Abstract
Strand displacement cascades are commonly used to make dynamically assembled structures. Particularly, the concept of “toehold-mediated DNA branch migration reactions” has attracted considerable attention in relation to dynamic DNA nanostructures. However, it is a challenge to obtain and control the formation of pure 1:1 ratio DNA duplexes with toehold structures. Here, for the first time, we report a photocontrolled toehold formation method, which is based on the photocleavage of 2-nitrobenzyl linker-embedded DNA hairpin precursor structures. UV light irradiation (λ≈365 nm) of solutions containing these DNA hairpin structures causes the complete cleavage of the nitrobenzyl linker, and pure 1:1 DNA duplexes with toehold structures are easily formed. Our experimental results indicate that the amount of toehold can be controlled by simply changing the dose of UV irradiation and that the resulting toehold structures can be used for subsequent toehold-mediated DNA branch migration reactions, e.g., DNA hybridization chain reactions. This newly established method will find broad application in the construction of light-powered, controllable and dynamic DNA nanostructures or large-scale DNA circuits.
Keywords: Photocontrol, DNA branch migration, DNA circuit, Toehold formation
INTRODUCTION
DNA branch migration was discovered in renatured circular molecules1 and has been well known in molecular biology. Branch migration describes the ability of a DNA strand partially paired with its complement in a duplex to extend its pairing by displacing the resident strand with which it is homologous. Extending this concept to DNA nanotechnology,2,3 Yurke et al. constructed a type of dynamic DNA-fueled molecular machine,4 which they termed as “toehold-mediated DNA strand displacement”. In this machine, a single-stranded DNA in a double-stranded complex is displaced by another single-stranded DNA with the help of a short sequence of contiguous bases called a “toehold”, and today this concept prevails in dynamic DNA nanostructures5–7 worldwide. For example, toehold-mediated branch migration has been used to trigger the assembly of DNA species,8–10 build DNA machines,11–15 and initiate the synchronized assembly of gold nanoparticles and construction of logic gates.16 Most notably, it has been applied to the construction of catalytic circuits,17–19 logic circuits20,21 and chemical reaction networks.22
In most dynamic DNA nanostructures and DNA circuit systems, oligonucleotide chains act as catalysts or as inputs that fuel the DNA-based molecular machine by a series of toehold-mediated branch migration reactions. For DNA circuits, each toehold-bearing DNA duplex must be purified in order to remove extra single strands when the stoichiometry is not perfect. This step avoids undesired reactions with other components in the circuit.21,23 Thus, pure 1:1 ratio toehold-bearing DNA duplexes are a prerequisite for the control of toehold-mediated branch migration reactions and their utilization in building large-scale circuits. Two methods, including polyacrylamide gel electrophoresis (PAGE) purification and digestion by DNA restriction enzymes,21 have been used to prepare the pure 1:1 ratio DNA duplexes. However, the PAGE purification methods are time-consuming and inefficient, and while it is more efficient, the restriction enzyme digestion method is limited by the use of restriction DNA enzymes and the generation of nonspecific by-products. Furthermore, it is hard to totally remove extra single strands using PAGE purification, especially in the autocatalyst DNA circuit.18 As a result, it remains a challenge to obtain and control the formation of pure 1:1 ratio toehold-bearing DNA duplexes for branch migration reactions.
We have addressed these issues by extending the previously reported strategy to generate sticky ends using a caging group flanked by DNA,25 which was advanced from the concept of “hidden toehold”. In contrast to the well-established overhanging-toehold systems, the “hidden toehold” concept (e.g., the ATP-activated displacement reaction developed by Liu et al.)24 allows regulation of a DNA branch migration reaction by environmental stimuli. However, Liu’s model requires the addition of ATP and subsequent removal of waste DNA molecules. In comparison, light is a clean energy source with controllable intensity, and it can activate a “hidden toehold” without the addition of any chemicals or generation of waste DNA molecules. We report here, for the first time, a new light-triggered, controllable toehold formation method capable of initiating and controlling the formation of pure 1:1 ratio DNA duplexes with overhanging-toehold structures. Compared to the well-established overhanging-toehold DNA duplexes prepared from two separate DNA strands, and different from the light-activated caged DNA base,26 we use a commercially available photocleavable nitrobenzyl linker27–31 (PC linker) to connect two complementary DNA strands, as shown in Figure 1a. This engineering design results in the formation of one long single-stranded DNA with two complementary parts (a and a*) and toehold (T) connected by the PC linker. After annealing, the linked DNA strand can form a hairpin precursor (Figure 1b), leading to a pure 1:1 ratio toehold-bearing DNA duplex upon UV light-induced photocleavage of the nitrobenzyl linker. The hairpin precursor prevents incorrect binding, and the stoichiometry is perfect because the two complementary parts are on the same initial strand.
Figure 1.

Photocleavage and photocontrolled toehold formation system. a) The chemical structure and principle of photocleavage of PC linker-connected DNA strands (shown as unannealed). b) Annealed linked DNA hairpin precursor, irradiated at 365 nm to form DNA duplex with toehold T.
RESULTS AND DISCUSSION
Validation of the photocontrolled toehold formation and evaluation of the photocleavage fraction of the PC linker
To study photocontrolled toehold formation and evaluate the photocleavage fraction of the PC linker, fluorescence spectroscopy experiments were performed using a quencher/fluorophore-labeled hairpin precursor. As shown in Figure 2, a 6-FAM-labeled DNA strand a and a Dabcyl-labeled DNA strand a* were connected using the PC linker. After annealing in the reaction buffer (20 mM Tris-HCl, 5 mM MgCl2, 300 mM NaCl, pH = 7.5), the DNA strand formed a hairpin precursor that resulted in quenching the FAM fluorescence. The toehold was hidden in this OFF state, thereby preventing the branch migration reaction. However, UV irradiation caused complete cleavage of the PC linker and subsequent release of the “hidden toehold”. The invading DNA strand then interacted with the toehold and displaced the FAM-labeled DNA strand, resulting in a significant increase in fluorescence intensity (ON state with recovered FAM fluorescence, as shown in Figure 2a).
Figure 2.
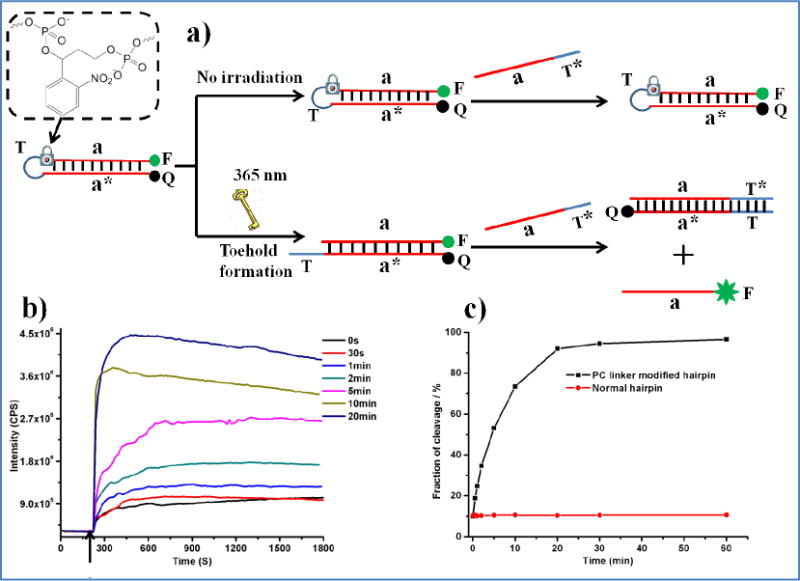
Photocontrolled toehold formation for toehold-mediated DNA branch migration reaction. a) The principle of photocontrolled hidden-toehold activation. b) Fluorescence test of toehold-mediated DNA branch migration reaction with different irradiation times. In a typical experiment, PC-linker-modified DNA hairpin precursor in buffer solution (150 μL, 200 nM) was irradiated with 365 nm light for different times. Then, 99 μL of photoirradiated hairpin was placed in a cuvette, and the invading strand (1 μL, 20 μM) was added to initiate the branch migration reaction. c) Plot of photocleavage fraction versus UV irradiation time. The arrow in Fig. 2 b shows the time that the invading strand was added.
Both time-dependent and static fluorescence spectra were measured (λex = 494 nm, λem = 519 nm). As expected, the fluorescence intensities increased with increasing UV irradiation time because more hidden toeholds were opened and available to the invading DNA. Even without UV irradiation, a slight fluorescence increase could also be observed. This may have occurred because a part of the “hidden toehold” could still interact with an invading DNA strand, causing partial opening of the hairpin precursor (9.5%, as shown in Figure 2c).
To determine the exact amount of released “hidden toehold”, the photocleavage fractions were compared by using a calibration curve. For calibration, different concentrations (20 nM, 40 nM, 100 nM and 200 nM) of quencher/fluorophore-labeled DNA duplexes with exposed toehold structure (the same situation as shown in Figure 2a after irradiation) were incubated with the invading DNA strand (200 nM) at room temperature for 30 min in reaction buffer. The fluorescence intensities at 519 nm were then recorded (λex = 494 nm). To obtain the standard curve, the fluorescence intensities were plotted versus the initial concentration of duplex with exposed toehold, and, as shown in Figure 3, the linear model fits the experimental data very well. For the photocleavage experiments, PC-linker-modified hairpin precursors or normal hairpin without PC linkers (200 nM) were irradiated at 365 nm for different times. After irradiation, the invading DNA strand (200 nM) was added, and the mixtures were incubated at room temperature for 30 min, followed by fluorescence measurement. Based on the fluorescence standard curve and the measured fluorescence intensity, the concentration of released toehold was determined and was divided by the initial hairpin concentration (200 nM) to obtain the fraction of cleaved PC linker for different irradiation times. As shown in Figure 2c, after 20 min of UV irradiation, the normal hairpin structure remained intact, and 92% of the hidden toehold was released. For subsequent studies, we choose to use 20 min as an optimized time, since this short irradiation time of irradiation provides sufficient toehold.
Figure 3.
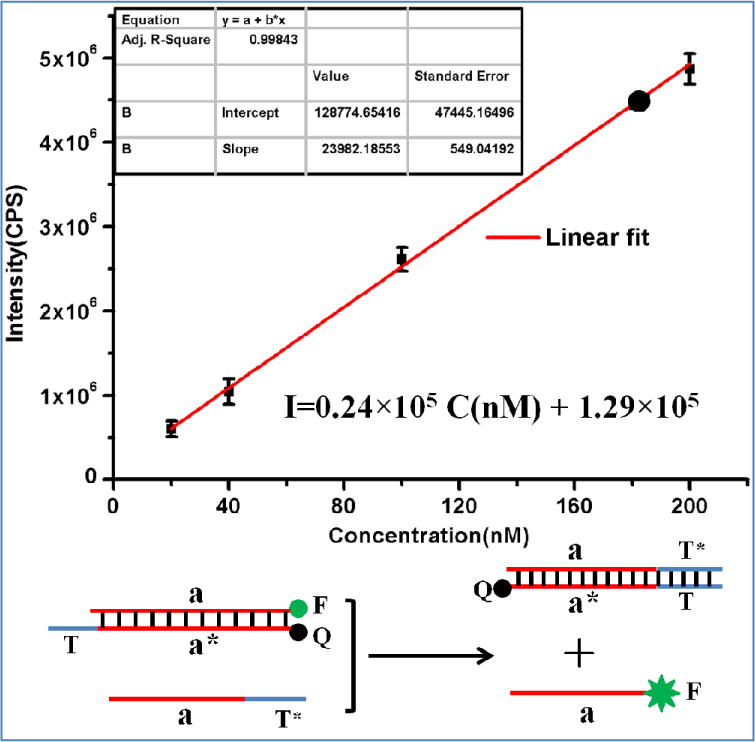
Standard calibration curve. Plot of fluorescence intensity of released strand a versus initial concentration of duplex with exposed toehold T [slope = (2.40±0.05) × 104; y-intercept = (1.29±0.47) × 105; r2 = 0.99843]. The reaction buffer contains 20 mM Tris-HCl, 5 mM MgCl2 and 300 mM NaCl, pH = 7.5. This experiment was repeated three times. The small black circle on the standard curve corresponds to 92% release of hidden toehold after 20 min irradiation.
Photocontrolled toehold formation and subsequent toehold-mediated branch migration reaction
To further verify whether the toehold-mediated branch migration reaction proceeded as designed, a PAGE experiment was carried out (Figure 4). In order to distinguish the DNA bands, a 10 T segment was added to the invading DNA to increase the molecular weight of the newly formed DNA duplex. First, the snap-cooled hairpin precursor (10 μM, heated to 95°c for 5 minutes, incubated on ice for 1 minute and left at room temperature for 30 minutes) was UV-irradiated for different times. After irradiation, the T10-tailed-invading DNA (10 μM) was added and incubated at room temperature for 30 minutes. As indicated in Figure 4, the band with the smallest migration in lanes 4 to 7 corresponds to the newly formed DNA duplex with T10 tail, while the band with the largest migration in lanes 4 to 7 corresponds to the displaced small DNA fragment. By increasing UV irradiation time, the bands for the hairpin precursor and T10-invading DNA became less intense, but the bands for the newly formed DNA duplex with T10 tail and the displaced small DNA fragment became more intense. These results further proved that the amount of released hidden toehold could, indeed, be controlled by regulating irradiation dosage. Moreover, in lane 1 of the Figure 4, we can see the PC-linker-modified DNA hairpin precursor without invading strand after 20 min photoirradiation; in lane 3, we see the PC-linker-modified DNA hairpin precursor without irradiation. The bands in lane 1 and 3 have the same migration, indicating that the method can indeed produce pure 1:1 ratio toehold-bearing DNA duplex without the formation of free single strands and without damage to the DNA structures.
Figure 4.

Native PAGE (20%) analysis of the branch migration reaction after UV irradiation for different times. In a typical experiment, photoirradiated PC-linker-modified DNA hairpin precursor (10 μM) was mixed with the invading strand (10 μM) and incubated at room temperature for 30 min. Lane 1: photoirradiated (20 min) PC-linker-modified DNA hairpin; lane 2: the invading strand; lane 3: PC-linker-modified DNA hairpin precursor (no irradiation); lane 4–8: PC-linker-modified DNA hairpin precursor irradiated for different times and incubation afterwards with the invading strand.
Photocontrolled DNA hybridization chain reaction
The power of this method was further demonstrated in a photocontrolled DNA hybridization chain reaction (HCR) system, i.e., using a toehold-bearing DNA duplex to initiate an autonomous polymerization of two different hairpins (H1 and H2; see Table S1 for sequences).9 HCR, based on a chain reaction of recognition and hybridization events between two sets of DNA hairpin molecules, is a new signal amplification technique, which can be used to sensitively detect DNA and other molecules,32,33 probe spatial organization of DNA strands,34in situ map mRNA expression35 and mediate cell death.36 In our design, the PC linker was employed to form a hairpin precursor (H3) with two hidden toeholds (Figure 5a). The toeholds (a* and x) are released after UV irradiation, forming the activated H3, with the amount of activated H3 regulated by changing the UV irradiation time. This activated H3 first interacts with H1 through hybridization between the released toeholds (a* and x) and the toeholds on the H1 hairpin (a and x*). The H1 hairpin is opened via branch migration, releasing hidden toehold (c) on H1 (Figure 5b). As shown in Figure S1, the formation of a band with small migration in the gel electrophoresis (lanes 3–8 in Figure S1) indicates that the H3·H1 complex was formed after UV irradiation; as the irradiation time increased, more and more complex formed, as demonstrated by the increasing intensity of the slow-moving band. As a control, in the absence of UV irradiation, no H3·H1 complex band was observed (lane 2 in Figure S1). The H3·H1 complex could further interact with another hairpin, H2, triggering a hybridization chain reaction (Figure 5a). As expected, the hairpins do not polymerize in the absence of UV irradiation, and different irradiation times can generate various amounts of activated H3 to fine-tune the HCR process in a UV irradiation dose-dependent manner (Figure 5c). As a control, HCR was performed using a DNA duplex as initiator (the same structure with activated H3), and the PAGE experiment was carried out (Figure S2). As indicated in Figure S2 the gel bands for addition of 4 μM and 6 μM DNA duplex were similar with those for 10 min and 20 min UV irradiation of 6 μM H3. In other words, the reaction efficiency for DNA branch migration with light activation is comparable to that for pure DNA duplexes.
Figure 5.
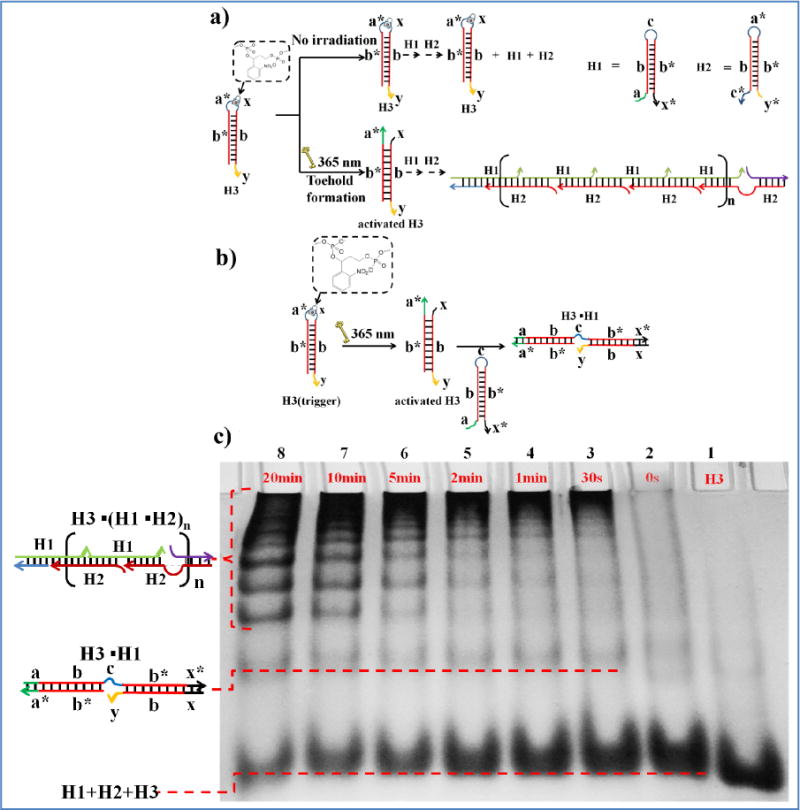
Photocontrolled toehold formation for DNA hybridization chain reaction. a,b) Principle of photocontrolled toehold formation for DNA hybridization chain reaction (HCR). c) Native PAGE (10%) analysis of the photocontrolled HCR. In a typical experiment, after different photoirradiation times, H3 (6 μM) was mixed with H1 (12 μM) and H2 (12 μM) and incubated at room temperature overnight. Lane 1: H3; lane 2: H3 mixed with H1 and H2 (no irradiation); lanes 3 to 8: H3 photoirradiated for different times and mixed with H1 and H2.
Kinetics of the photocontrolled DNA branch migration reaction
Finally, to better understand the kinetics of the photocontrolled DNA branch migration, fluorescence measurements were further employed using pyrene-modified H1 and H2 strands. Pyrene acts as a spatially sensitive fluorescent dye, which can form an excimer structure when an excited-state molecule is brought into close proximity to a ground state pyrene. Excimer formation results in a fluorescence emission shift to longer wavelength (380–400 nm for the monomer, 475 nm for the excimer).37 The fluorescence emission spectra (λex = 340 nm) of the stabilized HCR system, containing Pyrene-H1, Pyrene-H2 and H3 (see Table S2 for sequences), were measured after a series of UV irradiation times. With increasing irradiation time, the results (Figure S3) show that the excimer fluorescence intensity at 475 nm increased, while the monomer fluorescence intensity at 380 nm and 400 nm decreased. Pyrene-H1 and Pyrene-H2 could spatially separate the pyrene moieties and prevent them from forming the excimer structure. However, addition of activated H3 to the solution initiated DNA polymer formation, thereby forcing the pyrene moieties much closer to each other (Figure 6a) to generate the excimer signal. These kinetics results indicate that the hybridization chain reaction (HCR) rate increases with increasing UV irradiation time, further proving that the branch migration reaction-based HCR process can be controlled by fine-tuning the UV irradiation dose. For the HCR kinetics, a single point module was used to record the pyrene excimer fluorescence intensity at 475 nm with excitation at 340 nm at different times. Although the irradiation time is very short, some of the PC linker could still be photocleaved, which resulted in the continuous increase of fluorescence intensity corresponding to “0 min” in Figure 6b from 5 min to 45 min.
Figure 6.

Kinetics of photocontrolled DNA hybridization chain reaction system. a) Experimental design. In a typical experiment, a mixture of Pyrene-H1 (45 μL, 3 μM) and Pyrene-H2 (45 μL, 3 μM) were placed in a cuvette, and then photoirradiated H3 (15 μL, 3 μM) was added to initiate the reaction. b) Plot of excimer emission intensity versus polymerization reaction time for different H3 irradiation times. The fluorescence intensities at 475 nm were recorded at different times (λex = 340 nm).
CONCLUSION
We have presented here a new photocontrolled toehold formation method to generate 1:1 ratio DNA duplexes for toehold-mediated branch migration reactions. Different from previously reported overhanging-toehold systems, light is employed to activate the hidden toehold without addition of any chemicals or formation of waste DNA molecules. More importantly, the amount of released toehold can be easily controlled by fine-tuning the irradiation dose, allowing the rate of the toehold-mediated branch migration reaction to be regulated by changing the initial UV irradiation time. Our system shows potential for the construction of light-responsive dynamic DNA nanostructures and DNA circuits.38–40 Moreover, with the development of DNA microarray technology,41,42 parallel synthesis of a large amount of hairpin precursors on one microchip is possible. Thus, this toehold formation method is potentially crucial for making large-scale circuits on a single DNA microchip.
EXPERIMENTAL SECTION
Materials
The materials for DNA synthesis were purchased from Glen Research (Sterling, VA), including 6-(3′,6′-dipivaloylfluoresceinyl-6-carboxamido)-hexyl-phosphoramidite(6-FAM), 3-(4,4′-Dimethoxytrityl)-1-(2-nitrophenyl)-propan-1-yl-[(2-cyanoethyl)-(N, N-diisopropyl)]-phosphoramidite (PC-linker phosphoramidite), and 1-Dimethoxytrityloxy-3-[O-(N-4′-sulfonyl-4(dimethylamino)-azobenzene)-3-aminopropyl]-propyl-2-O succinoyl-long chain alkylamino-CPG (3′-Dabcyl CPG). Other chemicals were purchased from Sigma-Aldrich. All reagents for buffer preparation and HPLC purification came from Fisher Scientific. Unless otherwise stated, all chemicals were used without further purification.
DNA synthesis
All oligonucleotides were synthesized using an ABI 3400 DNA synthesizer (Applied Biosystems, Inc., Foster City, CA) at the 1.0 micromolar scale. DNA oligomers were deprotected in 2.5 mL AMA (ammonium hydroxide/40% aqueous methylamine 1:1) solution at 65°C for 20–30 min. The deprotected oligomers were then mixed with 250 μL 3.0 M NaCl and 6.0 mL ethanol and placed in a −20 °C freezer for precipitation. After centrifugation at 4000 rpm at 4°C for 15–30 minutes, the precipitated DNA products were dissolved in 400 μL 100 mM triethylamine acetate buffer (TEAA, pH = 7.5) and purified on a ProStar HPLC system (Varian, Palo Alto, CA) with a C-18 reversed-phase column (Alltech, 5 μm, 250 mm × 4.6 mm) using acetonitrile (0–40 min, 10–100%) and TEAA buffer (100 mM, pH 7.5) as eluent. The collected DNA products were dried and detritylated by dissolving in 200 μL 80% acetic acid for 20 minutes at room temperature, precipitated with 20 μL 3.0 M NaCl and 500 μL ethanol at −20°C, and dried by a vacuum dryer. All DNA concentrations were characterized with a Cary Bio-300UV spectrometer (Varian) using the absorbance of DNA at 260 nm.
UV Irradiation
To photoregulate PC linker cleavage, all samples were irradiated in reaction buffer (20 mM Tris-HCl, 5 mM MgCl2, 300 mM NaCl, pH = 7.5) at 365 nm with a UV-B lamp (SANKYO DENKI, Japan) with a 365 nm photochemical optical filter (Oriel Instruments (a Newport Corp. brand), Stratford, CT). The power of the UV light source had been measured by power meter (Newport Corp., Irvine, CA) with 1.33±0.1 mW cm−2 at the irradiated sample position.
Native PAGE analysis
Toehold-mediated branch migration reaction and HCR were observed using native PAGE gel. For the branch migration reaction, the gel was run in 20% acrylamide (containing 19/1 acrylamide/bisacrylamide) solution with 1 × TBE/15 mM Mg2+ buffer, at 100 V constant voltage for 4 h. For the HCR, the gel was run in 10% acrylamide solution at 80 V constant voltage for 4–5 h or 100 V constant voltage for 1 h (for H3, H1 interaction). All the gels were run at 4°C and were stained 30 min using Stains-All (Sigma-Aldrich) to image the position of DNA. Photographic images were obtained under visible light with a digital camera.
Fluorescence measurements
A FluoroMax-4 spectro-fluorometer (Jobin Yvon) was used for all steady-state or time-dependent fluorescence measurements. For the branch migration reaction, the kinetics module was used to measure time-dependent fluorescence intensity at 519 nm using excitation at 494 nm. For the HCR kinetics, the single point module was used to record the pyrene excimer fluorescence intensity at 475 nm using excitation at 340 nm at different times.
Supplementary Material
Acknowledgments
This work is supported by grants awarded by the National Institutes of Health (GM079359 and CA133086), by the National Key Scientific Program of China (2011CB911000), NSFC (Grant 21221003) and China National Instrumentation Program 2011YQ03012412, and by the National Natural Science Foundation of China (Grant Nos. 20934004 and 91127046); NBRPC (Grant Nos. 2012CB821500 and 2010CB934500).
Footnotes
ASSOCIATED CONTENT
The DNA sequences used in the work; synthesis of pyrene phosphoramidite; PAGE result of H3, H1 interactions; PAGE result of HCR initiated by pure DNA duplexes and fluorescence spectra of HCR products. This material is available free of charge via the Internet at http://pubs.acs.org.
Contributor Information
Haojun Liang, Email: hjliang@ustc.edu.cn.
Weihong Tan, Email: tan@chem.ufl.edu.
References
- 1.Lee CS, Davis RW, Davidson N. J Mol Biol. 1970;48:1–22. doi: 10.1016/0022-2836(70)90215-9. [DOI] [PubMed] [Google Scholar]
- 2.Seeman NC. Angew Chem Int Edit. 1998;37:3220–3238. doi: 10.1002/(SICI)1521-3773(19981217)37:23<3220::AID-ANIE3220>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Seeman N. C Nature. 2003;421:427–431. doi: 10.1038/nature01406. [DOI] [PubMed] [Google Scholar]
- 4.Yurke B, Turberfield AJ, Mills AP, Simmel FC, Neumann JL. Nature. 2000;406:605–608. doi: 10.1038/35020524. [DOI] [PubMed] [Google Scholar]
- 5.Bath J, Turberfield AJ. Nat Nanotechnol. 2007;2:275–284. doi: 10.1038/nnano.2007.104. [DOI] [PubMed] [Google Scholar]
- 6.Liu H, Liu DS. Chem Commun. 2009:2625–2636. doi: 10.1039/b822719e. [DOI] [PubMed] [Google Scholar]
- 7.Krishnan Y, Simmel FC. Angew Chem Int Edit. 2011;50:3124–3156. doi: 10.1002/anie.200907223. [DOI] [PubMed] [Google Scholar]
- 8.Dirks RM, Pierce NA. P Natl Acad Sci USA. 2004;101:15275–15278. doi: 10.1073/pnas.0407024101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkataraman S, Dirks RM, Rothemund PWK, Winfree E, Pierce NA. Nat Nanotechnol. 2007;2:490–494. doi: 10.1038/nnano.2007.225. [DOI] [PubMed] [Google Scholar]
- 10.Yin P, Choi HMT, Calvert CR, Pierce NA. Nature. 2008;451:318–U314. doi: 10.1038/nature06451. [DOI] [PubMed] [Google Scholar]
- 11.Yan H, Zhang XP, Shen ZY, Seeman NC. Nature. 2002;415:62–65. doi: 10.1038/415062a. [DOI] [PubMed] [Google Scholar]
- 12.Tian Y, Mao CD. J Am Chem Soc. 2004;126:11410–11411. doi: 10.1021/ja046507h. [DOI] [PubMed] [Google Scholar]
- 13.Yin P, Yan H, Daniell XG, Turberfield AJ, Reif JH. Angew Chem Int Edit. 2004;43:4906–4911. doi: 10.1002/anie.200460522. [DOI] [PubMed] [Google Scholar]
- 14.Bath J, Green SJ, Turberfield AJ. Angew Chem Int Edit. 2005;44:4358–4361. doi: 10.1002/anie.200501262. [DOI] [PubMed] [Google Scholar]
- 15.You MX, Chen Y, Zhang XB, Liu HP, Wang RW, Wang KL, Williams KR, Tan WH. Angew Chem Int Edit. 2012;51:2457–2460. doi: 10.1002/anie.201107733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song TJ, Liang HJ. J Am Chem Soc. 2012;134:10803–10806. doi: 10.1021/ja304746k. [DOI] [PubMed] [Google Scholar]
- 17.Seelig G, Yurke B, Winfree E. J Am Chem Soc. 2006;128:12211–12220. doi: 10.1021/ja0635635. [DOI] [PubMed] [Google Scholar]
- 18.Zhang DY, Turberfield AJ, Yurke B, Winfree E. Science. 2007;318:1121–1125. doi: 10.1126/science.1148532. [DOI] [PubMed] [Google Scholar]
- 19.Zhang DY, Winfree E. J Am Chem Soc. 2008;130:13921–13926. doi: 10.1021/ja803318t. [DOI] [PubMed] [Google Scholar]
- 20.Seelig G, Soloveichik D, Zhang DY, Winfree E. Science. 2006;314:1585–1588. doi: 10.1126/science.1132493. [DOI] [PubMed] [Google Scholar]
- 21.Qian LL, Winfree E. Science. 2011;332:1196–1201. doi: 10.1126/science.1200520. [DOI] [PubMed] [Google Scholar]
- 22.Soloveichik D, Seelig G, Winfree E. P Natl Acad Sci USA. 2010;107:5393–5398. doi: 10.1073/pnas.0909380107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian L, Winfree E, Bruck J. Nature. 2011;475:368–372. doi: 10.1038/nature10262. [DOI] [PubMed] [Google Scholar]
- 24.Xing YZ, Yang ZQ, Liu DS. Angew Chem Int Edit. 2011;50:11934–11936. doi: 10.1002/anie.201105923. [DOI] [PubMed] [Google Scholar]
- 25.Zhang KJ, Taylor JS. J Am Chem Soc. 1999;121:11579–11580. [Google Scholar]
- 26.Prokup A, Hemphill J, Deiters A. J Am Chem Soc. 2012;134:3810–3815. doi: 10.1021/ja210050s. [DOI] [PubMed] [Google Scholar]
- 27.Ordoukhanian P, Taylor JS. J Am Chem Soc. 1995;117:9570–9571. [Google Scholar]
- 28.Bai XP, Li ZM, Jockusch S, Turro NJ, Ju JY. P Natl Acad Sci USA. 2003;100:409–413. doi: 10.1073/pnas.242729099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seo TS, Bai XP, Ruparel H, Li ZM, Turro NJ, Ju JY. P Natl Acad Sci USA. 2004;101:5488–5493. doi: 10.1073/pnas.0401138101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruparel H, Bi LR, Li ZM, Bai XP, Kim DH, Turro NJ, Ju JY. P Natl Acad Sci USA. 2005;102:5932–5937. doi: 10.1073/pnas.0501962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo TS, Bai XP, Kim DH, Meng QL, Shi SD, Ruparelt H, Li ZM, Turro NJ, Ju JY. P Natl Acad Sci USA. 2005;102:5926–5931. doi: 10.1073/pnas.0501965102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong J, Cui X, Deng Y, Tang Z. Biosens Bioelectron. 2012;38:258–263. doi: 10.1016/j.bios.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Hong CY, Lin Y-H, Chen J-H, Chen GN, Yang H-H. Anal Chem. 2012;84:8277–8283. doi: 10.1021/ac3017828. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Jiang Y, Chen X, Ellington AD. J Am Chem Soc. 2012;134:13918–13921. doi: 10.1021/ja300984b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi HMT, Chang JY, Trinh LA, Padilla JE, Fraser SE, Pierce NA. Nat Biotechnol. 2010;28:1208–U1103. doi: 10.1038/nbt.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venkataraman S, Dirks RM, Ueda CT, Pierce NA. P Natl Acad Sci USA. 2010;107:16777–16782. doi: 10.1073/pnas.1006377107. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 37.Huang J, Wu YR, Chen Y, Zhu Z, Yang XH, Yang CJ, Wang KM, Tan WH. Angew Chem Int Edit. 2011;50:401–404. doi: 10.1002/anie.201005375. [DOI] [PubMed] [Google Scholar]
- 38.Chen X. J Am Chem Soc. 2012;134:263–271. doi: 10.1021/ja206690a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Genot AJ, Zhang DY, Bath J, Turberfield AJ. J Am Chem Soc. 2011;133:2177–2182. doi: 10.1021/ja1073239. [DOI] [PubMed] [Google Scholar]
- 40.Zhang DY. J Am Chem Soc. 2011;133:1077–1086. doi: 10.1021/ja109089q. [DOI] [PubMed] [Google Scholar]
- 41.Hughes TR, Mao M, Jones AR, Burchard J, MartonMJShannon KW, Lefkowitz SM, Ziman M, Schelter JM, Meyer MR, Kobayashi S, Davis C, Dai HY, He YDD, Stephaniants SB, Cavet G, Walker WL, West A, Coffey E, Shoemaker DD, Stoughton R, Blanchard AP, Friend SH, Linsley PS. Nat Biotechnol. 2001;19:342–347. doi: 10.1038/86730. [DOI] [PubMed] [Google Scholar]
- 42.Heller MJ. Annu Rev Biomed Eng. 2002;4:129–153. doi: 10.1146/annurev.bioeng.4.020702.153438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


