Abstract
Roux-en-Y gastric bypass (RYGB) surgery has become an accepted treatment for excessive obesity. We conducted a longitudinal study to assess regional body composition, muscle proteolysis, and energy expenditure before RYGB, and 6 and 12 months after RYGB. Whole-body and regional fat mass (FM) and lean mass (LM) were assessed via dual-energy X-ray absorptiometry (DXA), and myofibrillar protein degradation was estimated by urinary 3-methylhistidine (3-MeH) in 29 subjects. Energy expenditure and substrate oxidation were also determined using a whole-room, indirect calorimeter in 12 of these subjects. LM loss constituted 27.8 ± 10.2% of total weight loss achieved 12 months postoperatively, with the majority of LM loss (18 ± 6% of initial LM) occurring in the first 6 months following RYGB. During this period, the trunk region contributed 66% of whole-body LM loss. LM loss occurred in the first 6 months after RYGB despite decreased muscle protein breakdown, as indicated by a decrease in 3-MeH concentrations and muscle fractional breakdown rates. Sleep energy expenditure (SEE) decreased from 2,092 ± 342 kcal/d at baseline to 1,495 ± 190 kcal/day at 6 months after RYGB (P < 0.0001). Changes in both LM and FM had an effect on the reduction in SEE (P < 0.001 and P = 0.005, respectively). These studies suggest that loss of LM after RYGB is significant and strategies to maintain LM after surgery should be explored.
INTRODUCTION
Weight loss, even moderate amounts, is an established means to improve or ameliorate numerous obesity-related disease risk factors (1). Lifestyle interventions (diet, exercise, and behavioral therapy) for weight loss are challenging modalities, especially in the excessive obese population, and weight recidivism is frequent (2). As a result, surgical approaches to weight loss are now considered acceptable means for long-term treatment of obesity. One of the most predominant bariatric surgical procedures in the United States is Roux-en-Y gastric bypass (RYGB). Weight loss due to RYGB is a consequence of calorie restriction and nutrient malabsorption and results in ~60% excess body weight loss (3).
A recent systematic review of the literature by Chaston et al. reported that fat-free mass (FFM) loss comprised 31.3% of the weight loss subsequent to RYGB (4). FFM consists of skeletal muscle, organs, bone, and connective tissue (5). Skeletal muscle and organs (nonbone lean mass (LM)) are the most metabolically active components of FFM and are the major determinants of resting energy expenditure (REE) (5). REE decreases after RYGB (6–10), and some studies suggest that a decrease in REE after weight loss can be accounted for solely by FFM loss (7–9). Das et al. determined that fat mass (FM) also contributed to the reduced REE expenditure after RYGB (9).
There is a paucity of literature detailing the nature of LM losses incurred with RYGB. The regional distribution of LM and FM changes with RYGB has only been described in a small cohort of adolescents (11), which constitutes a special sub-population of RYGB patients. Furthermore, tissue protein breakdown has not been investigated after RYGB. A reduced protein turnover has been described after gastroplasty (12); however, restrictive procedures result in less LM loss than RYGB (4). Thus, the objective of our study was to explore temporal and regional changes in body tissue composition and to measure myofibrillar protein breakdown at 6 and 12 months after RYGB. We also report longitudinal changes in whole-body tissue composition after RYGB and the association with energy expenditure.
METHODS AND PROCEDURES
Subjects
Our study population consisted of 29 subjects with extreme obesity who were approved and scheduled for RYGB and were between the ages of 18 and 65 years. Exclusion criteria included a history of type 1 diabetes, current use of oral anticoagulants, intercurrent infection, a prior gastric operation, or a positive pregnancy test. The study protocol was approved by the Vanderbilt Institutional Review Board, and informed consent was obtained from each subject.
Study protocol
Each patient was admitted to the Vanderbilt Clinical Research Center three times: preoperatively, 6 months postoperatively, and 12 months postoperatively. Subjects were not under any dietary restrictions prior to the preoperative study. Oral antidiabetic medications and long-acting insulin were discontinued 5 days before each study visit, and short-term insulin therapy was initiated. Postoperatively, each patient was counseled by a bariatric registered dietitian to consume a balanced, postgastrectomy diet of 900–1,000 kcal/day and was encouraged to exercise. At each visit, subjects underwent a history and physical examination, and anthropometric and body composition measurements. A 24-h urine collection was obtained from each subject and was maintained at 4 °C until long-term storage at −80 °C. Twelve of these subjects also participated in a 24-h metabolic study in a whole-room indirect calorimeter after a supervised overnight fast.
Anthropometric measurements
Weight was measured on the same digital scale (Seca, Hamburg, Germany) to the nearest 0.1 kg. Height was measured with a wall-mounted stadiometer (Perspective Enterprises, Portage, MI) to the nearest 0.5 cm. To resolve difficulties in locating the true waist due to differences in muscle tone and the presence of a pannus, waist circumference was measured as the minimum diameter between the iliac crest and the rib cage. Hip circumference was taken at the widest point near the level of the femoral greater trochanter. All measurements were obtained by the same investigator.
Body composition
Body composition was measured by dual-energy X-ray absorptiometry (DXA) using narrow fan-beam technology (Lunar Prodigy, enCore software version 10.5; GE Medical Systems, Madison, WI). The mean coefficients of variation from two scans in 72 subjects (>50% with a BMI >30 kg/m2) are 0.96 ± 0.76% for FM and 0.74 ± 0.91% for LM. Half-body scans were performed for the right side from which whole-body composition was extrapolated (13, 14). FM and nonbone LM were determined for the whole body and for the regions of interests: extremities (arms and legs), trunk, and abdomen. Extremity and trunk regions were defined via existing software parameters. The abdominal region was manually defined, using the top of the external iliac crest as the lower limit and half the distance from there to the acromion as the upper limit. The DXA was calibrated daily using a manufacturer-provided phantom. All DXA measurements and analyses were conducted in concert by the same two investigators.
Energy expenditure and substrate oxidation
Energy expenditure and substrate oxidation were measured using a whole-room indirect calorimeter as previously described (15). Total energy expenditure (TEE) was defined as the total energy spent during the approximately 24-h stay in the room calorimeter. Sleep energy expenditure (SEE, kcal/min) was delineated as the period of time spent lying on mattress at night between 12:00 and 4:00 am without any significant movement determined by the force platform and calculated by an automatic algorithm in data processing. SEE was extrapolated to 24 h to obtain total SEE, which is a proxy for total REE but is measured over an extended period (~4 h). Total respiratory quotient (RQ) was calculated as the average minute-by-minute ratio of CO2 production to O2 consumption measured during the room calorimeter stay. Sleep RQ was calculated as the average minute-by-minute RQ measured during sleep. Substrate oxidation was calculated from the VO2 and RQ over 1-min intervals during the ~24-h stay (16). Total urine nitrogen concentration was used to determine 24-h protein oxidation.
Biochemical analyses
The 24-h urine samples were assayed for 3-methylhistidine (3-MeH), 1-MeH, and nitrogen. Excreted 3-MeH is a marker of myofibrillar protein breakdown (17), and 1-MeH serves as an indicator of dietary meat intake that could confound the 3-MeH data (18). Subjects were not instructed to maintain a meat-free diet. Urinary MeH levels were determined in duplicate using modification of a gas chromatography-mass spectrometry method previously reported (19). The daily fractional breakdown rate of muscle was calculated as the ratio of 3-MeH in the urine to the muscle precursor pool (0.6 μmol/g lean tissue) (17). Total urine nitrogen was measured using the Antek 9000 NS Analyzer (Antek Instruments, Houston, TX).
Statistical analyses
Study data were collected and managed using REDCap, a research electronic data capture tool hosted at Vanderbilt (20). Data are expressed as the mean ± s.d. Using previously published data (10), a sample size of 29 subjects would allow detection of a 3% change in LM, and a sample size of 12 would allow detection of an 11% change in SEE (α = 5 and 90% power) (21). Pairwise comparisons were made with the Wilcoxon signed ranks due to non-normally distributed data. A Holm adjustment was employed in the case of multiple comparisons. Differences in regional LM and FM losses were analyzed with the Kruskal–Wallis test. Mixed-model regression analysis was performed to determine the relationship between SEE and body composition. All statistical analyses were performed in R2.6.2 (http://www.r-project.org).
RESULTS
Baseline characteristics and anthropometric measurements
Our study population of 29 subjects with extreme obesity (BMI >40.0 kg/m2) predominately consisted of white females (72%) (Table 1). Approximately half of the subjects (45%) had a clinical diagnosis of type 2 diabetes preoperatively. One year after RYGB, subjects lost an average of 44.3 ± 10.2 kg of body weight, with ~80% of this weight loss occurring in the first 6 months (Table 2). This cohort of RYGB patients resided at the lower range of obesity class I (BMI = 30.0–34.9 kg/m2) 12 months postoperatively. The 25% reduction in waist circumference and 6% reduction in waist-to-hip ratio observed at 12 months after RYGB also primarily occurred in the first 6 months.
table 1.
Subject characteristics
| Age (years) | 43.8 ± 9.6 |
|---|---|
| Gender (male/female) | 4/25 |
| Race (AA/white) | 5/24 |
| Diabetes (yes/no) | 13/16 |
| BMI (kg/m2) | 46.3 ± 5.5 |
Values are mean ± s.d.
AA, African-American.
table 2.
Anthropometric measurements
| Measurement | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| Weight (kg) | 128.5 ± 16.1 | 92.9 ± 13.3* | 84.2 ± 13.3* |
| BMI (kg/m2) | 46.3 ± 5.5 | 33.5 ± 4.8* | 30.4 ± 4.7* |
| Waist circumferencea(cm) | 127.3 ± 11.5 | 102.7 ± 12.4* | 95.6 ± 10.4* |
| Waist-to-hip ratioa | 0.89 ± 0.08 | 0.85 ± 0.08* | 0.84 ± 0.07 |
Data are mean ± s.d.
n = 26.
P < 0.0003; baseline vs. 6 months and 6 months vs. 12 months.
Whole-body composition
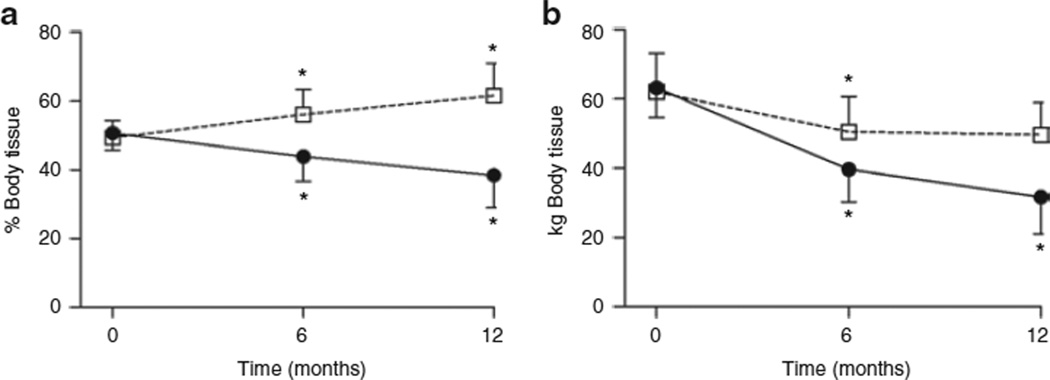
At baseline, the whole-body tissue mass in these obese subjects was equally distributed between FM and LM (Figure 1a). RYGB resulted in a decrease in percent body fat from 50.7 ± 5.0% to 38.4 ± 9.4% throughout the year subsequent to surgery (P < 0.0001). FM loss contributed 31.5 ± 7.9 kg of total body weight loss during this time (Figure 1b), or a 50.3 ± 13.3% reduction in FM (Table 3). Despite a steady increase in percent LM, overall LM declined by 12.2 ± 5.4 kg or 19.4 ± 7.3% of initial LM (Figure 1b and Table 3). LM loss contributed to 27.8 ± 10.2% of total weight loss during the 1-year follow-up. This loss of LM occurred almost entirely during the first 6 months after RYGB (Figure 1b and Table 3); LM loss constituted 32.5 ± 10.6% of weight loss during this time. Although all subjects lost LM in the first 6 months, the response in the second 6 months was variable: 16 subjects lost LM (2.4 ± 1.9 kg); 12 subjects gained LM (1.2 ± 1.2 kg); and one subject maintained her LM.
Figure 1.
Whole-body fat and lean tissue before and after gastric bypass surgery. (a) Percent body fat (closed circles) and lean (open squares) tissue and (b) fat (closed circles) and lean (open squares) tissue mass at baseline (time 0 months), and at 6 and 12 months after surgery. Values are mean ± s.d. *P < 0.0001, baseline vs. 6 months and 6 months vs. 12 months.
table 3.
Percent changes in fat and lean tissue mass after gastric bypass surgery
| Region | 0–6 Months | 6–12 Months | 0–12 Months |
|---|---|---|---|
| Whole body | |||
| Fat | −38 ± 9 | −21 ± 13 | 50 ± 13 |
| (−62, −21) | (−45, 2) | (−79, −29) | |
| Lean | −18 ± 6 | −1 ± 5 | −19 ± 7 |
| (−29, −6) | (−13, 11) | (−35, −4) | |
| Extremities | |||
| Fat | −35 ± 13 | −20 ± 12 | −47 ± 15 |
| (−59, 21) | (−39, 7) | (−74, 4) | |
| Lean | −14 ± 12 | −2 ± 5 | −17 ± 11 |
| (26, 40) | (−13, 16) | (−29, 33) | |
| Trunk | |||
| Fat | −39 ± 14 | −23 ± 17 | −53 ± 16 |
| (−67, −7) | (57, 7) | (−84, −29) | |
| Lean | −22 ± 11 | −0.2 ± 7 | −22 ± 12 |
| (−38, −0.5) | (−15, 17) | (−45, 2) | |
| Abdomen | |||
| Fat | −42 ± 11 | −25 ± 19 | −56 ± 16 |
| (−67, −26) | (−62, 13) | (−87, −26) | |
| Lean | −20 ± 9 | 2 ± 9 | −18 ± 12 |
| (−38, 0.6) | (−12, 22) | (−40, 5) | |
| Nonabdominal trunk | |||
| Fat | −37 ± 17 | −22 ± 17 | −50 ± 17 |
| (−69, 13) | (−55, 4) | (−83, −24) | |
| Lean | −23 ± 13 | −1 ± 8 | −24 ± 14 |
| (−46, 3) | (−17, 14) | (−50, 4) |
Data are percent tissue mass change during the indicated time periods, and are expressed as mean ± s.d. (minimum, maximum).
Regional body composition
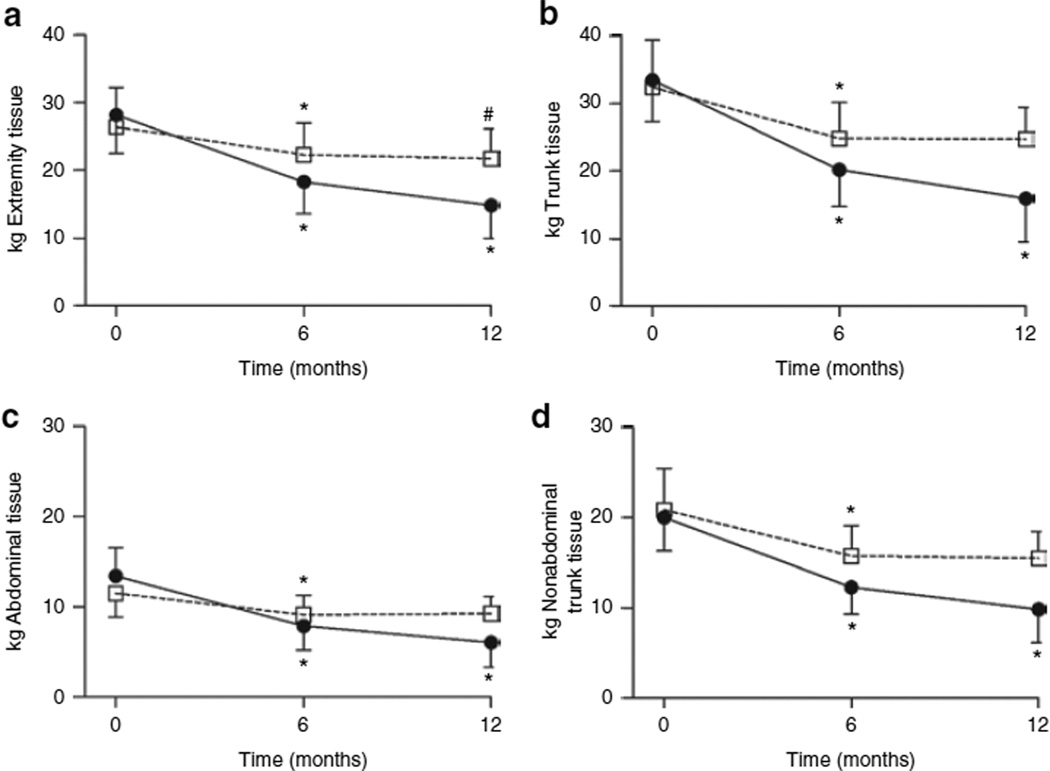
The regional distribution of changes in FM and LM resulting from RYGB were also analyzed (Figure 2 and Table 3). Overall, the trunk region accounted for more whole-body weight loss than the extremities (57 ± 10% vs. 41 ± 12%, respectively; P < 0.0001). FM loss in the extremities and trunk occurred both in the first (P < 0.0001) and second 6 months (P < 0.0001) after RYGB, and LM loss in both regions occurred primarily in the first 6 months (P < 0.0001). The change in LM in the extremities reached significance in the second 6 months after RYGB (P = 0.0006), even though the average change in LM was a decrement of 0.6 ± 1.1 kg or 2.3 ± 5.5% (Table 3). LM loss from the trunk accounted for ~66% of whole-body LM loss 6 months after RYGB. During this period, LM loss in the trunk region also incurred a greater proportional loss in LM compared to the extremities (22 ± 11% vs. 14 ± 12%, respectively; P = 0.009). Percent FM loss during this same time frame was comparable between the trunk and extremities (39 ± 14% vs. 35 ± 13%, respectively; P = 0.082).
Figure 2.
Changes in regional fat and lean tissue mass after gastric bypass surgery. Data are expressed as mean ± s.d. for fat (closed circles) and lean (open squares) tissue mass in the (a) extremities, (b) trunk, (c) abdomen, and (d) nonabdominal trunk at baseline (time 0 months), and at 6 and 12 months after surgery. *P < 0.0001 and #P = 0.006, baseline vs. 6 months and 6 months vs. 12 months.
The trunk region was further divided into the abdominal region and nonabdominal trunk region. The nonabdominal trunk region accounted for ~45% of whole-body LM loss in the first 6 months after RYGB. Percent LM loss 6 months after RYGB in the nonabdominal trunk region was greater than the abdominal region (23 ± 13% vs. 20 ± 9%, respectively; P = 0.053). FM loss exhibited the opposite tendency, with the abdominal region experiencing a larger proportional decrease in FM than the non-abdominal trunk region (42 ± 11% vs. 37 ± 17%, respectively; P = 0.030). During the year after RYGB, the losses of LM and FM were similar among the trunk, extremities, abdomen, and nonabdominal trunk regions (P = 0.121 and 0.312, respectively). Overall, the greatest regional percentage of LM loss occurred in the nonabdominal trunk (24 ± 14%), and the greatest percentage of FM loss occurred in the abdomen (56 ± 16%).
Estimates of muscle proteolysis
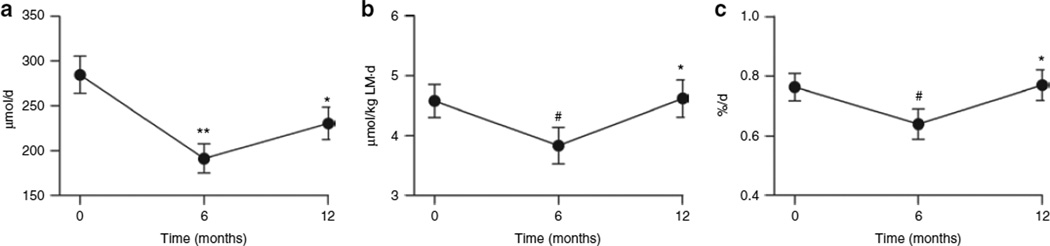
Daily urinary excretion of 3-MeH (a measure of myofibrillar protein breakdown) (Figure 3a) decreased from baseline at 6 months after RYGB (P < 0.0001) and increased thereafter (P = 0.003). When daily 3-MeH excretion was expressed relative to LM (Figure 3b), the same trends were observed, except 12-month postoperative levels had returned to baseline. The daily fractional breakdown rate of muscle tissue decreased at 6 months after RYGB (P = 0.045) and then returned to preoperative levels by 12 months after RYGB (P = 0.003). To ensure that the measured 3-MeH was from degraded muscle and not dietary meat intake, urinary 1-MeH was also determined. Although the 3-MeH:1-MeH ratio declined over time (0.60 ± 0.80, 0.49 ± 0.55, and 0.38 ± 0.40), the changes during the first and second 6 months after RYGB were not significant (P = 0.142 and 0.721, respectively).
Figure 3.
Estimates of muscle tissue breakdown with Roux-en-Y gastric bypass surgery. (a) 24-h 3-methylhistidine (3-MeH) levels, (b) amount of 3-MeH per kg of lean mass, and (c) fractional breakdown rate of muscle at baseline (time 0 months), and at 6 and 12 months after surgery. Data are expressed as mean ± s.d. *P < 0.005, **P < 0.0001, and #P < 0.05, baseline vs. 6 months and 6 months vs. 12 months.
Energy expenditure
In a group of 12 subjects, both TEE and SEE (Table 4) decreased 6 months following RYGB (P = 0.003 and 0.006, respectively), with no further changes in the subsequent 6 months (P ≥ 0.444). The decrease in TEE at 12 months after RYGB was 790 ± 334 kcal/day, and SEE declined by 563 ± 208 kcal/day. In the mixed-model regression analysis, the intercept model showed a decrease in SEE from baseline to 12 months (P < 0.0001). When body composition changes were entered into the model, the change in SEE was not independent of the changes in body composition. The change in LM and FM were significant contributors to changes in SEE (P < 0.001 and P = 0.005, respectively). For every 1 kg of LM lost, SEE decreased by 18.6 kcal/day, and for every 1 kg of FM lost, SEE decreased by 8.5 kcal/day.
table 4.
Energy metabolism and substrate oxidation
| Measurement | Baseline | 6 Months | 12 Months |
|---|---|---|---|
| Total EE (kcal/day) | 2,768 ± 474 | 2,010 ± 260* | 1,987 ± 228 |
| Sleep EE (kcal/day) | 2,092 ± 342 | 1,495 ± 190* | 1,513 ± 192 |
| Total RQ | 0.89 ± 0.04 | 0.84 ± 0.04** | 0.82 ± 0.04 |
| Sleep RQ | 0.90 ± 0.06 | 0.86 ± 0.05 | 0.81 ± 0.05† |
| Nonprotein RQ | 0.92 ± 0.06 | 0.85 ± 0.05 | 0.84 ± 0.06† |
| Carbohydrate oxidation (g/kg·day) | 3.2 ± 0.8 | 2.3 ± 0.7** | 2.2 ± 0.9 |
| Fat oxidation (g/kg·day) | 0.6 ± 0.4 | 1.0 ± 0.3 | 1.1 ± 0.5† |
| Protein oxidation (g/kg·day) | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.8 ± 0.3† |
Data are mean ± s.d.
EE, energy expenditure; RQ, respiratory quotient.
P < 0.02 and
P = 0.049 for baseline vs. 6 months and
P ≤ 0.006 for baseline vs. 12 months.
Substrate oxidation
All measures of RQ (total, sleep, and nonprotein) in 12 subjects were decreased (P < 0.02) 12 months after RYGB (Table 4). At 6 months after RYGB, total RQ was marginally lower than baseline (P = 0.049). Accordingly, carbohydrate oxidation rates (g/kg·day) were decreased 6 months after surgery (P = 0.048), and remained stable during the second 6 months after RYGB. Fat oxidation exhibited a corresponding increase after RYGB, but did not attain significance until 1 year (P = 0.003). Energy derived from fat increased from 27 to 45% at 12 months after RYGB (P = 0.073), and energy from carbohydrates decreased from 64 to 38% (P = 0.003). Protein oxidation increased at 12 months (P = 0.003) as did percent energy from protein (12–16%, P = 0.015).
DISCUSSION
With the increasing popularity of bariatric surgery as a means for weight loss, it is imperative to understand the impact of the rapid weight loss incurred with this procedure. Our finding that LM accounted for 27.7% of total weight loss achieved 1 year after RYGB is slightly higher than reports of 21.2% (9), 24.8% (7), and ~20% (22) of weight loss from LM. The 31.3% contribution of FFM to weight loss after RYGB cited by Chaston et al. was derived from four studies, the majority of which examined early postoperative time points (4). Indeed, we observed a 32.7% contribution of LM to total weight loss in the first 6 months after RYGB. This figure is in agreement with one study that reported a 33.5% contribution of LM to overall weight loss 6 months after RYGB (7), but discordant with the 22.9% described by another group (8). Of the aforementioned studies, ours was the only one to use DXA, a three compartmental model of body composition analysis. DXA does not distinguish between water loss and LM loss (23), although the effect of hydration status on tissue estimation errors was deemed minimal (24). Several studies have concluded that measurement of FM and LM by DXA corresponds well with other techniques during periods of moderate weight loss (25, 26), whereas others disagree (27); comparison of different body composition techniques after bariatric surgery has not been reported.
Inge et al. performed DXA measurements on five adolescents before and 1 year after RYGB (11). They determined that 17% of tissue mass lost in the trunk consisted of LM. In our study cohort, LM loss in the trunk comprised 36% of total trunk tissue loss (Table 3). This disparity could be due to differences in sample size, or that our study population is ~25 years older. Less preservation of LM after dramatic weight loss in an older population is plausible given the established loss of lean tissue with aging. Interestingly, our data show that LM reduction in the trunk accounted for 66% of whole-body LM loss. The use of DXA for estimates of regional tissue mass measurements has not been extensively validated, and the arrangement of bones and high bone content of certain regions may interfere with accurate tissue mass measurements (23). However, none of the other commonly used methods for body composition are capable of such determinations. Other methods of regional body composition, such as magnetic resonance imaging, are cost and size prohibitive.
The mechanism(s) of accelerated LM loss in the first 6 months following gastric bypass surgery in comparison to the second 6 months remains unclear. We measured urinary 3-MeH as a marker of myofibrillar breakdown. 3-MeH is an amino acid formed from the methylation of histidine in actin and myosin chains. Once released from muscle protein stores, 3-MeH cannot be reutilized for protein synthesis and is excreted in the urine (17). Contrary to expectations during LM loss, the 3-MeH data suggest that muscle breakdown is decreased 6 months after gastric bypass. These findings are similar to what had been reported in response to prolonged fasting (28). During periods of malnourishment, rates of protein turnover are diminished and attributed to decreases in both rates of whole-body protein synthesis and degradation (29). From 6 to 12 months after RYGB, the rate of protein degradation was diminished and returned to preoperative levels, coincident with attenuation of LM loss. This pattern of decreased and then increased myofibrillar proteolysis is similar to the progression of protein breakdown in response to starvation with protein synthesis inhibition in the rat (30). In contrast to our findings in RYGB, protein breakdown was reduced 3 months after vertical banded gastroplasty and remained depressed at 12 months (12). Regardless, the compensatory protein sparing mechanism invoked after RYGB does not appear to counter-balance the LM losses.
Low protein turnover rates during a 3-week, low-energy diet (~500 kcal/day) in obese subjects can be offset by adequate dietary protein intake (50 g/day) (31). A prospective study revealed that RYGB subjects self-reported insufficient protein intake related to protein intolerance 1 year after surgery (32). Nonetheless, protein malnutrition (hypoalbuminuria) after RYGB appears rare (33) and related to postoperative complications (34, 35) or a Roux limb length >150 cm (36). Although dietary protein intake was not recorded in our study, patients receive dietetic counseling to consume 70 g/day protein, and albumin levels were normal after RYGB (3.6 ± 0.2 g/ dl; range 3.2–4.7 g/dl), indicating no clinical protein malnutrition. Participants in a hypocaloric diet program who maintained a higher amount of protein intake incurred less LM loss (37). Similar prospective randomized studies have not been conducted in the bariatric surgery population; thus, it is presently unclear whether inadequate protein consumption and/or absorption contributes to LM loss after RYGB.
The first 6 months after RYGB are characterized by a reduction in carbohydrate oxidation; this has been proposed to drive the metabolic response to fasting, i.e., increased protein oxidation and lipolysis (38). Our data do not show a significant increase in protein or fat oxidation until 12 months after surgery, although the increase in fat oxidation occurred mostly in the first 6 months after RYGB without a major change in the second 6 months. Considering the most substantial changes in diet and weight occur in the first 6 months after surgery, a larger sample size might reveal earlier changes in these variables. The detectable change in carbohydrate oxidation in our 12 subjects suggests that this is a more robust effect of RYGB on substrate metabolism than protein oxidation.
One strength of our study is the use of the whole-room indirect calorimeter to measure energy expenditure and substrate oxidation, allowing for measurements over a long time period during free movement (15). Several studies, including the present work, have described a reduced REE after RYGB (6–10). There is controversy as to whether decreases in REE are larger than expected with respect to tissue losses (7–9). Our data support the contribution of LM loss changes in REE, and further suggest that a reduced FM also contributes to the reduced SEE following RYGB, although, on a per kg basis, LM loss has a greater effect on the reduction of SEE. TEE was also reduced in our subjects after RYGB, which, in addition to SEE, accounts for energy expended during activity. Physical activity measured in free living with Actical accelerometers (Respironics, Bend, OR) did not change 1 year after RYGB (data not shown). A recent observational study by Faria et al. suggests that subjects with weight regain 2 years after RYGB have a lower REE (39), indicating that elevating REE after RYGB might enhance weight loss. Physical activity may be an effective means to increase LM and thus REE after RYGB (40); however, exercise intervention studies following RGYB are lacking.
It is not known how LM loss affects anthropometric and metabolic outcomes after bariatric surgery; larger studies are needed to make such assessments. Although substantial weight loss is achieved with surgery, patients often remain overweight or obese. Further research is warranted to determine whether minimizing lean tissue mass loss, especially in the trunk region, during the first 6 months following gastric bypass might improve clinical outcomes.
ACKNOWLEDGMENTS
This work was supported by the following National Institutes of Health grants: National Institute of Diabetes and Digestive and Kidney Diseases RO1-DK070860 to N.N.A., the Vanderbilt CTSA grant 1 UL1 RR024975 from the National Center for Research Resources, DK20593 to the Vanderbilt Diabetes Research and Training Center, and DK058404 to the Vanderbilt Digestive Disease Research Center. We thank James M. Isbell and Julia P. Dunn for their advice on this project.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Phelan S, Wadden TA, Berkowitz RI, et al. Impact of weight loss on the metabolic syndrome. Int J Obes (Lond) 2007;31:1442–1448. doi: 10.1038/sj.ijo.0803606. [DOI] [PubMed] [Google Scholar]
- 2.Wadden TA, Butryn ML, Byrne KJ. Efficacy of lifestyle modification for long-term weight control. Obes Res. 2004;12(Suppl):151S–162S. doi: 10.1038/oby.2004.282. [DOI] [PubMed] [Google Scholar]
- 3.Buchwald H, Estok R, Fahrbach K, et al. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248.e5–256.e5. doi: 10.1016/j.amjmed.2008.09.041. [DOI] [PubMed] [Google Scholar]
- 4.Chaston TB, Dixon JB, O’Brien PE. Changes in fat-free mass during significant weight loss: a systematic review. Int J Obes (Lond) 2007;31:743–750. doi: 10.1038/sj.ijo.0803483. [DOI] [PubMed] [Google Scholar]
- 5.Müller MJ, Bosy-Westphal A, Kutzner D, Heller M. Metabolically active components of fat-free mass and resting energy expenditure in humans: recent lessons from imaging technologies. Obes Rev. 2002;3:113–122. doi: 10.1046/j.1467-789x.2002.00057.x. [DOI] [PubMed] [Google Scholar]
- 6.Bobbioni-Harsch E, Morel P, Huber O, et al. Energy economy hampers body weight loss after gastric bypass. J Clin Endocrinol Metab. 2000;85:4695–4700. doi: 10.1210/jcem.85.12.7083. [DOI] [PubMed] [Google Scholar]
- 7.Carey DG, Pliego GJ, Raymond RL. Body composition and metabolic changes following bariatric surgery: effects on fat mass, lean mass and basal metabolic rate: six months to one-year follow-up. Obes Surg. 2006;16:1602–1608. doi: 10.1381/096089206779319347. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco F, Papapietro K, Csendes A, et al. Changes in resting energy expenditure and body composition after weight loss following Roux-en-Y gastric bypass. Obes Surg. 2007;17:608–616. doi: 10.1007/s11695-007-9117-z. [DOI] [PubMed] [Google Scholar]
- 9.Das SK, Roberts SB, Kehayias JJ, et al. Body composition assessment in extreme obesity and after massive weight loss induced by gastric bypass surgery. Am J Physiol Endocrinol Metab. 2003;284:E1080–E1088. doi: 10.1152/ajpendo.00185.2002. [DOI] [PubMed] [Google Scholar]
- 10.Olbers T, Björkman S, Lindroos A, et al. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg. 2006;244:715–722. doi: 10.1097/01.sla.0000218085.25902.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inge T, Wilson KA, Gamm K, et al. Preferential loss of central (trunk) adiposity in adolescents and young adults after laparoscopic gastric bypass. Surg Obes Relat Dis. 2007;3:153–158. doi: 10.1016/j.soard.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 12.van Gemert WG, Westerterp KR, van Acker BA, et al. Energy, substrate and protein metabolism in morbid obesity before, during and after massive weight loss. Int J Obes Relat Metab Disord. 2000;24:711–718. doi: 10.1038/sj.ijo.0801230. [DOI] [PubMed] [Google Scholar]
- 13.Rothney MP, Brychta RJ, Schaefer EV, Chen KY, Skarulis MC. Body composition measured by dual-energy X-ray absorptiometry half-body scans in obese adults. Obesity (Silver Spring) 2009;17:1281–1286. doi: 10.1038/oby.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tataranni PA, Ravussin E. Use of dual-energy X-ray absorptiometry in obese individuals. Am J Clin Nutr. 1995;62:730–734. doi: 10.1093/ajcn/62.4.730. [DOI] [PubMed] [Google Scholar]
- 15.Sun M, Reed GW, Hill JO. Modification of a whole room indirect calorimeter for measurement of rapid changes in energy expenditure. J Appl Physiol. 1994;76:2686–2691. doi: 10.1152/jappl.1994.76.6.2686. [DOI] [PubMed] [Google Scholar]
- 16.Froidevaux F, Schutz Y, Christin L, Jéquier E. Energy expenditure in obese women before and during weight loss, after refeeding, and in the weight-relapse period. Am J Clin Nutr. 1993;57:35–42. doi: 10.1093/ajcn/57.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Young VR, Munro HN. Ntau-methylhistidine (3-methylhistidine) and muscle protein turnover: an overview. Fed Proc. 1978;37:2291–2300. [PubMed] [Google Scholar]
- 18.Sjölin J, Hjort G, Friman G, Hambraeus L. Urinary excretion of 1-methylhistidine: a qualitative indicator of exogenous 3-methylhistidine and intake of meats from various sources. Metab Clin Exp. 1987;36:1175–1184. doi: 10.1016/0026-0495(87)90245-9. [DOI] [PubMed] [Google Scholar]
- 19.Rathmacher JA, Link GA, Flakoll PJ, Nissen SL. Gas chromatographic/mass spectrometric analysis of stable isotopes of 3-methylhistidine in biological fluids: application to plasma kinetics in vivo. Biol Mass Spectrom. 1992;21:560–566. doi: 10.1002/bms.1200211107. [DOI] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupont WD, Plummer WD Jr. Power and sample size calculations. A review and computer program. Control Clin Trials. 1990;11:116–128. doi: 10.1016/0197-2456(90)90005-m. [DOI] [PubMed] [Google Scholar]
- 22.Palazuelos-Genis T, Mosti M, Sánchez-Leenheer S, et al. Weight loss and body composition during the first postoperative year of a laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2008;18:1–4. doi: 10.1007/s11695-007-9311-z. [DOI] [PubMed] [Google Scholar]
- 23.Roubenoff R, Kehayias JJ, Dawson-Hughes B, Heymsfield SB. Use of dual-energy X-ray absorptiometry in body-composition studies: not yet a “gold standard”. Am J Clin Nutr. 1993;58:589–591. doi: 10.1093/ajcn/58.5.589. [DOI] [PubMed] [Google Scholar]
- 24.Pietrobelli A, Wang Z, Formica C, Heymsfield SB. Dual-energy X-ray absorptiometry: fat estimation errors due to variation in soft tissue hydration. Am J Physiol. 1998;274:E808–E816. doi: 10.1152/ajpendo.1998.274.5.E808. [DOI] [PubMed] [Google Scholar]
- 25.Frisard MI, Greenway FL, Delany JP. Comparison of methods to assess body composition changes during a period of weight loss. Obes Res. 2005;13:845–854. doi: 10.1038/oby.2005.97. [DOI] [PubMed] [Google Scholar]
- 26.Thomson R, Brinkworth GD, Buckley JD, Noakes M, Clifton PM. Good agreement between bioelectrical impedance and dual-energy X-ray absorptiometry for estimating changes in body composition during weight loss in overweight young women. Clin Nutr. 2007;26:771–777. doi: 10.1016/j.clnu.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Minderico CS, Silva AM, Keller K, et al. Usefulness of different techniques for measuring body composition changes during weight loss in overweight and obese women. Br J Nutr. 2008;99:432–441. doi: 10.1017/S0007114507815789. [DOI] [PubMed] [Google Scholar]
- 28.Hoffer LJ, Forse RA. Protein metabolic effects of a prolonged fast and hypocaloric refeeding. Am J Physiol. 1990;258:E832–E840. doi: 10.1152/ajpendo.1990.258.5.E832. [DOI] [PubMed] [Google Scholar]
- 29.Millward DJ. Protein deficiency, starvation and protein metabolism. Proc Nutr Soc. 1979;38:77–88. doi: 10.1079/pns19790011. [DOI] [PubMed] [Google Scholar]
- 30.Lowell BB, Ruderman NB, Goodman MN. Regulation of myofibrillar protein degradation in rat skeletal muscle during brief and prolonged starvation. Metab Clin Exp. 1986;35:1121–1127. doi: 10.1016/0026-0495(86)90025-9. [DOI] [PubMed] [Google Scholar]
- 31.Garlick PJ, Clugston GA, Waterlow JC. Influence of low-energy diets on whole-body protein turnover in obese subjects. Am J Physiol. 1980;238:E235–E244. doi: 10.1152/ajpendo.1980.238.3.E235. [DOI] [PubMed] [Google Scholar]
- 32.Moize V, Geliebter A, Gluck ME, et al. Obese patients have inadequate protein intake related to protein intolerance up to 1 year following Roux-en-Y gastric bypass. Obes Surg. 2003;13:23–28. doi: 10.1381/096089203321136548. [DOI] [PubMed] [Google Scholar]
- 33.Shah M, Simha V, Garg A. Review: long-term impact of bariatric surgery on body weight, comorbidities, and nutritional status. J Clin Endocrinol Metab. 2006;91:4223–4231. doi: 10.1210/jc.2006-0557. [DOI] [PubMed] [Google Scholar]
- 34.Faintuch J, Matsuda M, Cruz ME, et al. Severe protein-calorie malnutrition after bariatric procedures. Obes Surg. 2004;14:175–181. doi: 10.1381/096089204322857528. [DOI] [PubMed] [Google Scholar]
- 35.Rinaldi Schinkel E, Pettine SM, Adams E, Harris M. Impact of varying levels of protein intake on protein status indicators after gastric bypass in patients with multiple complications requiring nutritional support. Obes Surg. 2006;16:24–30. doi: 10.1381/096089206775222168. [DOI] [PubMed] [Google Scholar]
- 36.Brolin RE, LaMarca LB, Kenler HA, Cody RP. Malabsorptive gastric bypass in patients with superobesity. J Gastrointest Surg. 2002;6:195–203. doi: 10.1016/s1091-255x(01)00022-1. discussion 204. [DOI] [PubMed] [Google Scholar]
- 37.Gordon MM, Bopp MJ, Easter L, et al. Effects of dietary protein on the composition of weight loss in post-menopausal women. J Nutr Health Aging. 2008;12:505–509. doi: 10.1007/BF02983202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein S, Wolfe RR. Carbohydrate restriction regulates the adaptive response to fasting. Am J Physiol. 1992;262:E631–E636. doi: 10.1152/ajpendo.1992.262.5.E631. [DOI] [PubMed] [Google Scholar]
- 39.Faria SL, Kelly E, Faria OP. Energy expenditure and weight regain in patients submitted to Roux-en-Y gastric bypass. Obes Surg. 2009;19:856–859. doi: 10.1007/s11695-009-9842-6. [DOI] [PubMed] [Google Scholar]
- 40.Metcalf B, Rabkin RA, Rabkin JM, Metcalf LJ, Lehman-Becker LB. Weight loss composition: the effects of exercise following obesity surgery as measured by bioelectrical impedance analysis. Obes Surg. 2005;15:183–186. doi: 10.1381/0960892053268381. [DOI] [PubMed] [Google Scholar]