Abstract
Direct injection of agents into the dorsal root ganglia (DRGs) offers the opportunity to manipulate sensory neuron function at a segmental level to explore pathophysiology of painful conditions. However, there is no described method that has been validated in detail for such injections in adult rats. We have found that 2 (µl of dye injected through a pulled glass pipette directly into the distal DRG, exposed by a minimal foraminotomy, produces complete filling of the DRG with limited extension into the spinal roots. Injection into the spinal nerve required 3 µl to achieve comparable DRG filling, produced preferential spread into the ventral root, and was accompanied by substantial leakage of injected solution from the injection site. Injections into the sciatic nerve of volumes up to 10 (µl did not reach the DRG. Transient hypersensitivity to mechanical stimulation at threshold (von Frey) and noxious levels (pin) developed after 2 µl saline injection directly into the DRG that was in part attributable to the surgical exposure procedure alone. Only minimal astrocyte activation in the spinal dorsal horn was evident after DRG saline injections. Injection of adeno-associated virus (AAV) vector conveying green fluorescent protein (GFP) transgene resulted in expression as soon as 1 day after injection into the DRG, including fibers in the spinal dorsal horn and columns. AAV injection into the DRG produced additional thermal hypersensitivity and withdrawal from the stroke of a brush and compromised motor performance. These findings demonstrate a method for selective injection of agents into single DRGs for anatomically restricted actions.
Keywords: Dorsal root ganglion, Gene therapy, Neuropathic pain, Regional anesthesia, Pain treatment, Injection technique
1. Introduction
Pain resulting from nerve injury is often persistent and difficult to treat. Although neuropathic pain is accompanied by altered function in the spinal cord and brain, numerous studies have established that the primary sensory neuron and its soma in the dorsal root ganglion (DRG) are important sites for pathogenic functional changes leading to pain, including that which follows nerve trauma (Devor and Seltzer, 1999; Fuchs et al., 2007; Hogan et al., 2000; McCallum et al., 2003; Sapunar et al., 2005). Underlying these alterations in neuronal excitability are shifts in peptide expression, including membrane receptors, ion channels, and intracellular signaling molecules. Ultimately, DRGs proximal to nerve injury show selective neuronal loss through apoptosis (Ekstrom, 1995). Recent discoveries have highlighted a pathogenic role also for glia in the DRG, which are activated following peripheral nerve injury and thereafter may produce soluble factors that perpetuate the painful condition (Watkins and Maier, 2002).
Although critical changes leading to chronic pain reside in the DRG, no treatments for chronic pain have been devised that target this organ. Manipulating neuronal function at one or several DRGs would have the clear benefits of optimizing efficacy while limiting influences on other neuronal populations, thereby avoiding side effects that inevitably accompany systemic medications. While intrathecal injection is a simple and relatively noninvasive means to deliver agents to sensory neurons, it is not possible to restrict injected solution to a single side or to a limited longitudinal range of vertebral levels. Material injected into peripheral nerves may be conveyed to the DRGs (Moore et al., 1954), but this pathway may also result in nerve and spinal cord damage (Hogan, 2008), and would convey agents to multiple DRGs proximal to the injection site. Direct injection into the DRG has the benefits of specific targeting of sensory pathways on a segmental basis, and clinical methods for injecting drugs directly to selected DRGs are well established (Hogan, 1998; Pfirrmann et al., 2001). Chronic conditions would require repeated injections for most therapeutic agents, and technical challenges limit the clinical applicability of continuous infusion directed at a single DRG in humans. However, developments in molecular biology may resolve these limitations by allowing prolonged gene modification by the administration of viral vectors.
For research applications, various approaches have been employed to deliver agents directly to the DRGs of rats. Topical application may be achieved by catheter implantation in the area of the DRG after laminectomy (Lyu et al., 2000; Rueter et al., 2003) or installation of a cannula through the laminar bone (Zhang et al., 2001), but penetration of agents into the DRG cannot be assured by this approach. A percutaneous method has been described for injection into the lumbar DRG of rats (Ferrari et al., 2007), but we have found that overlying laminar bone allows injection by this method only into the spinal nerve rather than the DRG per se (Puljak et al., 2009). Injection within the DRG has also been performed by tunneling a cannula via a subepineural path within the spinal nerve (Ma et al., 2010; Zhou et al., 2000), but this may traumatize both a segment of the spinal nerve and the DRG, and the terminus of the catheter cannot be observed, so the location of injection is only inferred. Direct injection into the DRG exposed by laminectomy has been performed in rats (Puljak et al., 2009) and rabbits (Palay et al., 1982), and for injection of viral vectors (adeno-associated virus, AAV; Mason et al., 2010), but there has been no validation of the distribution of different volumes of injected solution, characterization of the required volume, or analysis of histological and behavioral changes that might indicate trauma or inflammation.
In the present study, we describe the development of a microinjection technique for delivering agents into the DRG. Because of its location, bone removal is required to expose any of it to view, so we determined the minimal extent of foraminotomy to allow direct injection. We additionally assessed sciatic nerve injection since it is a readily accessible peripheral site, and evaluated injection into the spinal nerve just distal to the DRG, where the nerve emerges from the intervertebral foramen and can be approached without bone removal. We performed trial injections with dye to examine the extent of solution spread and to identify the optimal volume for filling the ganglion without excessive spread to other structures. Injections of phosphate buffered saline (PBS) were carried out to evaluate the effect of injection on sensory and motor behavior as well as DRG histological response to injection. Because gene therapy could be applied for treatment of sensory disorders through DRG injection, we additionally examined the behavioral and histological consequences of injecting an AAV vector encoding for the marker green fluorescent protein (GFP).
2. Materials and methods
2.1. Animal subjects
All experiments were conducted using male Sprague-Dawley rats (130–150 g at the start of the study) obtained from a single vendor (Charles River Laboratories Inc., Wilmington, Massachusetts). Animals were housed individually in a room maintained at constant temperature (22 ± 0.5 °C) and relative humidity (60 ± 15%) with an alternating 12 h light-dark cycle. All experiments were conducted during the light phase. Food and water were available ad libitum throughout the experiments. All procedures were approved by the Animal Care and Use Committees of the Zablocki VA Medical Center and Medical College of Wisconsin (Milwaukee, Wisconsin) or the Ethical Committee of the University of Split School of Medicine.
2.2. Injections
2.2.1. Surgical exposure
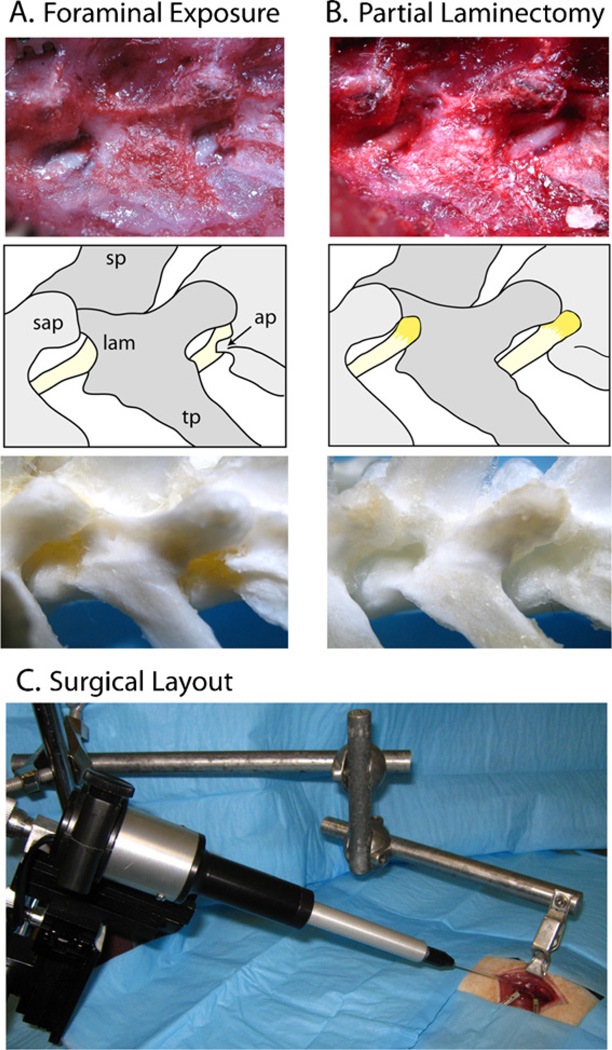
Animals were anesthetized with isoflurane (4% for induction 1.5–2% maintenance). For sciatic nerve injection, the right sciatic nerve was exposed at the mid-thigh level by blunt dissection. The injection site was 1 cm proximal to the trifurcation of the nerve. For spinal nerve and DRG exposure, an approximately 3 cm incision was made in the skin just to the right of the dorsal midline starting from the superior iliac crest. The superficial muscular fascia was incised and the paraspinal muscles separated by a combination of sharp and blunt dissection, exposing the lateral aspect of the fourth lumbar (L4) to L6 vertebrae and the dorsal aspect of the medial portion of their transverse processes. Exposure was maintained using retractors mounted on magnetic holders (Fine Science Tools, Foster City, California). While using binocular magnification (5–15 ×), the L4 and L5 spinal nerves as well as the intervertebral foramina from which they emerge were gently exposed. This alone was adequate to reveal the L5 spinal nerve just distal (approximately 2 mm) from the DRG, which remained covered by laminar bone (Fig. 1 A). At the L4 level, the foramen and spinal nerve are partially obscured by the accessory process that descends from the base of the L4 transverse process, which is readily removed by a using a 1 mm rongeur (Friedman–Pearson rongeur, Fine Science Tools). For direct injection to the DRG, the foramen was slightly enlarged in a cephalad direction using the rongeur to remove a 1 mm-deep crescent of laminar bone, exposing the distal third of the DRG (Fig. 1B). While exposure performed this way initially produces local bleeding, this stopped promptly in all animals without any specific intervention.
Fig. 1.
Paravertebral surgical exposure for ganglionic injection, showing the operative field (top panel), reference diagram (middle panel), and cleaned vertebral bones (bottom panel). (A) Initial dissection of soft tissues at the level of the fourth lumbar (L4) and L5 spinal nerves (yellow) shows the superior articular processes (sap), spinous processes (sp) and transverse processes (tp), as well as the laminar bone (lam) and accessory process on L4 (ap). The dorsal root ganglia are covered by laminar bone. (B) Removal of laminar bone superior to the foramen and the L4 accessory process reveals the distal dorsal root ganglion, recognized by its broader diameter and brownish-orange color. (C) The motorized injection system is mounted on a magnetic stand via a manual micromanipulator. The rat vertebral column is stabilized by clamping the spinous process of L5 using a clip mounted on an articulated arm that is attached to the same magnetic stand. (This would be draped during actual surgery.) Two retractors are also shown, which maintain exposure within the wound. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
2.2.2. Injection technique
During injection, the spinal column was stabilized by immobilizing the L4 spinous process with a clamp fashioned from an alligator clamp attached to an articulated arm that was held on a magnetic base (Fig. 1C). With the exception of large volume (10 µl) sciatic nerve injections, all injections were performed using a microprocessor-controlled injection system employing direct piston displacement (Nanoliter 2000, World Precision Instruments, Sarasota, Florida) mounted on a micromanipulator, equipped with a pulled glass capillary injection tip. These were prepared with a long taper without regard for tip diameter, and were then forced through a laboratory cleaning tissue held taut perpendicular to the pipette. This reliably produced sharp tips, and those with somewhat beveled tips with diameters of 40–60 µm were chosen for use. During injection, the pipette was oriented at an angle as close to tangential with the injection surface as possible (25–30° from horizontal), and advanced until the tip began to indent the nerve epineurium or DRG capsule. The tip was advanced further in small increments until the tip pierced the tissue (nerve epineurium or DRG capsule). Once penetration was achieved, the injector was backed off until the compression of the tissue was not evident, in order to lessen tissue pressure on the pipette aperture. Following injection, the pipette tip was left in place for 3 min to help seal tissue around the tip. The injection was performed at a rate of 20 nl/s. After the injection was completed, the pipette was left in place for 5 min before removal to allow fluid to distribute and the pressure within the tissue to equalize. The wound was closed in layers using 6-0 chromic gut fascial sutures and surgical skin staples.
2.3. Spinal nerve ligation
In a subset of animals, a nerve injury was performed to provide a positive control for comparison to the effects of DRG or spinal nerve injection. To perform spinal nerve ligation (SNL) (Kim and Chung, 1992), a comparable exposure was performed, but without laminar bone removal but with removal of the L6 transverse process. The L5 and L6 spinal nerves were ligated using 4-0 silk suture, and sectioned and the wound closed.
2.4. Evaluation of injectate distribution
In order to examine the spread of an aqueous solution after injection, the extent and pattern of injection distribution was examined in one group of animals after injection of varying volumes of 0.1% Fast Green FCF (Sigma–Aldrich, St. Louis, Missouri). Dye was injected into the sciatic nerve at volumes of 3 and 10µl, or into the spinal nerve or DRG at 1, 2, 3, and 5 µl. Animals were maintained under anesthesia for 30 min following completion of injection, which allowed enough time for dye to spread but not long enough for removal by vascular uptake, and then were sacrificed by decapitation. After complete dorsal laminectomy at levels T10 to L6, the injected tissue was first inspected in situ to determine the extent of stained tissue. DRGs were then removed, along with the attached spinal roots and peripheral nerves, and measurements were taken to determine the distance traveled by the dye within each structure. The DRGs were further examined by making axial sections at multiple levels along their length, and distribution in the anterior/posterior dimension was determined under magnification (15×). Following DRG removal, the remaining spinal roots and dura were inspected for staining to determine if dye leaked into the intrathecal space. The level of the spinal cord at which the L4 and L5 roots enter was examined to detect the presence of dye.
2.5. Localization of injection by DAPI staining
To evaluate the feasibility of the inclusion of a fluorescent dye for post-mortem evaluation of injectate distribution, diamidino-phenylindole (DAPI, Sigma) was injected at a concentration of 0.25 µg/|µl directly into the DRGs of 7 rats. Rats were sacrificed after 7 or 14 days, and the injected ganglia harvested and embedded in Optimal Cutting Temperature (OCT) freezing medium (Tissue Tek, Tokyo, Japan). Ganglia were cut into 10 µm thick sections and examined for the presence or absence of DAPI staining by fluorescence microscopy.
2.6. Behavioral testing
In other animals, behavior was examined to determine the consequences of injection into either the spinal nerve or the DRG. Sensory and motor testing were performed 3 days prior to surgery and on days 1,3,7, and 17 following surgery. Tests were applied in the sequence presented below. Experimental groups (n = 5 animals each) included one in which the skin was incised and then closed (skin incision group), one in which the foramen was exposed and enlarged by bone removal as described above, but no injections were made (exposure group), ones in which PBS was injected into either the DRG (PBS/DRG group) or into the spinal nerve (PBS/nerve group), and ones in which AAV vector was injected into either the DRG (virus/DRG group) or into the spinal nerve (virus/nerve group).
2.6.1. Dynamic mechanical stimulation (brush)
Animals were placed on a wire mesh platform, and a camel hair brush (2.5 mm width, Ted Pella Inc., Redding, CA) was passed along the bottom of the hind paw from front to back in a smooth motion at a rate of approximately 2 cm/s. Each hind paw was tested 3 times in alternating fashion. Any withdrawal from the stimulus was considered a positive response.
2.6.2. Cooling stimulation (cold)
Acetone was expelled from a syringe attached to PE220 tubing to make a meniscus that was touched to the plantar surface of the hind paw, such that the drop spread out on the plantar surface of the paw without contact of the tubing to the skin. Each hind paw was tested 3 times in alternating fashion. Any withdrawal was considered a positive response.
2.6.3. Punctate mechanical stimulation (von Frey)
Mechanical withdrawal threshold was determined using calibrated monofilaments (Patterson Medical, Bolingbrook, Illinois). Filaments with approximate forces (in g) of 0.3, 0.5, 0.8,1.0, 2.8, 5, 9, 14, and 24 were applied in an up-down fashion (Chaplan et al., 1994). Briefly, beginning with the 2.8 g filament, filaments were applied with just enough force to bend the fiber and held for 1 s. If a response was observed, the next smaller filament was applied, and if no response was observed, the next larger was applied, until a reversal occurred, defined as a withdrawal after a previous lack of withdrawal, or vice versa. Following a reversal event, four more stimulations were performed following the same pattern. The forces of the filaments before and after the reversal, and the four filaments applied following the reversal, were used to calculate the 50% withdrawal threshold according to the method of Dixon (1980). Rats not responding to any filament were assigned a score of 25 g.
2.6.4. Heating stimulation (heat)
Using a device designed for the purpose of identifying thermal sensitivity (Paw Thermal Stimulator System, University Anesthesia Research & Development Group, San Diego, CA). Rats were placed on a temperature-regulated glass platform heated to 30 °C and the hind paws stimulated with a radiant heat source (50 W halogen bulb) directed through an aperture. The time elapsed from initiation of the stimulus until withdrawal (withdrawal latency) as detected by a series of photocells was measured. Each hind paw was tested 4 times and the withdrawal latency values averaged.
2.6.5. Noxious mechanical stimulation (pin)
A 22 g spinal anesthesia needle was applied to the plantar surface of the hind paw with enough force to indent, but not puncture the skin. This was applied 5 times and then repeated after at least 2 min, for a total of 10 stimulations. This evokes either a simple withdrawal response with immediate return of the foot to the cage floor, or a response characterized by sustained elevation with grooming (e.g. licking or chewing the toes) and possibly shaking, lasting at least 1 s. This latter behavior was referred to as hyperalgesia behavior (Hogan et al., 2004), and has been specifically associated with an aversive experience (Wu et al., 2010).
2.6.6. Motor testing (rotarod)
Motor function was assessed using an accelerating RotaRod apparatus (IITC Life Sciences, Woodland Hills, California). Rats were placed on a rotating drum and allowed to acclimate. The drum was then gradually accelerated from 4 to 40 rpm over 300 s. The time elapsed and speed of rotation when the rat fell was recorded. For training, each animal had three initial trials at least 10 min prior to the recorded d0 trial. Subsequently, only one trial was performed for each time point.
2.7. Histological examination
2.7.1. Tissue harvest
On day 1,7, or 17 after injection with 2 µl saline or virus, or exposure of the DRG without injection, animals were anesthetized with isoflurane and transcardially perfused with cold saline followed by 4% paraformaldehyde in PBS. L4 and L5 DRGs were harvested from both sides. The segment of the spinal cord where the dorsal and ventral roots of the L4 and L5 ganglia entered the cord was collected as well. Tissue was then post-fixed overnight in 4% PFA and stored in PBS containing 0.1% sodium azide at 4°C.
2.7.2. Histology
Following washes in 0.1 M PBS and overnight cryoprotection in 30% sucrose, ganglia and spinal cords were embedded in OCT and sectioned on a cryostat (Thermo Shandon Cryotome, Pittsburgh, PA, USA). Sections 10 µm thick were cut and placed on silane-coated glass slides. All incubations were done overnight at room temperature and 0.2% Triton X-100 in PBS was used for rinsing. Normal goat serum (3%) was used to prevent non-specific antibody binding.
For detection of activated DRG satellite cells and dorsal horn astrocytes, sections were treated with rabbit anti-glial fibrillary acidic protein (GFAP) polyclonal antibody (1:1000, Chemicon, Cat. No. AB5804). This polyclonal antibody detects GFAP of rat, human, bovine and canine origin (manufacturer’s technical data sheet) and has been previously characterized. Neuronal injury was identified with rabbit polyclonal anti-ATF-3 antibody (1:250, Santa Cruz, sc-22798). ATF-3 antibody has been previously characterized (Hamamura and Yokota, 2007). In Western blot analyses, this antibody recognizes a single band of ∼20 kD protein corresponding to the expected molecular weight of ATF-3 (manufacturer’s technical data sheet). GFP detection was performed using anti-GFP rabbit polyclonal IgG antibody (1:100) (Santa Cruz, sc-8334, Lot no 12206) raised against amino acids 1–238 representing full length GFP of Aequorea victoria origin. Western blot analysis of GFP fusion protein expression in transfected COS cells reveals a single band of expected molecular weight (manufacturer’s information).
Secondary detection was performed using goat anti-rabbit IgG-TR (Santa Cruz, sc-2780), goat anti-rabbit IgG-FITC (Santa Cruz, sc-2012), and biotinylated goat anti-rabbit secondary antibody IgG-B (1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA; Cat. No. sc-2040) and followed by Streptavidin Alexa Fluor 488 conjugate (1:500; Molecular Probes, Inc., Eugene, Oregon, USA; Cat. No. S-32354). After final rinsing in PBS, slides were mounted, air-dried and coverslipped (Immuno-Mount, Shandon, Pittsburgh, PA, USA). Staining controls included omission of primary antibody from the staining procedure, which resulted in no staining in DRG or dorsal horn tissue.
2.7.3. Quantitative analysis of histological observations
Every fourth section of each DRG (3–4 from each rat) was examined under an upright microscope (Olympus, BX51) equipped with DP71 camera (Olympus, Tokyo, Japan). Microphotographs were captured using Cell A, Imaging Software for Life Sciences Microscopy (Olympus, Tokyo, Japan), under the same time exposition, binning and gain for each image. No background subtraction was performed. All modifications to the microphotographs were performed in an identical fashion for all DRGs or dorsal horns on the same slide. Dorsal horn images were then imported into MetaMorph software (ver. 6.2.r5, Molecular Devices, Downing-ton, PA, USA) and analyzed as monochrome microphotographs (2040 × 1536 pixels, 8 bit, 0–256 grey levels). Fluorescence intensity values were acquired along a line positioned between dorsal root entry zone and central canal (Line scan function, scan width 15).
Each DRG photo was divided into 200 × 200 µm squares with at least 80% surface occupied by ganglion neurons. Only the neurons with visible nuclei and those underlying the right and upper borders of the square were counted. The procedure included determination of the total number of ganglion neurons and the number of GFP positive neurons from each area of interest. Cell numbers were determined under fluorescence and in dubious cases fluorescence was replaced with differential interference contrast (DIC). No counterstain was used. Percentage was calculated by dividing GFP expressing neurons by the total number of neurons. The diameters were calculated by summing the length and width of neurons with a visible nucleus and dividing the sum by two.
2.8. Recombinant AAV (rAAV) vectors
The AAV vector plasmid, pdsAAV-CMV-GFP (Wang et al., 2003), generates double-stranded (ds) rAAV vectors expressing enhanced green fluorescent protein (eGFP) driven by the CMV enhancer/promoter (kindly provided by Dr. X. Xiao, UNC-Chapel Hill). The AAV8 packaging plasmid, p5E18-VD2–8, was kindly provided bye Drs. G. Gao and J. M. Wilson (University of Pennsylvania, Philadelphia, PA). The dsAAV8-CMV-GFP vector was produced in human embryonic kidney 293 cells by the triple-transfection method, purified by two cycles of cesium chloride gradient cen-trifugation, and concentrated as described elsewhere (Grimm et al., 2003). The final viral preparation was kept in phosphate-buffered saline (PBS) containing 5% sorbitol. The physical particle titer, determined by a quantitative dot-blot assay was 1.2 × 1013 vector genomes/ml. Unless otherwise noted, all vector injections were 2 µ1 for each DRG.
2.9. Primary sensory neuron culture
To test if transgene expression persists in sensory neurons after DRG dissociation, the AAV-injected L4 and L5 DRGs from one rat were cultured as described previously (Malin et al., 2007). Briefly, the animal was sacrificed by decapitation under isoflurane anesthesia and the injected DRGs were removed after laminectomy. Each DRG was separately digested for 30 min in 0.5 mg/ml Blendzyme 2 (Roche Applied Sciences, Indianapolis, IN), followed by 30 min in 0.2 mg/ml trypsin (Sigma–Aldrich, St. Louis, MO). A single cell suspension was achieved by gentle trituration, and plated on poly-L-lysine coated coverslips. Cells were cultured in Neurobasal-A medium supplemented with 1X B27 supplement, 100 U/ml penicillin, 100 mg/ml streptomycin (all from Invitrogen, Carlsbad, CA) and 25 ng/ml NGF-β (Sigma–Aldrich).
2.10. Statistical analysis
For each behavior, the time series data within an experimental group was analyzed in order to determine if there was an effect of the surgical intervention upon that behavior by the parametric repeated measures ANOVA (for parameters with continuous variable measures, i.e. von Frey, heat, rotarod) with post hoc comparisons (only between the baseline day, hereafter termed d0, and each subsequent day) using Tukey’s test, or by the nonparametric Friedman’s test (for parameters with discrete variable measures, i.e. brush, cold, pin). To compare effects between groups for each test, each animal’s behavior score was normalized to d0 and the area under the curve (AUC) for that animal’s subsequent behavior was determined. The AUC scores were analyzed by single-sample Wilcoxon signed rank test to determine if there was a significant difference from zero. AUC for each group were compared using the Mann–Whitney U-test for paired comparisons between the exposure group and each other group. Statistical calculations were performed using Statistica 8 (StatSoft, Tulsa, OK) or Prism (GraphPad Software Inc., La Jolla, California). Data are presented as mean ± SD.
3. Results
3.1. Dye injections
3.1.1. Spinal nerve injections
With experience, paravertebral exposure of the L4 and L5 injection sites required approximately 30 min to complete, and each injection required an additional 8 min. In all spinal nerve injections, Fast Green preferentially spread proximally into the DRG rather than distally into the spinal nerve beyond the injection site (Table 1). At the lowest volume (1 µl), staining was concentrated at the distal third of the DRG and its adjacent ventral root. Injections of 2 µl successfully stained the DRG completely in longitudinal and anterior/posterior dimensions in only 2 of 5 injections, with heavier staining of the ventral root adjacent to the DRG. When the injection volume was increased to 3 µl, the preference for ventral root staining became more apparent, with spread to the ventral root proximal to the DRG within the spinal canal in 4 of 5 injections, as far as 2.5 mm. Injections of 3 µl and 5 µl often produced readily apparent staining of both the dorsal and ventral roots, as well as leakage into the intrathecal space demonstrated by visible staining of the inner aspect of the ventral dura and the adjacent nerve roots. With the exception of 2 of the 1 µl injections, all spinal nerve injections produced some degree of staining of tissue adjacent to the injection site during injection, indicating leakage or diffusion of dye from the injection site.
Table 1.
Distribution of dye injected into dorsal root ganglion (DRG) or spinal nerve.
| DRG inject ion |
Spinal nerve
injection |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Spread to | Volume (total n) | 1 µl(5) | 2 µ1(5) | 3 µl(5) | 5 µ1(5) | 1 µ1(5) | 2 µ1(5) | 3 µ1(5) | 5 µ1(5) |
| Complete DRG | Affected n | 0 | 5 | 4 | 5 | 1 | 2 | 4 | 4 |
| Dorsal root | Affected n (spread, mm) | 0 | 5(0.5–5) | 4(5–15) | 5(0.5–20) | 1(1.5) | 2(1) | 2(1) | 4(1–5) |
| Ventral root | Affected n (spread, mm) | 0 | 1(1.5) | 4(2–15) | 5(4–15) | 2(0.5–1.5) | 1(0.5) | 4(1.5–2.5) | 3(3–5) |
| Spinal cord | Affected n | 0 | 0 | 2 | 4 | 0 | 0 | 0 | 0 |
| Spinal nerve | Affected n (spread, mm) | 0 | 3(1–1.5) | 3(0.5–1.5) | 2(1) | 5(1.5–2) | 5(1.5–5) | 5(1.5–2) | 5(1–2) |
| Dura | Affected n | 0 | 2 | 4 | 5 | 0 | 1 | 3 | 2 |
| Injection site leakage | Affected n | 0 | 0 | 1 | 3 | 2 | 5 | 4 | 4 |
3.1.2. Intraganglionic injections
We first measured the dimensions of L4 and L5 DRGs collected from a group of rats comparable to those that received injections, in order to evaluate the suitability of combining data from uninjured DRGs at the L4 and L5 levels. Although we found a minor difference in length (L4 DRG 2.34 ± 0.05 mm, n = 19; L5 DRG 2.56 ±0.05 mm, n = 18; P < 0.01), there were no differences in width (L4 1.57±0.03mm, n = 19; L5 1.52 ± 0.03mm, n = 18; P= 0.21) or mass (L4 2.59 ± 0.11 mg,n = 19; L5 2.64 ± 0.09 mg,n = 18, P=0.73). We therefore combined data from L4 and L5 DRGs in the analysis of injectate distribution. We note, however, that ganglia at other levels may differ. For instance, the adjacent L6 DRGs are smaller (length 2.03 ±0.04 mm, width 1.17 ±0.02 mm, mass 1.22 ± 0.06 mg; n = 19). Thus, our specific findings regarding injection volumes should not be generalized to other vertebral levels.
Injection directly into the DRG after partial foraminotomy resulted in relatively less staining of the ventral root, especially with injection of 1 µl (Table 1). Injection of 2 µl resulted in complete staining of the DRG with spread into the dorsal root but minimally into the ventral root. When injection volume was increased to 3 µl, staining of both roots became more apparent, even as far proximally as the spinal cord (approximately 20 mm from the DRG), as did evidence of leakage into the intrathecal space as shown by staining of the dura. Injecting 5 µl into the ganglion resulted in heavy staining of the DRG and ventral root, as well as significant proximal transit of dye in the spinal roots to the cord in 4 out of 5 injections.
3.1.3. Sciatic nerve injections
Injection of 3 µl of dye into the sciatic nerve (n = 3) resulted in staining of a 10–13 mm segment of the nerve, with greater proximal staining (5–8 mm) than distal (2–5 mm). Injection of 10µl (n = 3) stained 18–23 mm of the nerve, with proximal transit of 13–18 mm and distal spread of 5–8 mm. Dye did not reach the DRG, which is approximately 55 mm distant, after any intraneural injection. While the injections appeared to stain the entire nerve on gross inspection, transverse sectioning of the nerve showed a ring of peripheral staining in 2 of 3 injections at each volume, indicating that the injection was extrafascicular.
3.1.4. DAPI injection
It may be necessary in some experimental protocols to confirm post mortem where the injectate was deposited. Accordingly, we examined the ability of diamidino-phenylindole (DAPI), a nontoxic vital dye, to remain identifiable after injection (n = 7 rats, n = 12 DRGs). We injected 2 µl per DRG using a concentration (0.25 µg/ µl) that was nontoxic to cultured HeLa cells over the period of a week (data not shown). This concentration is also less than the reported cytoxic levels (Bakunov et al., 2008). DAPI staining, especially of glial satellite cells, was clearly evident without diminution up to 14 days (data not shown).
3.2. Behavioral testing
3.2.1. General observations
Rats appeared normal during the entire observation period. Specifically, there was no excessive grooming or mutilation of the ipsilateral paw (autotomy), and no obvious gait disorder that would indicate paresis. Responses to specific stimuli and motor ability are summarized in figures depicting time-course data (Fig. 2) and area under the curve data (Fig. 3). Analysis of AUC data showed no changes that were significantly different from baseline (Wilcoxon test), although significant paired comparisons (Mann–Whitney U-test) are mentioned below.
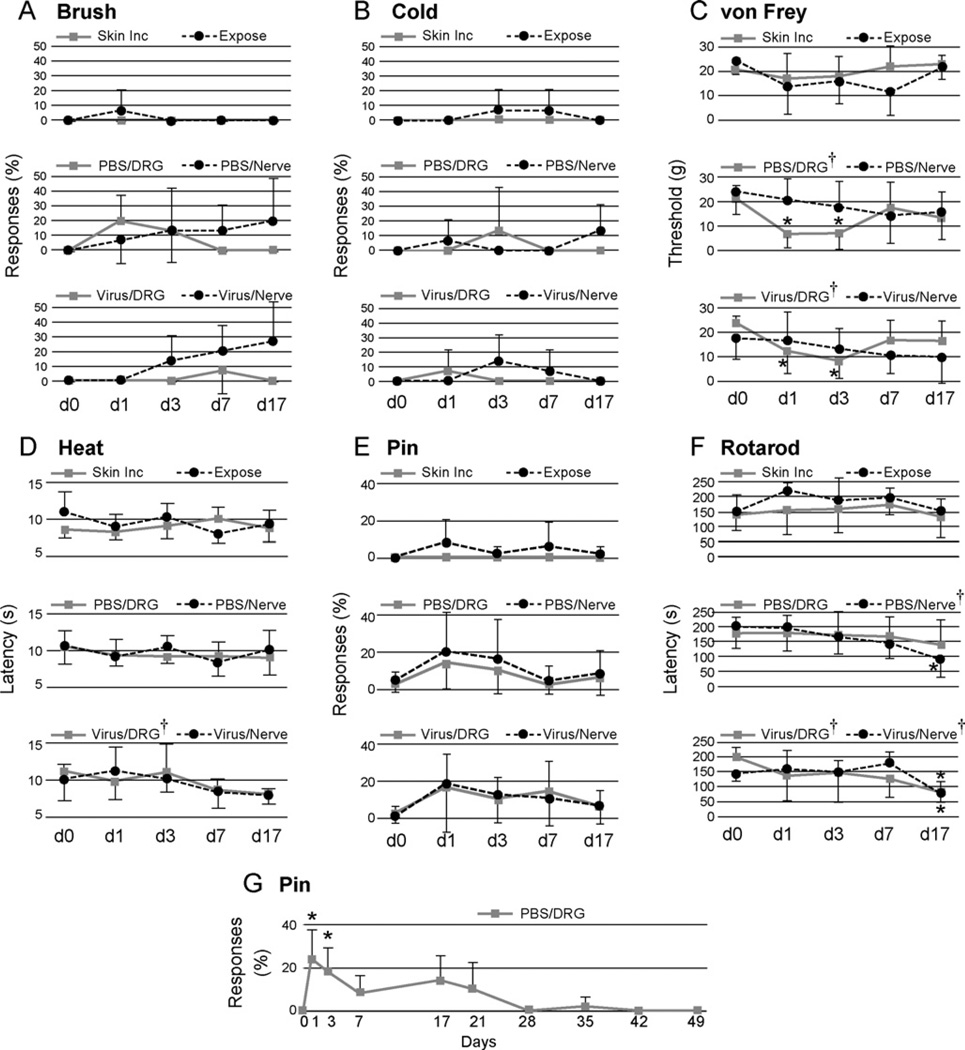
Fig. 2.
Sensory and motor function following surgery and injections evaluated over time. Testing was performed upon the plantar surface of the right paw ipsilateral to injection of either phosphate-buffered saline (PBS) or AAV viral vector (Virus) into the fourth lumbar (L4) and L5 dorsal root ganglion (DRG) or L4 and L5 spinal nerves (Nerve), or surgical exposure but no injection (Expose), or skin incision only (Skin Inc). Tests included determining frequency of withdrawal from stroking with a soft brush (A. Brush), frequency of withdrawal after applying a drop of acetone (B. Cold), threshold of withdrawal from touch with fibers of graduated force (C. von Frey), latency of withdrawal from radiant heat (D. Heat), and frequency of complex, sustained hyperalgesia behavior after touch with a pin (E. Pin). Motor function was tested by determining the duration of ambulation upon a rolling drum (F. Rotarod). Pin responses were also determined in a separate group over a longer time course (G.) † represents a significant main effect for time by repeated measures ANOVA, and* indicates a significant paired comparison to baseline (day 0, dO)byTukey’s test. Mean ± SD, n = 5 animals in each group.
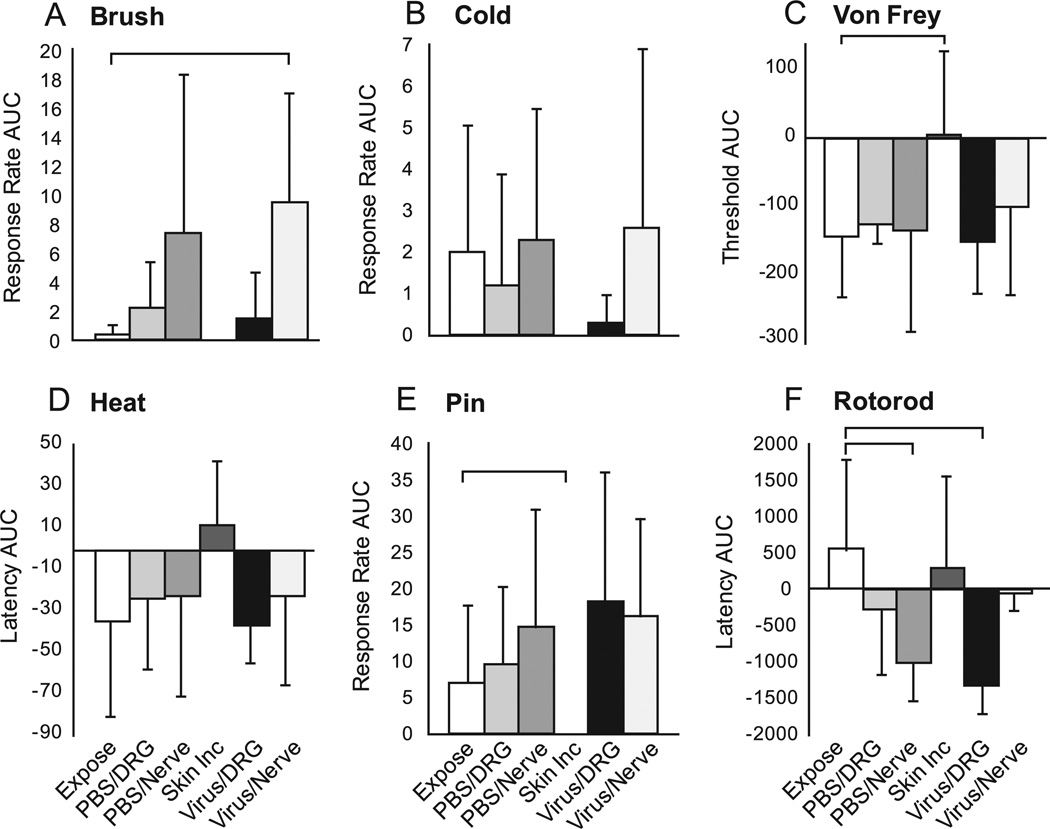
Fig. 3.
Sensory and motor function following surgery and injections evaluated using the area under the curve (AUC) across time for measures normalized to the baseline (day 0, d0). The groups are the same as defined for Fig. 2. Brackets indicate significant differences by Mann-Whitney (U-test for comparisons between Exposure and each other group. Mean ± SD. Group sizes are n = 5 animals.
3.2.2. Brush
Animals in the skin incision and exposure groups showed only rare responses to brush. No groups showed statistically proven changes over time. However, combination of the two nerve injection groups provided enough power to show a significant increase in withdrawal from brush (P < 0.01) that appeared progressively with time after injection. AUC calculations showed that responses became more frequent in the virus/nerve group compared to the exposure group (P < 0.05)
3.2.3. Cold
Withdrawal from cold was not affected in any of the experimental groups.
3.2.4. von Frey
Analysis of time course data showed that skin incision had no effect on the force threshold for withdrawal from punctate mechanical stimulation. Exposure also had no proven effect, although the significance level (P = 0.06) suggests the possibility of an influence. A significant reduction in threshold was seen after DRG injection of PBS (P < 0.05) or virus (P < 0.01), in a pattern that showed initial allodynia that subsequently resolved. AUC analysis documented a decreased withdrawal threshold in the exposure group compared to skin incision (P < 0.05).
3.2.5. Heat
Latency for withdrawal from a radiant heat source was affected only by viral vector injection into the DRG (P < 0.05). There were no differences between groups by paired comparisons.
3.2.6. Pin
Hyperalgesia responses to noxious pin stimulation were not seen in skin incision animals, but were observed rarely in the exposure group. No group showed a significant change after injection. There was a significant difference (P< 0.05) between exposure and skin incision comparing AUCs, but none of the injection groups differed from the exposure group by this measure.
3.2.7. Rotarod
Time course data showed that slowly progressive motor impairment was observed after nerve injections with either PBS (P < 0.001) or viral vector (P < 0.01), and also after viral injection into the DRG (P < 0.05). Compared to the exposure group, AUC was significantly reduced in the PBS/nerve group (P < 0.05) and in the virus/DRG (P < 0.01), the latter of which was confirmed by 2-way ANOVA (P < 0.05).
3.2.8. Prolonged observation of recovery
Since hyperalgesia responses to pin are very unusual in normal animals (Hogan et al., 2004), the persistence of hyperalgesia behavior following PBS injection into the DRG, even at a low level, was further pursued by following a separate group of animals (n = 5) for a greater duration to identify the course of this effect. There was a significant effect of injection (P < 0.001), with a course that showed full resolution after 21 days (Fig. 2G).
3.2.9. Sensory response to SNL
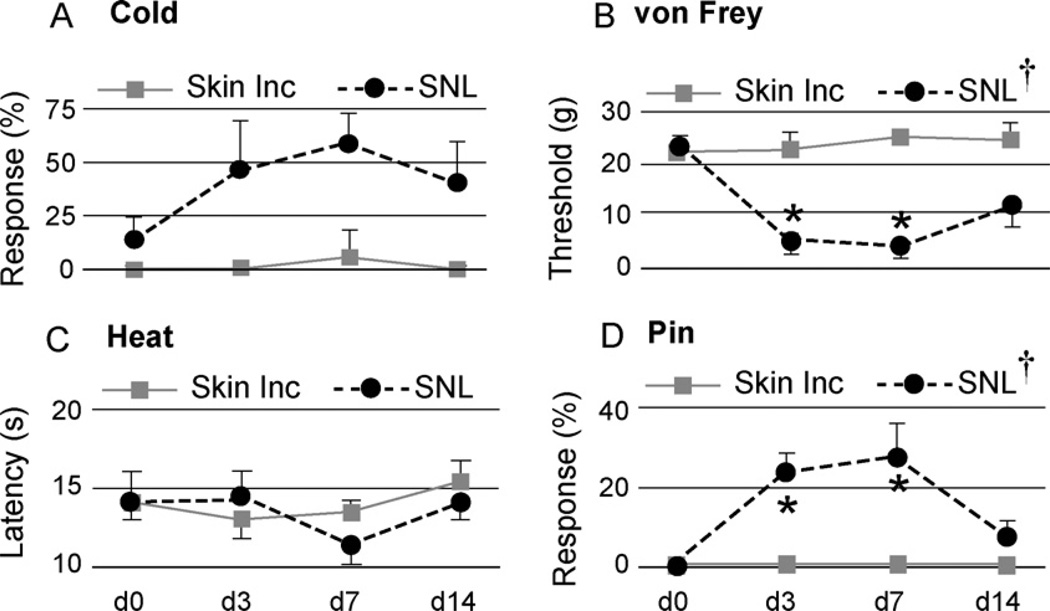
To compare the findings after DRG injections to a known standard, we tested animals subjected to the conventional neuropathic pain model of SNL. Response rates to von Frey and pin increased after SNL but not in control animals (Fig. 4). This contrasts with the lack of changes in pin responses after DRG injections. Also, the increased sensitivity to von Frey stimulation after SNL is greater than after DRG injection. (We did not test motor function after SNL since SNL involves transection of L5 motor fibers and thus is not comparable to DRG injections.)
Fig. 4.
Sensory function following nerve injury evaluated over time. Testing was performed upon the plantar surface of the right paw ipsilateral to spinal nerve ligation (SNL) or skin incision only (Skin Inc; a different group than that used for experiments shown in Figs. 2 and 3). Tests included determining frequency of withdrawal after applying a drop of acetone (A. Cold), threshold of withdrawal from touch with fibers of graduated force (B. von Frey), latency of withdrawal from radiant heat (C. Heat), and frequency of complex, sustained hyperalgesia behavior after touch with a pin(D. Pin), † represents a significant main effect for time by repeated measures ANOVA, and * indicates a significant paired comparison to baseline (day 0, d0) by Tukey’s test. Mean ± SD, n = 5 animals in each group.
3.3. Anatomic observations
3.3.1. Macroscopic DRG appearance
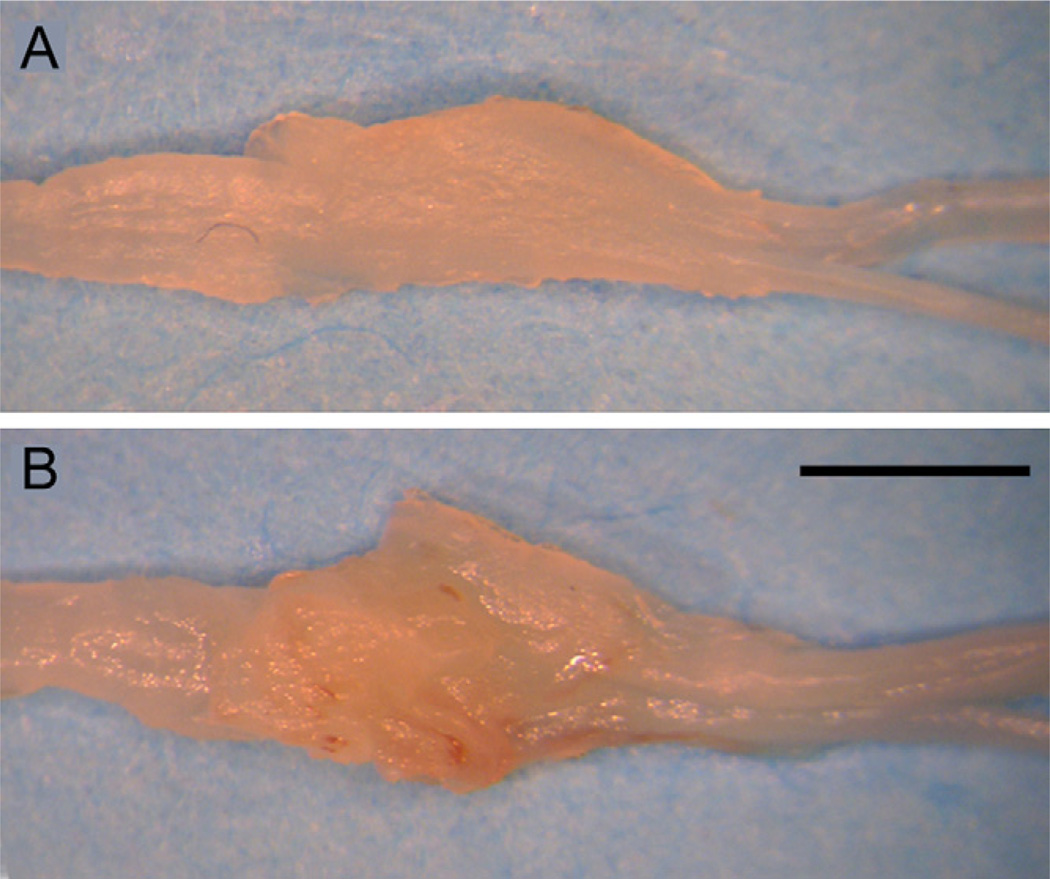
DRGs harvested at 7 days or more following exposure or injection showed a moderate accumulation of connective tissue (Fig. 5), regardless of whether saline or virus solution was injected. This additional tissue readily peeled away from the underlying DRG, which otherwise appeared normal.
Fig. 5.
Excised fifth lumbar dorsal root ganglia, harvested 7 days after exposure surgery (A) and after adeno-associated virus injection (B) and fixation. Scale bar= 1 mm.
3.3.2. Histologic consequences of DRG injection
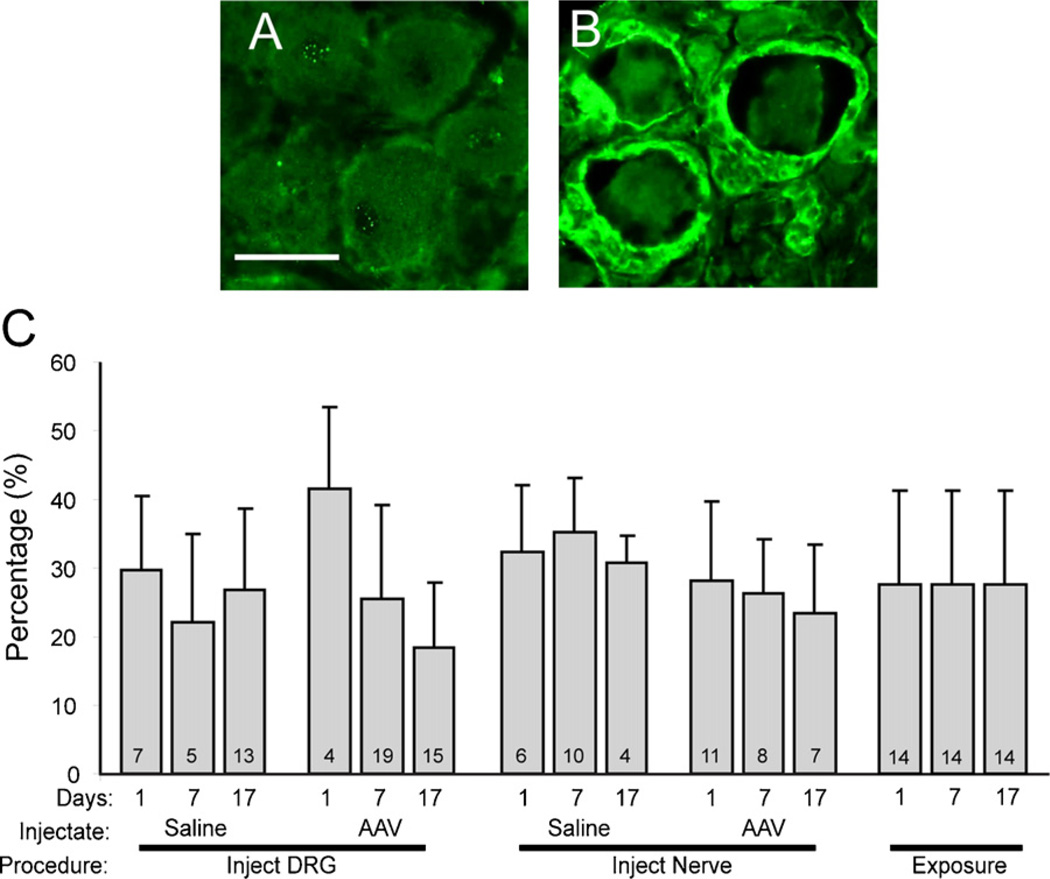
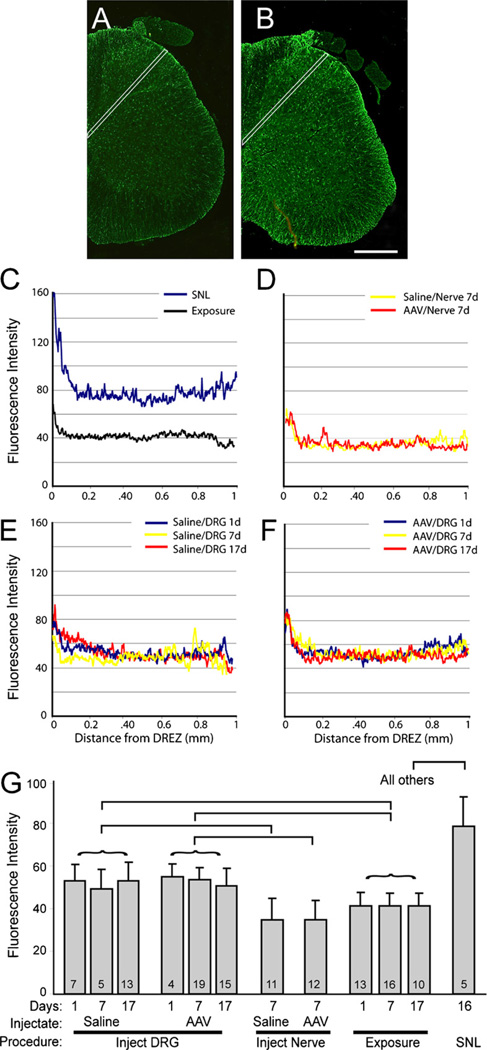
There was no evidence of structural damage or cell infiltration on microscopic examination. Specifically, no neurons were positive for ATF3 (Fig. 6), which demonstrates the absence of a population of axotomized neurons. Activation of satellite glial cells in the DRG was measured as the frequency of ring-like profiles with elevated GFAP staining (Fig. 7A, B). Injections of saline or AAV into either the spinal nerve or the DRG directly did not affect this measure compared to non-injected exposure controls (Fig. 7C). In the dorsal horn of the spinal cord (Fig. 8), line scan analysis of GFAP staining intensity showed only minimal elevation of GFAP above exposure alone for both nerve and DRG injections of either saline or AAV, compared to the substantial elevation produced by axotomy by SNL.
Fig. 6.
Histological appearance of dorsal root ganglion 17 days following adeno-associated virus vector expressing green fluorescent protein (GFP). (A) Fluorescence of GFP is predominantly in neurons, with occasional evidence of GFP in satellite glial cells that surround neuronal somata (arrows). (B) Staining for ATF3, an indicator of neuronal damage, is negative. (C) The same field in differential interference contrast imaging, showing neurons and satellite glial cells (arrows), which encircle the neuronal soma and appear as crescentic profiles that may also show the nucleus at the cell’s thickest part (yellow arrow). Scale bar=50µm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
Fig. 7.
Identification of activated satellite glial cells in the dorsal root ganglion (DRG) as an indication of inflammation. (A) DRG tissue stained with anti-glial fibrillary acidic protein (GFAP) antibody 17 days after surgical exposure alone shows no surrounding rings of GFAP-positive sells. (B) DRG 17 days after AAV injection shows positive GFAP staining in rings of activated satellite glial cells surrounding neuronal somata. (C) Bars indicate the percentage of neuronal somata surrounded by GFAP-positive rings under different conditions, including different times after injections of saline or adeno-associated viral (AAV) vector, or exposure of the ganglia without injection. The numbers in the bars indicate the number of fields quantified, from two animals in each case. Mean ± SD. Statistical analysis (ANOVA) showed no differences between groups when different days were combined into a single group.
Fig. 8.
Activation of dorsal horn astrocytes detected by immunofluorescence intensity of staining with anti-glial fibrillary acidic protein (GFAP) antibody. (A) Dorsal horn tissue harvested 17 days after exposure of dorsal root ganglia (DRG) at the fourth lumbar (L4) and L5 levels. (B) A similar section 17 days after injection of the L4and L5 DRG with adeno-associated viral (AAV) vector. In both cases, the white lines indicate the path along which fluorescence intensity was measured. (C) Sample traces are shown for animals subjected to spinal nerve ligation (SNL) and surgical exposure alone. Similar traces are presented for injection of saline or AAV into the spinal nerve (D), saline into the DRG with harvest 1, 7, or 17 days later (E), and AAV injected into the DRG 1, 7, or 17 days prior to harvest (F). Summary data are shown in (G). The numbers in the bars indicate the number of fields quantified, from two animals in each case. Mean ± SD. Statistical analysis after grouping data obtained at different days following injection or exposure (represented by the curved brackets) showed a significant main effect of group (ANOVAP< 0.001). Square brackets indicate significant paired comparisons.
3.3.3. GFP expression in DRG
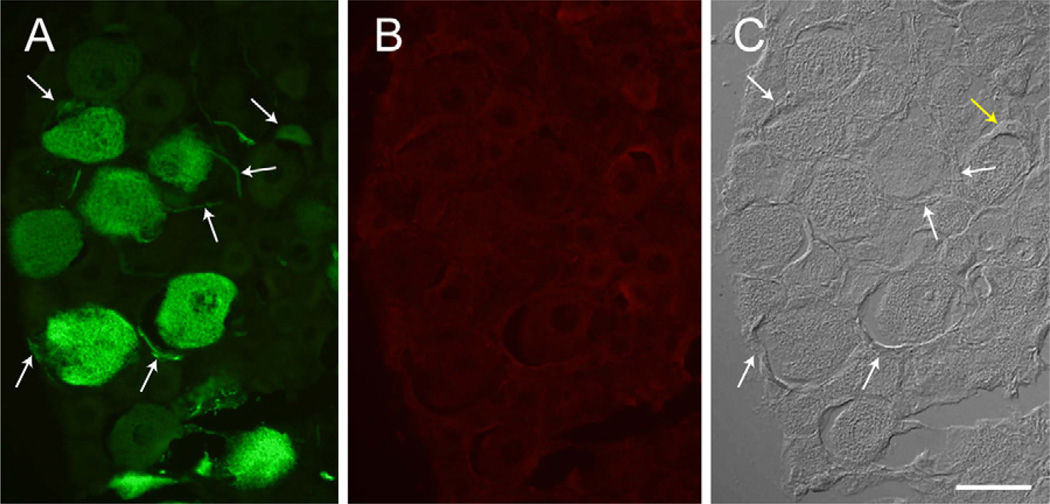
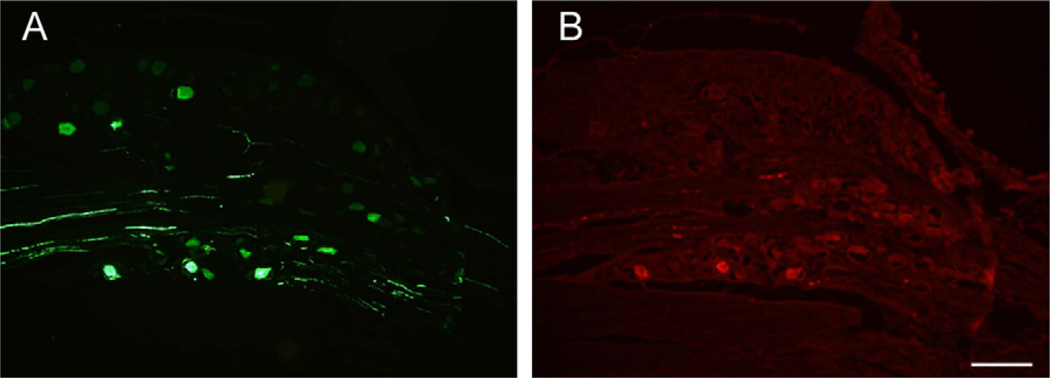
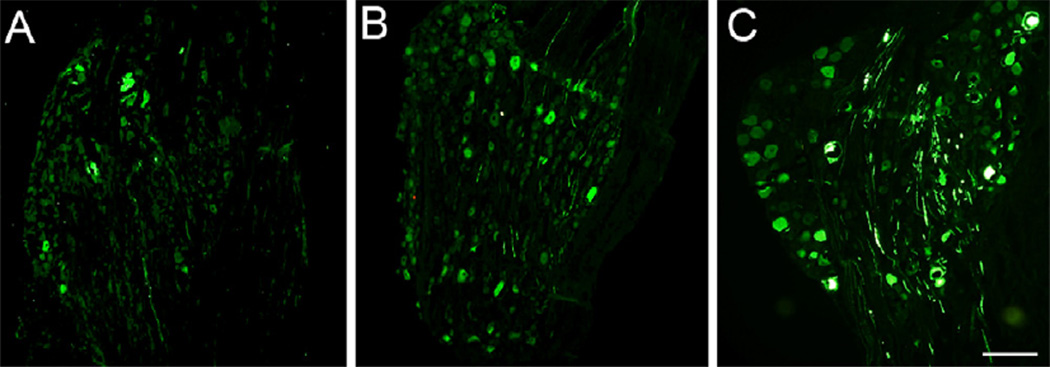
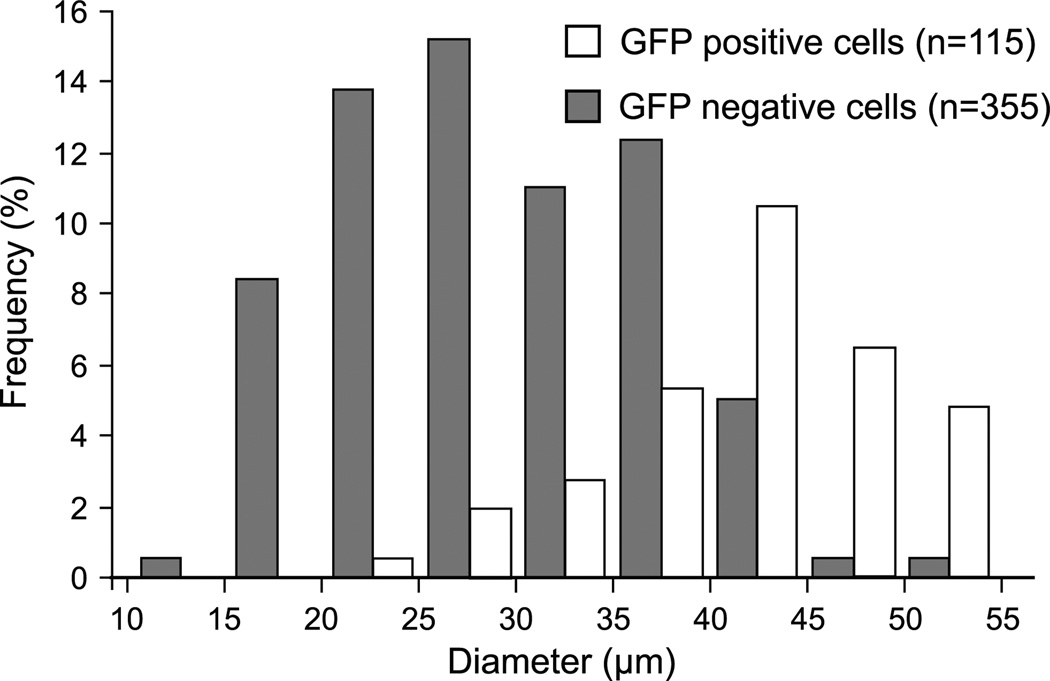
Fluorescence of the GFP transgene product was readily evident following DRG administration of the AAV vector (2 DRGs from 2 rats at each of three post-injection intervals). Positive cell counts were not increased by use of anti-GFP antibody (Fig. 9), so counts were performed using direct GFP fluorescence. Transduced neurons expressing GFP were evident as soon as 1 day after injection (Fig. 10), and the rate of positive cells, as a percentage of all cells, increased thereafter (18 ±13% at 1 day, n = 5 fields, 20 ±12% at 7 days, n = 14; 32 ± 13% at 17 days, n = 18). Expression was predominantly cytoplasmic and was variable among transduced neurons, with only occasional expression in satellite glial cells (Fig. 6). The AAV vector showed a distinct tropism for larger neurons (diameter for positive neurons 41.0 ± 0.7 µm, for negative neurons 33.4 ± 0.5 µm, P< 0.001; Fig. 11).
Fig. 9.
Green fluorescent protein detected in a DRG 7 days after injection of adeno-associated virus (AAV) vector. (A) Fluorescence of GFP. (B) The same section, in which GFP is identified by immunostaining. Scale bar=200 µm.
Fig. 10.
Identification of green fluorescent protein in dorsal root ganglion 1 day (A), 7 days (B), or 17 days (C) after intraganglionic injection of adeno-associated virus (AAV) vector. Scale bar=200 µm.
Fig. 11.
Frequency histogram describing the diameter of dorsal root ganglion somata that showed green fluorescent protein (GFP) fluorescence and those that lack GFP following adeno-associated virus (AAV) vector injection either 1, 7, or 17 days prior to tissue harvest. Since the diameter of transduced neurons was not different on these different days, the data are combined.
3.3.4. Distant GFP expression
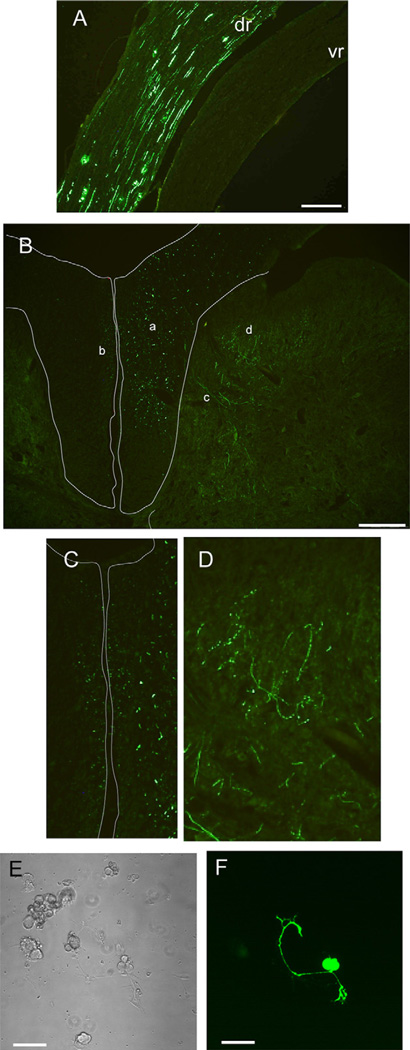
Although GFP signal was seen in the proximal portions of contralateral DRGs after injection of 3 µl of AAV vector (n = 6 rats, data not shown), this was likely due to intrathecal spread from excessive injection volume since contralateral GFP was not seen with 2 µl injections (in 2 rats in which both L4 and L5 DRGs were injected). Strong GFP signal was evident in the dorsal root attached to the injected DRG, but minimal GFP was seen in the ventral roots (in the roots of 4 injected DRGs of 2 rats; Fig. 12A). In the dorsal spinal cord (Fig. 12B), numerous large fibers were labeled by GFP and seen in section throughout the ipsilateral dorsal column (fasciculus gracilis). Additionally, small ascending fibers expressing GFP were seen in the very medial periseptal area of the dorsal columns bilaterally (Fig. 12C). Primary afferents entering the dorsal horn were also identified, many with features suggesting varicosities (Fig. 12D) that are typical of nonmyelinated nociceptive fibers. Other than in the periseptal dorsal column, GFP was not seen in the contralateral spinal cord (n = 4 DRG injections, n = 2 rats).
Fig. 12.
Green fluorescent protein expression 17 days after adeno-associated virus (AAV) vector injection into dorsal root ganglia. (A) Transduced neuronal units are evident predominantly in the dorsal root (dr), with minimal representation in the ventral root (vr). (B) Fluorescent fibers in the dorsal spinal cord are evident in the dorsal column (a, the area enclosed by the thin outline), including small fibers bilaterally in the periseptal medial dorsal column (b; shown enlarged in C). Fibers are seen entering the dorsal horn (c), including those with series of expansions suggesting varicosities (d; shown enlarged in D). Dorsal root ganglion neurons in culture for two days show fluorescence from GFP after in vivo transduction by AAV vector injection (C. bright field view, D. fluorescence view). Scale bars are 200 µm (A and B)and 50 µm (C and D)
3.3.5. Dissociation of transduced DRG neurons
One of the benefits of in situ genetic modification of sensory neurons is the ability to perform physiological analysis without prolonged culture, as would be necessary if transduction/transfection were performed following dissociation. After 2d in culture, neurons dissociated from DRGs following in vivo AAV injection showed distinct GFP fluorescence (n = 2 DRGs, 20% of n = 60 neurons were positive for GFP; Fig. 12E, F), demonstrating the persistence of the transgene expression following dissociation.
4. Discussion
We have evaluated a technique for selective administration of an injected agent to a single DRG. Our findings indicate that direct injection of 2 µl fills the adult rat DRG with only minor spread of the injectate to adjacent structures, produces minimal evidence of inflammatory changes, and has only mild and transient effects on sensory and motor function. The use of this approach for the anatomically selective administration of a viral vector showed genetic modification of sensory neurons in the segment of the injected DRG, thus confirming the utility of our technique for research on sensory mechanisms and the possibility of therapeutic translation for the treatment of chronic pain.
4.1. Injection technique
The described procedure has several benefits compared to other methods for delivery of agents to the DRG. Use of a pulled glass micropipette allows a much smaller tip to be used than can be achieved with a metal needle, possibly resulting in less trauma to the DRG. The injection apparatus we used employs positive displacement of a noncompressible fluid to drive injection, which makes solution delivery independent of variable resistance at the injection tip, and results in injection performance that is more predictable than we encountered using air pressure-driven systems. Movement of the interface between the mineral oil and the injectate provides a direct and measurable indicator of injection. The entire apparatus is small enough to be mounted on a micromanipulator, providing a stable platform for injection and the achievement of a shallow angle of incidence to the nerve surface, which reduces the likelihood of piercing the DRG completely and injecting into the ventral root.
Alternative injection sites for DRG treatment include intrathecal injection via lumbar puncture, which is easily performed. However, administration of a relatively large dose of agent is required, and this route inevitably delivers the agent to multiple segmental levels bilaterally (Xu et al., 2006). Furthermore, diffusion into the entire DRG is not assured, even in rats with short diffusion distances compared to humans. Sciatic nerve injection as a delivery pathway likewise targets several DRGs, and intrafascicular injection is damaging to peripheral nerves in contrast to direct injection into the DRG. We were not able to deliver dye to the DRG from injection into the sciatic nerve in the thigh, although this does not eliminate the possibility of axonal uptake and eventual transport to the DRG.
We have found that intraganglionic injection of 2 µl of dye results in even staining of the entire DRG, without the extensive transit into the spinal roots seen with larger injections. There is no physical barrier between the ganglion and the dorsal spinal root, and a small amount of staining was seen in the most proximal portion of the dorsal root. If it is essential to limit spread of injectate only to the ganglion itself, a reduction in volume to 1 µl is reliable for that purpose. Use of the spinal nerve as an injection site has the advantage that it does not require bone removal, but volumes of 3 µl or more are required to fill the DRG, and also result in substantial spread to the ventral root and more leakage at the injection site. Injecting into a peripheral nerve also carries the risk of an intrafascicular injection and nerve damage.
4.2. Behavioral effects of injection
Since experimental injections into the DRG are likely to be for the purpose of modulating sensory function, it is critical to ascertain what the consequences are of the injection per se. Our AUC data indicate that hyperalgesia in response to noxious mechanical stimulation (pin) and hypersensitivity to threshold punctate stimulation (von Frey) develop after surgical exposure of the ganglion, compared to skin incision controls. Since these changes were not made worse by injection of saline, exposure would be a suitable control for studies involving DRG injection, provided the vehicle is no more noxious than saline. Time course data demonstrate that hypersensitivity following injection resolves within 7 days for von Frey and within 28 days for pin testing, so observations following those time intervals would be optimal for identifying effects of injected substances. In all cases, these changes are much smaller in magnitude than those that follow nerve injury (Hogan et al., 2004), however our data also demonstrate substantial inter-individual variability in responses. Disordered motor function after saline injection is evident only when it is injected into the spinal nerve, which may indicate the relative ability to avoid motor dysfunction by directing injections to the DRG compared to nerve injection.
4.3. Histological effects of injection
Although there is substantial inflammatory response surrounding the DRG after surgical exposure, there is no evidence of infiltrate and only minimal evidence of glial activation in the DRG or astrocyte activation in the dorsal horn. GFAP expression in astrocytes of the CNS and satellite glial cells of the DRG may be activated by many triggering pathological events, but inflammation and axonal trauma at both sites are reliably reflected in elevated GFAP expression in these glial populations (Aldskogius and Kozlova, 1998; Watkins and Maier, 2002). Therefore, our histological data, together with the behavioral observations, support the view that the trauma of exposure and injection do not result in problematic axonal injury or inflammation, supporting the potential use of this technique in a clinical setting.
4.4. Viral vector effects
Viral vector-mediated gene therapy is a promising application of segmental DRG injection. Our initial findings show that substantial transduction can be achieved with restriction to the neurons in the targeted DRG. Although histologic evidence of inflammation was no different than after saline injection or exposure alone, DRG injection produced thermal hypersensitivity and withdrawal from the stroke of a brush only with AAV. Additionally, injection of AAV into the DRG resulted in motor dysfunction, which could be due to either adverse effects on the motor neuron fibers passing anterior to the DRG or to a proprioceptive sensory deficit, which is also suggested by the preferential tropism of the AAV towards large sensory neuronal somata. It has been reported that GFP expression by AAV vector of this serotype can produce toxicity in some CNS neuronal types (Klein et al., 2006). The absence of toxicity in that study with administration of an empty AAV8 vector (Klein et al., 2006) indicates that toxicity was due to GFP expression, so our results may similarly reflect the expression of GFP rather than vector activity per se.
Because somatic transduction results in GFP fluorescence throughout the entire neuron, the use of a GFP transgene provides a means of tracing both the proximal and distal projections of sensory neurons from a single ganglion. In this study, we observed fluorescence in the gracile fasciculus on either side of the dorsal median fissure. The possibility exists that this may represent a novel ascending tract of small primary afferent fibers.
4.5. Limitations
The described procedure has limitations. The surgery is time consuming, although the durations are acceptable after experience is acquired. Care must be taken not to damage the spinal nerve or DRG during exposure. Exposure and injection of the DRG causes significant trauma of paravertebral tissue, which can result in scar formation around the DRG. This may complicate subsequent procedures, such as repeated injections or surgeries to generate neuropathy after injection. The small size of the rat DRG requires that injected volumes be kept quite small. Therefore, injectate may have to be highly concentrated to deliver an efficacious amount. For instance, for viral vector gene therapy, a high concentration of vector particles may be necessary, possibly requiring multiple concentration and purification steps.
4.6. Conclusions
Selective segmental injections into the peripheral sensory nervous system may have two important applications. First, this provides an opportunity to explore pathophysiological processes that underlie chronic pain. As an example, for pain that follows incomplete peripheral nerve injury, it is not yet clear what the relative contributions are from the neuronal population that suffers complete axotomy versus the adjacent but intact population. These populations are segregated in separate DRGs in the SNL model, which allows selective modulation of their function using segmental injections of vectors. A second strong incentive for developing segmental injection techniques in animal subjects is for the exploration of potential clinical approaches. Segmental sensory dysfunction, especially neuropathic pain, may be an ideal setting to apply rapidly evolving genetic therapies.
Acknowledgments
This study was funded in part by the VA Rehabilitation Research and Development grant 3690-03 (to Q Hogan), and NIH grant DK078388 (to H Nakai).
Contributor Information
Gregory Fischer, Email: gfischer@mcw.edu.
Sandra Kostic, Email: sandra.kostic1@gmail.com.
Hiroyuki Nakai, Email: nakaih@pitt.edu.
Frank Park, Email: fpark@mcw.edu.
Damir Sapunar, Email: damirsapunar@gmail.com.
Hongwei Yu, Email: hyu@mcw.edu.
Quinn Hogan, Email: qhogan@mcw.edu.
References
- Aldskogius H, Kozlova EN. Central neuron–glial and glial–glial interactions following axon injury. Prog Neurobiol. 1998;55:1–26. doi: 10.1016/s0301-0082(97)00093-2. [DOI] [PubMed] [Google Scholar]
- Bakunov SA, Bakunova SM, Wenzler T, Barszcz T, Werbovetz KA, Brun R, et al. Synthesis and antiprotozoal activity of cationic 2-phenylbenzofurans. J Med Chem. 2008;51:6927–6944. doi: 10.1021/jm800918v. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Devor M, Seltzer Z. Pathology of damaged nerves in relation to chronic pain. In: Wall PD, Melzack R, editors. Textbook of pain. Edinburgh: Churchill Livingstone; 1999. pp. 129–164. [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Ekstrom PA. Neurones and glial cells of the mouse sciatic nerve undergo apoptosis after injury in vivo and in vitro. Neuroreport. 1995;6:1029–1032. doi: 10.1097/00001756-199505090-00020. [DOI] [PubMed] [Google Scholar]
- Ferrari LF, Cunha FQ, Parada CA, Ferreira SH. A novel technique to perform direct intragangiolnar injections in rats. J Neurosci Methods. 2007;159:236–243. doi: 10.1016/j.jneumeth.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Fuchs A, Rigaud M, Hogan QH. Painful nerve injury shortens the intracellular Ca2+ signal in axotomized sensory neurons of rats. Anesthesiology. 2007;107:106–116. doi: 10.1097/01.anes.0000267538.72900.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm D, Zhou S, Nakai H, Thomas CE, Storm TA, Fuess S, et al. Preclinical in vivo evaluation of pseudotyped adeno-associated virus vectors for liver gene therapy. Blood. 2003;102:2412–2419. doi: 10.1182/blood-2003-02-0495. [DOI] [PubMed] [Google Scholar]
- Hamamura K, Yokota H. Stress to endoplasmic reticulum of mouse osteoblasts induces apoptosis and transcriptional activation for bone remodeling. FEBS Lett. 2007;581:1769–1774. doi: 10.1016/j.febslet.2007.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan Q, Sapunar D, Modric-Jednacak K, McCallum JB. Detection of neuropathic pain in a rat model of peripheral nerve injury. Anesthesiology. 2004;101:476–487. doi: 10.1097/00000542-200408000-00030. [DOI] [PubMed] [Google Scholar]
- Hogan QH. Injection for diagnosis and therapy of back disease. In: An H, editor. Principles and techniques of spine surgery. Baltimore: Williams and Wilkins; 1998. [Google Scholar]
- Hogan QH. Pathophysiology of peripheral nerve injury during regional anesthesia. Reg Anesth Pain Med. 2008;33:435–441. doi: 10.1016/j.rapm.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan QH, McCallum JB, Sarantopoulos C, Aason M, Mynlieff M, Kwok WM, et al. Painful neuropathy decreases membrane calcium current in mammalian primary afferent neurons. Pain. 2000;86:43–53. doi: 10.1016/s0304-3959(99)00313-9. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Klein RL, Dayton RD, Leidenheimer NJ, Jansen K, Golde TE, Zweig RM. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent proteins. Mol Ther. 2006:13. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu YS, Park SK, Chung K, Chung JM. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000;871:98–103. doi: 10.1016/s0006-8993(00)02451-3. [DOI] [PubMed] [Google Scholar]
- Ma C, Rosenzweig J, Zhang P, Johns DC, LaMotte RH. Expression of inwardly rectifying potassium channels by an inducible adenoviral vector reduced the neuronal hyperexcitability and hyperalgesia produced by chronic compression of the spinal ganglion. Mol Pain. 2010;6:65. doi: 10.1186/1744-8069-6-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin SA, Davis BM, Molliver DC. Production of dissociated sensory neuron cultures and considerations for their use in studying neuronal function and plasticity. Nat Protoc. 2007;2:152–160. doi: 10.1038/nprot.2006.461. [DOI] [PubMed] [Google Scholar]
- Mason MR, Ehlert EM, Eggers R, Pool CW, Hermening S, Huseinovic A, et al. Comparison of AAV serotypes for gene delivery to dorsal root ganglion neurons. Mol Ther. 2010;18:715–724. doi: 10.1038/mt.2010.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCallum JB, Kwok WM, Mynlieff M, Bosnjak ZJ, Hogan QH. Loss of T-type calcium current in sensory neurons of rats with neuropathic pain. Anesthesiology. 2003;98:209–216. doi: 10.1097/00000542-200301000-00032. [DOI] [PubMed] [Google Scholar]
- Moore DC, Hain RF, Ward A, Bridenbaugh LD. Importance of the perineural spaces in nerve blocking. JAMA. 1954;156:1050–1053. doi: 10.1001/jama.1954.02950110012005. [DOI] [PubMed] [Google Scholar]
- Palay HW, Tipnis UR, Yates AJ, Yates RR. A method for injecting dorsal root ganglia in rabbit. J Neurosci Methods. 1982;6:173–174. doi: 10.1016/0165-0270(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Pfirrmann CW, Oberholzer PA, Zanetti M, Boos N, Trudell DJ, Resnick D, et al. Selective nerve root blocks for the treatment of sciatica: evaluation of injection site and effectiveness—a study with patients and cadavers. Radiology. 2001;221:704–711. doi: 10.1148/radiol.2213001635. [DOI] [PubMed] [Google Scholar]
- Puljak L, Kojundzic SL, Hogan QH, Sapunar D. Targeted delivery of pharmacological agents into rat dorsal root ganglion. J Neurosci Methods. 2009;177:397–402. doi: 10.1016/j.jneumeth.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueter LE, Kohlhaas KL, Curzon P, Surowy CS, Meyer MD. Peripheral and central sites of action for A-85380inthe spinal nerve ligation model of neuropathic pain. Pain. 2003;103:269–276. doi: 10.1016/s0304-3959(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Sapunar D, Ljubkovic M, Lirk P, McCallum JB, Hogan QH. Distinct membrane effects of spinal nerve ligation on injured and adjacent dorsal root ganglion neurons in rats. Anesthesiology. 2005;103:360–376. doi: 10.1097/00000542-200508000-00020. [DOI] [PubMed] [Google Scholar]
- Wang Z, Ma HI, Li J, Sun L, Zhang J, Xiao X. Rapid and highly efficient transduction by double-stranded adeno-associated virus vectors in vitro and in vivo. Gene Ther. 2003;10:2105–2111. doi: 10.1038/sj.gt.3302133. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev. 2002;82:981–1011. doi: 10.1152/physrev.00011.2002. [DOI] [PubMed] [Google Scholar]
- Wu HE, Gemes G, Zoga V, Kawano T, Hogan QH. Learned avoidance from noxious mechanical simulation but not threshold semmes weinstein filament stimulation after nerve injury in rats. J Pain. 2010;11:280–286. doi: 10.1016/j.jpain.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JJ, Walla BC, Diaz MF, Fuller GN, Gutstein HB. Intermittent lumbar puncture in rats: a novel method for the experimental study of opioid tolerance. Anesth Analg. 2006;103:714–720. doi: 10.1213/01.ane.0000226100.46866.ea. [DOI] [PubMed] [Google Scholar]
- Zhang JM, Homma Y, Ackerman WE, Brull SJ. Topical application of acidic bupiva-cainetothe lumbar ganglion induces mechanical hyperalgesia in the rat. Anesth Analg. 2001;93:466–471. doi: 10.1097/00000539-200108000-00045. [DOI] [PubMed] [Google Scholar]
- Zhou XF, Deng YS, Xian CJ, Zhong JH. Neurotrophins from dorsal root ganglia trigger allodynia after spinal nerve injury in rats. Eur J Neurosci. 2000;12:100–105. doi: 10.1046/j.1460-9568.2000.00884.x. [DOI] [PubMed] [Google Scholar]