, pre-dialysis, secondary hyperparathyroidism, vitamin D
Introduction
Secondary hyperparathyroidism (SHPT) is an important complication of chronic kidney disease (CKD), which requires therapy, mainly to minimize the effects of high levels of parathyroid hormone (PTH) on bone, resulting in demineralization, loss of structural strength, fractures, and bone pain.[1,2]
In the mid-1980s, calcitriol was introduced and found to be effective in reducing the serum PTH levels in patients with renal failure requiring dialysis and also in pre-dialysis patients.[3,4,5,6,7] The principal toxicities of calcitriol that limit its use are hypercalcemia and hyperphosphatemia. Substantial evidence has accumulated over the last few years that it is possible to obtain some selectivity of the effects of vitamin D by the use of vitamin D analogs.[8,9] Three analogs have been marketed, i.e., paricalcitol, doxercalciferol, and maxacalcitol.
Doxercalciferol (1-α-hydroxyvitamin D2) is a D2 analog of vitamin D. D2 analogs appear to be less toxic than the D3 analogs. A trial in women with osteoporosis, which in fact was the first human study of doxercalciferol, demonstrated minimal calcemic effects of doxercalciferol even at doses as high as 5.0 μg/day.[10]
Since then, doxercalciferol has been used successfully in patients with SHPT due to CKD. Coburn et al. reported a study of 55 adults with stage 3 or 4 CKD. To date, it is the only placebo-controlled study of doxercalciferol in pre-dialysis patients.[11] In this study, the mean plasma iPTH level decreased by 46% from baseline after 24 weeks of doxercalciferol treatment (P < 0.001), but was unchanged with placebo. No clinically significant differences in mean serum calcium or phosphorus or incidence of hypercalcemia or hyperphosphatemia were noted between the groups.
Since the mid- to late-1990s, the experience with doxercalciferol in patients on dialysis has been more extensive. It has been found to be useful for patients also with a low risk of hypercalcemia and hyperphosphatemia.[12,13,14] In this study, we investigated the efficacy of oral doxercalciferol in the treatment of SHPT in Indian patients with CKD-4 as evidenced by its ability to reduce plasma iPTH levels over 18 weeks of treatment. We also studied the incidence of the side effects of oral doxercalciferol with emphasis on hypercalcemia, hyperphosphatemia, and increase in CaxP product.
Materials and Methods
A total of 35 patients with CKD-4 (glomerular filtration rate 15-29 mL/ min as calculated by the Cockroft–Gault formula) and iPTH >150 pg/mL from the Department of Nephrology, Postgraduate Institute of Medical Education and Research (PGIMER), Chandigarh, India, were enrolled in this prospective study from July 2009 to December 2010. The study was approved by the institute's Ethics Committee.
Inclusion criteria were age 20-75 years, a baseline iPTH value greater than 150 pg/mL, and no intake of either calcium-containing phosphate binders or vitamin D analogs within the previous 8 weeks. Exclusion criteria were current alcohol or drug abuse, pregnancy or nursing, history of hyperthyroidism or sarcoidosis, active malignancy requiring treatment, malabsorption syndromes, significant hepatic dysfunction, and treatment with steroids, anticonvulsants, bisphosphonates, fluoride, or lithium in the previous year.
Those subjects who satisfied the inclusion criteria and were willing to give an informed consent were included in the study. After inclusion in the study, the patient's particulars were entered into a proforma, with particular emphasis on the demographic profile, such as age and gender, clinical history, examination including general physical examination, measurement of blood pressure, and systemic examination and routine laboratory parameters that included complete hemogram, renal function tests, liver function tests, and alkaline phosphatase.
At baseline, all patients had two measurements of iPTH levels separated by 1 or 2 days, and the average was taken. iPTH was measured using the ADVIA Centaur Intact PTH assay, which is a two-site sandwich immunoassay using direct chemiluminometric technology. In addition, all patients had measurements of corrected serum calcium, phosphorus, and CaxP product. The above parameters were repeated at 6, 12, and 18 weeks in all patients.
Doxercalciferol was begun at a dose of 1.5 μg daily before breakfast. The drug was stopped temporarily if low iPTH levels (<70.0 pg/mL), moderate hypercalcemia (defined as corrected serum calcium level >10.7 mg/ dL), or severe hyperphosphatemia (defined as serum phosphorus level >8.0 mg/dL) occurred. These were then monitored weekly until the serum calcium levels normalized (<10.2 mg/dL) and/or the serum phosphorus decreased below 6.9 mg/dL. iPTH was retested after 6 weeks. In each case, the drug was restarted after 6 weeks at a dose reduced by by 0.5 μg/day. Only calcium acetate was used as the phosphate binder. Its dose was reduced if hypercalcemia occurred. If suppression of iPTH was less than 30% of baseline, with iPTH values greater than 200 pg/mL, the dosage was increased in steps of 1.0 μg/ day after subsequent estimations of iPTH. In patients with baseline iPTH between 150 and 200 pg/mL, the starting dose was 1 μg/day.
The values are reported as mean ± SD or SE. Also, the significance of the mean difference from baseline at each time was assessed by means of repeated measures analysis of variance using the Bonferroni method to correct for multiple comparisons. Paired t-test was used to compare the mean of specific abnormal values with the baseline. Multiple linear regression analysis was used to determine whether any relationship existed between the changes in either serum Ca or serum P and the changes in serum iPTH levels. A P-value of less than 0.05 was considered significant. All analyses were performed using SPSS 13.0 for Windows.
Results
The baseline characteristics of the study subjects are summarized in Table 1. Of the 35 patients, 21 were male and 14 were female. In all, 33 patients were followed up for the whole of 18 weeks, whereas the remaining two patients were followed up for 12 weeks. The principal results of the study are summarized in Table 2.
Table 1.
Baseline characteristics of the study patients
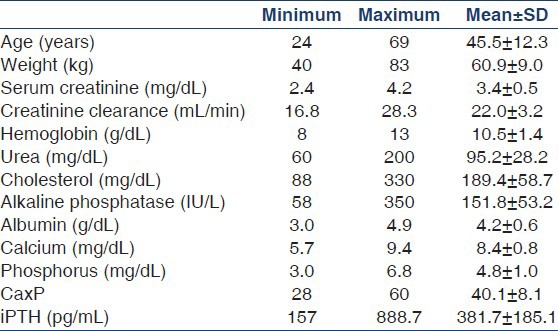
Table 2.
Summary of the biochemical parameters at important time points in the study (values as mean±SE)
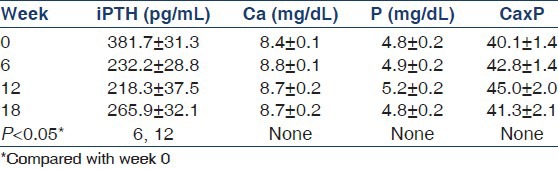
At baseline (week 0), the mean serum iPTH level was 381.7 ± 31.3 pg/mL, with a range of 157-888.7 pg/mL. The plasma iPTH levels were significantly suppressed at 6 and 12 weeks compared with the baseline levels, whereas the week 18 iPTH levels were not significantly different from the baseline levels. At the time of estimation of week 18 iPTH levels, 11 patients had been off treatment for 6 weeks and two patients for 12 weeks. This could explain the nonsignificant difference between the baseline and the week 18 iPTH levels. The iPTH levels at weeks 6, 12, and 18 were not significantly different from each other. The mean iPTH level from 6 weeks onward was 237.9 ± 25.7 pg/mL, and was significantly less than the baseline (P < 0.001) levels. The mean percentage change over the treatment period was 35.4 ± 4.4%. The maximum suppression of iPTH averaged 62.9 ± 4.9% (range 3.6-98.8%). In absolute terms, the iPTH level decreased maximally by 148.4 ± 26.1 pg/mL. Of all, 14 patients achieved at least 50% suppression by week 6, whereas another nine patients achieved this by week 12 and two more patients by week 18. The mean time to 50% suppression was 9 ± 4 weeks. Of the 35 patients, 29 (i.e., 83%) achieved at least a 30% suppression in iPTH at at least one follow-up visit. Three, six, and two patients achieved an iPTH level between 70 pg/mL and 110 pg/ mL at weeks 6, 12, and 18, respectively.
At the first follow-up visit, six patients had an iPTH level that was less than 70 pg/mL, necessitating temporary stoppage of treatment. On the next follow-up visit, all patients had recovered to an iPTH level greater than 70 pg/ mL, whereas eight other patients had developed a low iPTH. By week 18, all the eight patients had an iPTH level >70 pg/mL and five new patients had developed a low iPTH. Only one patient had two levels of iPTH <70 pg/ mL. The mean iPTH level on the visit following a low iPTH level was 277.8 ± 35.1 pg/ mL (range 107.9-500 pg/ mL). On three of the 19 above occasions, the patients had hypercalcemia (Ca >10.7 mg/dL) and on no occasion was severe hyperphosphatemia (P > 8.0 mg/dL) present.
Four patients developed hypercalcemia (Ca >10.7 mg/ dL) – one each at week 6 and week 12 and two at week 18. For the entire group, there were 0.64 episodes of hypercalcemia per 100 weeks of treatment. The mean high calcium level was 11.7 ± 0.4 mg/dL (range 10.9- 12.6 mg/dL). Three patients recovered to a calcium level <10.7 mg/dL within 1 week of stopping doxercalciferol and phosphate binders, and one patient recovered in 2 weeks. There was significant correlation between change in serum calcium and change in serum iPTH (R square = 0.141, P < 0.001) and insignificant correlation between changes in serum calcium and phosphorus (R square = 0.01, P = 0.304). Renal function did not deteriorate in any of the patients.
Two patients developed severe hyperphosphatemia (P > 8.0 mg/dL) at week 12. The levels were 9.0 mg/ dL and 8.2 mg/dL. One patient had an iPTH level of 753 pg/ mL (up from 631.2 pg/mL at baseline) and the other had an iPTH level of 89.5 pg/mL (down from 212.9 pg/mL at baseline). The week 6 phosphorus levels were 5.8 mg/dL and 7.0 mg/dL, and they had been on 2.5 μg/day and 1.5 μg/day of doxercalciferol since week 6, respectively. Both the patients had been on six tablets of phosphate binder for 6 weeks when severe hyperphosphatemia occurred. For the total group, there were 0.32 episodes of severe hyperphosphatemia per 100 weeks of treatment. Both the patients recovered to a phosphorus level <6.9 mg/dL within 2 weeks of temporarily stopping doxercalciferol. In neither instance was severe hyperphosphatemia accompanied by either hypercalcemia or low iPTH. The mean calcium binder dose for the whole group was 2.8 ± 0.2 tablets of calcium acetate/day.
The maximum daily doxercalciferol dose was 1 μg in two patients, 1.5 g in 18 patients, 2.5 μg in nine patients, and 3.5 μg in six patients. The mean of the maximum doxercalciferol doses employed was 2.0 ± 0.1 μg/day. The mean doxercalciferol dose throughout the study was 1.4 ± 0.1 μg/day. The mean doxercalciferol dose at maximal iPTH suppression was 1.6 ± 0.1 μg/day. In all, 24 patients were on 1.5 g of doxercalciferol when they achieved their minimum iPTH value. Four patients achieved their minimum iPTH at a dose of 1 μg and seven at 2.5 μg. Doxercalciferol was stopped temporarily in 21 patients on 22 occasions. The reasons were a low iPTH on 16 occasions, hypercalcemia once, both low iPTH and hypercalcemia on three occasions, and hyperphosphatemia twice. Six patients temporarily stopped treatment at week 6, of who four restarted their treatment at a reduced dose at week 12 and two at week 18. Eleven patients required stoppage of treatment at week 12, all but one of whom restarted their treatment at week 18. Four patients stopped treatment at week 18. Only one patient required stoppage of treatment after it had been restarted at a lower dose. Of a total of 618 patient-weeks of treatment, 120 weeks were spent in off-treatment time.
Discussion
Doxercalciferol is an attractive alternative to calcitriol for the management of SHPT in CKD patients, especially considering its low calcemic and phosphatemic effects. This study is one of the few prospective studies on the use of doxercalciferol in pre-dialysis CKD patients.
In this study, the mean iPTH at baseline was 381.7 ± 31.3 pg/ mL (range 157-888.7 pg/mL), which decreased by 35.4 ± 4.4% to 237.9 ± 25.7 pg/mL (P < 0.001). In all, 25 (72%) and 29 patients (83%) achieved at least 50% and 30% suppression of their baseline iPTH levels, respectively. Nineteen patients developed a low iPTH (<70 pg/mL) at sometime during their study. Of them, the 14 patients who had a repeat iPTH estimation after 6 weeks within the study period had all recovered to an iPTH >70 pg/mL. Remarkably, for such a significant decrease in iPTH, there were only four episodes of hypercalcemia and two episodes of severe hyperphosphatemia throughout the study period. All of these episodes recovered within a maximum of 2 weeks of stopping doxercalciferol and adjusting the phosphate binder dose.
These data are comparable to those of Coburn et al., whose study was conducted over a period of 24 weeks.[11] In the doxercalciferol group, the mean iPTH level decreased from a baseline level of 219 ± 22 pg/mL by 46.3 ± 7.6% to 118 ± 17 pg/mL at the end of the study (P < 0.001). In our study, the week 0 and week 18 levels were not significantly different probably because a significant number of patients (n = 13) had been off-treatment for at least 6 weeks at week 18. In that study, 74% of the patients had achieved a 30% suppression of baseline iPTH level compared with 83% in our study. One patient developed two episodes of hypercalcemia (Ca >10.7 mg/dL) in the study by Coburn et al., whereas in our study four patients had one episode each of hypercalcemia. One patient had hyperphosphatemia (P > 6.5 mg/dL) in that study, whereas in our study severe hyperphosphatemia was defined as P > 8.0 mg/dL, and two patients developed the abnormality. The iPTH level necessitating interruption of therapy in our study was 70 pg/mL, whereas in their study it was 15 pg/mL; hence, a comparison of the incidence of “low” iPTH is difficult. Also, the previous study used an older first-generation iPTH assay. In our study, the starting dose was 1.5 μg/day of doxercalciferol, and increments were in steps of 1.0 μg/day every 6 weeks, whereas in the study by Coburn et al., the starting dose was 1 μg/day and increments were in steps of 0.5 μg/day every 2-4 weeks. One would expect a higher incidence of hypercalcemia and severe hyperphosphatemia in our study, which in fact was the case, but the absolute numbers were still acceptable. Also, most patients promptly recovered their baseline biochemical values once doxercalciferol was temporarily stopped and the phosphate binder dose was modified. Treatment interruptions were more frequent in our study (n = 22) compared with twice in the other study. The difference, at least in part, could probably be attributed to a higher threshold for stopping treatment (iPTH <70 compared with iPTH <15) and also to the generally larger doses employed in our study.
Doxercalciferol had to be stopped temporarily in 21 patients, the most common reason, by far, being a low iPTH level. A more conservative dosing schedule with more frequent monitoring of biochemical values might have allowed a more calibrated control of SHPT. But, in the Indian setting, such monitoring might not be feasible because of significant financial constraints. From this point of view, the current study is a representation of what might happen in the “real world.” A smaller starting dose and gradual increase in the dose with monitoring of iPTH levels every 2 weeks would enable better achievement of the target iPTH level with lesser incidence of extreme biochemical values, and eliminate the need for stopping doxercalciferol because of oversuppression of the iPTH levels.
This study is one of the few prospective studies on the use of doxercalciferol in pre-dialysis CKD patients, and probably the first in a South Asian population. It confirms that doxercalciferol is effective in suppressing SHPT in patients with CKD-4 with an acceptable incidence of hypercalcemia and hyperphosphatemia. When low iPTH or hypercalcemia or severe hyperphosphatemia occurs necessitating temporary stoppage of treatment, the biochemical abnormalities revert promptly on dose adjustments.
Acknowledgments
Dr. Reddy's Laboratories Ltd., Hyderabad, India, provided doxercalciferol to the patients and arranged for iPTH estimations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Coco M, Rush H. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis. 2000;36:1115–21. doi: 10.1053/ajkd.2000.19812. [DOI] [PubMed] [Google Scholar]
- 2.Danese MD, Kim J, Doan QV, Dylan M, Griffiths R, Chertow GM. PTH and the risks for hip, vertebral, and pelvic fractures among patients on dialysis. Am J Kidney Dis. 2006;47:149–56. doi: 10.1053/j.ajkd.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 1,25-dihydroxy-cholecalciferol in uremic patients. J Clin Invest. 1984;74:2136–43. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989;321:274–9. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann P, Ritz E, Schmidt-Gayk H, Schäfer I, Geyer J, Nonnast- Daniel B, et al. Comparison of intermittent and continuous oral administration of calcitriol in dialysis patients: A randomized prospective trial. Nephron. 1994;67:48–53. doi: 10.1159/000187887. [DOI] [PubMed] [Google Scholar]
- 6.Nordal KP, Dahl E. Low dose calcitriol versus placebo in patients with predialysis chronic renal failure. J Clin Endocrinol Metab. 1988;67:929–36. doi: 10.1210/jcem-67-5-929. [DOI] [PubMed] [Google Scholar]
- 7.Baker LR, Abrams L, Roe CJ, Faugere MC, Fanti P, Subayti Y, et al. 1,25(OH)2D3 administration in moderate renal failure: A prospective double-blind trial. Kidney Int. 1989;35:661–9. doi: 10.1038/ki.1989.36. [DOI] [PubMed] [Google Scholar]
- 8.Brown AJ, Finch JL, Lopez-Hilker S, Dusso A, Ritter C, Pernalete N, et al. New active analogues of vitamin D with low calcemic activity. Kidney Int. 1990;29:S22–7. [PubMed] [Google Scholar]
- 9.Brown AJ, Dusso A, Slatopolsky E. Selective vitamin D analogs and their therapeutic applications. Semin Nephrol. 1994;14:156–74. [PubMed] [Google Scholar]
- 10.Gallagher JC, Bishop CW, Knutson JC, Mazess RB, DeLuca HF. Effects of increasing doses of 1alpha-hydroxyvitamin D2 on calcium homeostasis in postmenopausal osteopenic women. J Bone Miner Res. 1994;9:607–14. doi: 10.1002/jbmr.5650090504. [DOI] [PubMed] [Google Scholar]
- 11.Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43:877–90. doi: 10.1053/j.ajkd.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Tan AU, Jr, Levine BS, Mazess RB, Kyllo DM, Bishop CW, Knutson JC, et al. Effective suppression of parathyroid hormone by 1 alpha-hydroxy-vitamin D2 in hemodialysis patients with moderate to severe secondary hyperparathyroidism. Kidney Int. 1997;51:317–23. doi: 10.1038/ki.1997.39. [DOI] [PubMed] [Google Scholar]
- 13.Frazão JM, Elangovan L, Maung HM, Chesney RW, Acchiardo SR, Bower JD, et al. Intermittent doxercalciferol (1alpha-hydroxyvitamin D(2)) therapy for secondary hyperparathyroidism. Am J Kidney Dis. 2000;36:550–61. doi: 10.1053/ajkd.2000.16193. [DOI] [PubMed] [Google Scholar]
- 14.Maung HM, Elangovan L, Frazão JM, Bower JD, Kelley BJ, Acchiardo SR, et al. Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1alpha-hydroxyvitamin D(2)) in dialysis patients with secondary hyperparathyroidism: A sequential comparison. Am J Kidney Dis. 2001;37:532–43. [PubMed] [Google Scholar]


