Abstract
Background: There is growing evidence that despite the absence of marked deficiencies, diet during pregnancy has important implications for maternal and child health in industrialized countries. At present, relatively little is known about prevailing patterns of intake across groups with diverse sociodemographic and lifestyle patterns in these settings.
Objectives: The aims of this study were to develop methods for the standardization of food group intake data and to describe the process of applying these methods to existing pregnancy cohort studies, which included >200,000 women across Europe.
Design: The study developed a detailed standardization protocol to harmonize intakes of selected food groups, which included fruit, vegetables, meats, seafood, and dairy products. Standardization is necessary to facilitate valid comparisons of intake patterns and disparities across countries and will lead to the development of harmonized databases for possible future pooled analyses.
Results: On the basis of comparisons with previously coordinated multicountry studies, preliminary data suggest that the standardization process yielded sufficiently comparable intake data, which indicate differences in food cultures across the countries that participated.
Conclusions: This project provides lessons on the feasibility of harmonizing dietary intake data from existing studies, which can be applied in future post hoc standardization efforts. The data yielded in this analysis will also provide useful information for the development of food and nutrition policies for pregnant women in Europe, including the identification of population subgroups in which dietary inadequacies during pregnancy may be widespread.
INTRODUCTION
Dietary intakes are especially critical during pregnancy, because inadequate amounts of key nutrients may compromise fetal development or maternal health and may potentially influence longer-term risks to offspring health. Whereas there is a substantial literature that describes intakes of and risks associated with the dietary deficiencies that remain common among women in poorly nourished populations, there are limited published data on current patterns of dietary intake among pregnant women in industrialized countries (1). Recent studies suggest that although deficiencies may not be stark, maternal diet in these settings is related to various aspects of offspring health (2, 3).
There is a need for a better understanding of current patterns of dietary intake among pregnant women in industrialized countries, which includes the identification of any population subgroups with dietary inadequacies that may warrant targeted nutrition policies or programs. Comparable analyses across countries can facilitate the development of regional policies and programs related to diet during pregnancy, analogous to the ongoing development of regional guidelines for the general population (4). Given the cost and complexity of establishing multicountry studies, pooled or meta-analyses that take advantage of existing studies conducted in different countries provide a practical approach for the study of the health effects of maternal diet during pregnancy. Although previous publications on this topic have concluded that it is likely feasible to standardize intake data for selected food groups collected with the use of different methodologies, to our knowledge, research reports that describe the steps involved and experience with such an exercise have not been published (5). The focus of this first stage of the project was not to compare intakes directly, but rather to evaluate the feasibility of adequately harmonizing selected maternal dietary intake data collected with the use of different methods for application in future research. Planned applications of the harmonized data included assessments of whether and how maternal dietary intakes are associated with characteristics such as tobacco use in different countries and regions; to ensure adequate validity of such analyses, it was important to maximize the comparability of intake estimates from different studies by first harmonizing these data.
This study aimed to standardize maternal dietary intake data from existing pregnancy cohort studies across European countries with the use of a standardized approach. This article describes issues addressed and lessons learned during the harmonization process, including its limitations.
METHODS
The harmonization process was developed iteratively. A core protocol and analysis plan were developed based on assessments of dietary data from 4 mother–child cohort studies that participated in the Early Nutrition Programming Project (EARNEST) study: the Danish National Birth Cohort, the Norwegian Mother and Child Birth Cohort, the Avon Longitudinal Study of Parents and Children in England, and the Infancy and Environment Study in Spain. Details on study designs and sample sizes of these cohorts are available elsewhere (6–9). Briefly, these cohorts ranged in sample size from ≈2000 to nearly 100,000 participants, with food-frequency questionnaires (FFQs) comprising 50 to >300 items. These cohorts represented different regions and food cultures, as well as diverse study design characteristics, and thus provided a basis for the identification of many of the challenges involved in the harmonization process. An initial protocol was developed based on English-language versions of the FFQs used in these core cohorts. The food groups selected for study included meats, fruit, vegetables, seafood, and dairy products (milk and yogurt). These food groups are major contributors to intakes of several nutrients of particular importance during pregnancy, which include folate, calcium, and the n−3 (omega-3) fatty acid docosahexaenoic acid.
Standardization protocol
The initial protocol provided detailed, standardized definitions of key food groups that described the specific types of foods to be included in, and excluded from, each food group and subgroup. Participants were asked to provide detailed relevant descriptions in their food questionnaires, including the number of food items included within each food group and subgroup, an English-language description of each food item included in these food groups, and preliminary intake estimates for each group in grams/day. These data allowed the coordinator to identify errors in the assigning of food items to groups, and to assess whether aspects of the questionnaire design may have contributed artifactually to apparent intake disparities. Potentially relevant issues included the omission of key food items and disparities in the number of different items included within each food group.
Collaborating studies
Potential participants were identified through extensive literature and Internet searches, which included the birth cohorts’ website (www.birthcohorts.net). Invitation letters that explained the aims, methods, and data requirements were sent to potential collaborators. Interested participants were asked to provide, in addition to the data described above, basic information to determine eligibility, which included a brief description of the methods used to collect dietary data (trimester assessed, number of items overall and within food groups, total energy intakes estimated), and availability of information on the sociodemographic, lifestyle, and health characteristics to be examined. Eligible to be included were studies with data collection initiated after 1990, dietary questionnaires that included the food groups of potential interest and ideally included ≥50 items, and data on population characteristics of interest. To maximize participation, it was decided to ask for summary information (means and percentiles of intake) rather than raw intake data.
Data
In accordance with the protocol, eligible participants were asked to provide standardized intakes of different types of meats, vegetables, fruit, and dairy foods. Participants also provided previously calculated information on daily energy intakes, which provided a basis for the further evaluation of the overall comparability of dietary data. Preliminary intake data were requested initially for the sample as a whole, to evaluate whether data conformed adequately to the standardized food group definitions; adjustments to the initial food groupings made by each collaborator were requested by the coordinator as needed. The next step involved a workshop and joint discussion in which collaborators participated in evaluation of the standardization process. These discussions provided an opportunity to identify and correct any remaining inconsistencies in food groupings. At this workshop each collaborator was asked to describe his or her dietary data collection protocol and population characteristics relating to the factors of interest, and to present intake data, with the use of a standardized presentation protocol developed by the study coordinator, which ensured that all studies provided the same information. This process helped identify any remaining disparities in interpretation of the food standardization protocol and clarify doubts with regard to the classification of culture-specific foods. Subsequently, participants were asked to provide revised intake estimates stratified by the characteristics of interest in the main study: smoking during pregnancy, prepregnancy obesity, maternal education, and maternal age.
RESULTS
The analysis includes a substantial number of existing studies that represent pregnant women from 4 regions of Europe, as shown in Table 1. There was substantial heterogeneity with respect to study design, sample size, and dietary data collection methods.
TABLE 1.
Characteristics of participating studies1
| Country: cohort | Enrollment period | Dietaryassessmentmethod | No. of items |
| Northern Europe | |||
| Denmark: Danish National Birth Cohort (n = 70,133) | 1996–2002 | FFQ | 360 |
| England: Avon Longitudinal Study of Parents and Children2 (n = 11,877) | 1991–1992 | FFQ | 50 |
| Norway: Norwegian Mother and Child Cohort study (n = 54,350) | 1998–2008 | FFQ | 255 |
| Scotland: The SEATON study (n = 1710) | 1997–1999 | FFQ | 150 |
| Sweden: The ABIS cohort (n = 15,659) | 1997–1999 | FFQ | 22 |
| Western Europe | |||
| France: The EDEN cohort (n = 1610) | 2003–2005 | FFQ | 137 |
| Germany: The LISA study3 (n = 3039) | 1998–1999 | FFQ | 50 |
| Netherlands: Generation R study (n = 1203) | 2002–2006 | FFQ | 293 |
| Southern Europe | |||
| Italy: The GEPSII study (n = 597) | 2003–2004 | FFQ | 188 |
| Greece: The RHEA study (n = 1500) | 2007–2008 | FFQ | 250 |
| Portugal: Generation XXI (n = 327) | 2005–2007 | FFQ | 86 |
| Spain: The Infancy and Environment study cohort (n = 2585) | 2003–2008 | FFQ | 100 |
| Central/eastern Europe | |||
| Poland: The Krakow study (n = 450) | 2000–2002 | A single 24-h recall | NA |
| Poland: The Lublin study (n = 256) | 2007 | 7-d record | NA |
| Austria: Austrian pregnancy study (n = 800) | 2001 | A single 24-h recall | NA |
ABIS, All Babies In Southeast Sweden; EDEN, Etude sur les Déterminants du développement et de la santé de l'Enfant; FFQ, food-frequency questionnaire; GEPSII, Gene and Environment Prospective Study on Infancy in Italy (Italy); LISA, Influence of life-style factors on the development of the immune system and allergies in East and West Germany; NA, not applicable; SEATON, Study of Eczema and Asthma to Observe the effects of Nutrition.
Milk intakes not quantified.
Meat intakes excluded sausages and other processed meats.
Step 1: food item descriptions and initial classification
Before providing intake estimates, detailed descriptions of the number and types of items included in each questionnaire for the food groups of interest were provided by collaborators from each study, in keeping with the protocol distributed by the study coordinator (Table 2). Several collaborators reported having to recalculate intakes of food groups to meet these criteria, which included the exclusion of potatoes from vegetable intakes. In a few studies this information indicated that key food subgroups had been excluded from the dietary questionnaires, which may have contributed to underestimation of intakes (eg, poultry, sausages, and other processed meats were omitted in Germany, which contributed to underestimation of meat intakes). These studies were subsequently excluded from analyses on those specific food groups. In addition, the process showed that many studies lacked information on key subgroups, such as high- compared with lower-fat dairy products, or oily compared with lean fish. As a consequence the possibility of an examination of these subgroups in comparative analyses was precluded, and food group definitions and analysis plans were simplified in subsequent requests for intake estimates.
TABLE 2.
Standardized food group definitions in the protocol disseminated to participants
| Standardized food group definitions | No. of food items | Food items included from your cohort's questionnaire1 | |
| Meats (excluding poultry/game birds) | Total fresh, processed, organ, and other meats (sum of subgroups) | ||
| (a) Fresh red meats | Include game meats and content of mixed dishes; exclude poultry and organ meats (reported separately) | ||
| (b) Processed meats | Include sausages, ham, tinned meats, and similar items, including content of mixed dishes | ||
| (c) Organ meats | Liver and other organs, including content of mixed dishes | ||
| (d) Other red meats | Other meats not included above | ||
| Poultry and game birds | Total poultry and game birds, including content of mixed dishes | Also include rabbit, where eaten | |
| Seafood (fish and shellfish) | Total fresh and processed fish, including content of mixed dishes | ||
| (a) Fresh, frozen, or tinned fatty fish (>5 g fat/100 g) | Include content of mixed dishes and tinned fish in oil or water; exclude other processed fish (ie, salted, smoked, pickled) | ||
| (b) Fresh, frozen, or tinned lower-fat fish (≤5 g fat/100 g) | Include content of mixed dishes and tinned fish in oil or water; exclude other processed fish (ie, salted, smoked, pickled) | ||
| (c) Shellfish, squid, other seafood | Include fresh, frozen, or tinned seafood; exclude salted, smoked, or pickled | ||
| (d) Other processed or preserved fish and shellfish | Include salted, smoked, pickled fish and shellfish | ||
| Milk, cream, yogurt | Milk, cream, and yogurt, including with coffee/tea, etc | ||
| (a) Full-fat milk | Include reconstituted powdered milk (ie, with water content) | ||
| (b) Reduced/nonfat milk (≤2%) | Include reconstituted powdered milk (ie, with water content) | ||
| (c) Other milk products/types | Include cream, buttermilk, evaporated milk, etc | ||
| (d) Full-fat yogurt | Yogurt/yogurt drinks, plain/with fruit | ||
| (e) Reduced-fat yogurt | Yogurt/yogurt drinks, plain/with fruit | ||
| Fruit | Total fruit, including juices (do not include jams/jellies) | ||
| (a) Fresh fruit, excluding juices | All types of fresh fruit (citrus, berries, etc) | ||
| (b) Fruit juices | Fruit juices only (do not include flavored drinks with low fruit content, if possible to exclude)2 | ||
| (c) Canned fruit | |||
| (d) Dried fruit | Dried fruit such as apricots; exclude nuts and seeds (reported above) | ||
| Vegetables | Total vegetables, including all types of vegetables (leafy, fruiting, mushrooms, etc); include juices, if applicable | ||
| (a) Raw vegetables | |||
| (b) Cooked vegetables | |||
| (c) Vegetable juices (if consumed) | |||
| Σ fruit and vegetables combined | Combined intakes of fruit and vegetable food groups |
Please describe each item.
If fresh-squeezed available separately from other juices, please provide.
Step 2: evaluation of preliminary data
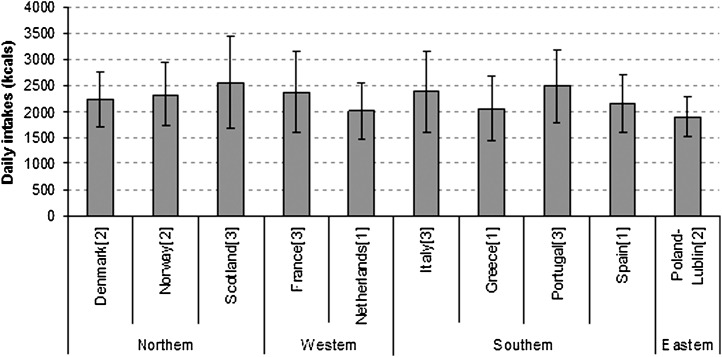
Reported energy intakes, requested to assess the overall comparability of intake data, were generally similar, with discrepancies that appeared largely to reflect small increases in energy needs in later trimesters (Figure 1). These data suggested that the trimester of assessment might contribute to fairly small differences in the quantity of reported intakes.
FIGURE 1.
Mean (±SD) energy intakes among participating countries with comprehensive dietary questionnaires. Numbers in brackets indicate trimester of intake data collection. Energy intake was not calculated in Germany, and complete energy intakes were not available in England because milk consumption was not quantified. Partial energy intakes, excluding milk consumption, equals 1180 ± 488 kcal in the United Kingdom.
To assess the comparability of intake estimates at the food group level, a workshop was held at which each collaborator presented details regarding how intakes of the simplified food groupings were estimated with the use of a standardized format. This process indicated that the majority of food items were classified similarly by all participants, because adherence to the protocol was high. However, a few disparities were identified and addressed during the workshop. For example, it was decided to analyze fruit intakes both including and excluding juices because it was not always possible to identify juice drinks, which may have limited fruit and high sugar content. In some countries, juices represented a substantial proportion of intakes, which included both countries where overall levels of intake were higher (eg, Norway: means of 173 and 275 g/d for juice and whole fruit, respectively) and lower (eg, Denmark: means of 175 and 147 g/d, respectively), whereas in other countries juice intakes were practically null (eg, Portugal: means of 8 and 391 g/d, respectively). In addition, because differences such as the use of additives and fillers in foods such as hamburgers led to substantial disparities in the proportion of meats classified as fresh red meats compared with processed meats, red and processed meats were assessed jointly (eg, 51% classified as processed in Scotland compared with 18% in Portugal). After this workshop the collaborators made any final revisions necessary to food group definitions; detailed intake data additionally stratified by maternal characteristics (not shown) were requested only after this step.
Step 3: assessment of standardized data
After standardization, overall intakes of the food groups were reviewed again. To aid in the evaluation of the comparability of standardized data, levels of intake were compared with those reported among women from the general population in countries that participated in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (Table 3). As shown, harmonized intakes appeared to largely reflect expected disparities in intake across food cultures, and to resemble those reported in EPIC.
TABLE 3.
Comparison of intakes of selected food groups in the Early Nutrition Programming Project (EARNEST) and the European Prospective Investigation into Cancer and Nutrition (EPIC)1
| Categories of mean intake |
||||||
| Fruit2 |
Vegetables3 |
Red and processed meat |
||||
| EPIC | EARNEST | EPIC | EARNEST | EPIC | EARNEST | |
| g/d | g/d | g/d | ||||
| Northern Europe | ||||||
| Denmark | ≤250 | ≤250 | ≤175 | ≤175 | >70 | >70 |
| England | ≤250 | ≤250 | ≤175 | ≤175 | ≤50 | ≤50 |
| Norway | ≤250 | >250–350 | ≤175 | ≤175 | >70 | >70 |
| Scotland | — | ≤250 | ≤175 | ≤175 | — | >70 |
| Sweden | ≤250 | — | ≤175 | ≤175 | >50–70 | >50–70 |
| Western Europe | ||||||
| France | ≤250 | ≤250 | >175–250 | >175–250 | >50–70 | >70 |
| Germany | ≤250 | ≤250 | ≤175 | ≤175 | >70 | — |
| Netherlands | ≤250 | ≤250 | ≤175 | ≤175 | >70 | >70 |
| Southern Europe | ||||||
| Italy | >300 | >300 | >175–250 | >175–250 | >50–70 | >70 |
| Greece | ≤250 | >300 | >175–250 | >175–250 | ≤50 | >50–70 |
| Portugal | — | >300 | — | >175–250 | — | >50–70 |
| Spain | >300 | >300 | >175–250 | >250 | >70 | >70 |
| Central/eastern Europe | ||||||
| Poland–Krakow | — | >300 | — | ≤175 | — | >50–70 |
| Poland–Lublin | — | >250–350 | — | >250 | — | >70 |
| Austria | — | ≤250 | — | ≤175 | — | >50–70 |
EPIC data were reported for women; ranges indicate the highest and lowest values across different centers. The UK data were reported for the general population cohort. The EPIC data were reported by Agudo et al (10; fruit and vegetables) and Pala et al (11; meats).
“Fruit” excludes juices.
“Vegetables” excludes potatoes.
For example, the range reported for mean daily fruit intakes (excluding juices) was generally lower in northern and western European countries than in southern or Mediterranean countries, with the exception of somewhat higher fruit intakes reported by pregnant women in Norway and Greece than those observed in EPIC (Table 2). In both EPIC and EARNEST, mean fruit intakes were relatively high among women from Spain and Italy (>300 g/d), and relatively lower (<250 g/d, ≈2 typical servings daily) in Denmark, England, and Netherlands (12). Similarly, in both projects, mean vegetable intakes were relatively lower in most northern European countries (Denmark, England, Norway, Sweden; <175 g/d, roughly one typical raw and one cooked serving daily), with higher levels in the Mediterranean (12).
In both EPIC and EARNEST, estimated intakes of red and processed meat intakes were substantially lower in Greece (35 and 63 g/d, respectively) and the United Kingdom (50 and 46 g/d, respectively) than elsewhere, where intakes generally exceeded 70 g/d (11) (Table 2). Markedly higher seafood intakes reported in Spain than elsewhere (including the other Mediterranean countries that participated in EPIC) were also observed in both projects [data not shown (13)].
Despite substantial differences in questionnaire design, there was no clear evidence of systematic differences in estimates of mean intake related to these issues. Notably, there were substantial disparities in the number of food items included in different questionnaires, and consequently marked variation in numbers of items used to estimate intakes of different food groups. For example, meat intakes were assessed with the use of 9 items in Spain compared with 22 items in Italy, and by 33 items (which included questions on consumption in sandwiches) in Norway compared with 11 items in Scotland. Nonetheless, estimated intakes of red and processed meats were similar in Spain (105 g/d) and Italy (107 g/d), as well as in Norway (87 g/d) and Scotland (83 g/d). Similarly, there was no clear evidence of systematic trends related to trimester of dietary assessment. For example, comparable fruit intakes were reported in Italy (third trimester, 316 g/d) and Spain (first trimester, 317 g/d), as well as in Portugal (third trimester, 391 g/d) and Greece (first trimester; 407 g/d).
DISCUSSION
To build on lessons learned in previous studies, the EARNEST European Diet in Pregnancy Project developed a detailed protocol for the standardization of food group intake data from existing studies in pregnant women across Europe to conduct a comparative study of dietary intakes during pregnancy (5, 14, 15). With the use of this protocol, reported intakes of the food groups selected for study (fruit, vegetables, meats, and milk and yogurt) were standardized within each participating study by collaborators familiar with their own food cultures. Ultimately, disparities across countries in estimated mean food group intakes were similar to those observed in previous studies, and appeared to largely reflect differences in food cultures.
Although not all countries were included in both projects, comparisons with the multicenter EPIC study, designed to obtain comparable estimates across countries, suggest the standardization yielded estimates that reflect differences in food cultures, albeit with some exceptions. For example, intakes of fruit and vegetables were markedly higher in most Mediterranean countries than elsewhere, as were intakes of seafood among Spanish and Portuguese women. However, fruit intakes among Norwegian women ranked somewhat higher in EARNEST than in EPIC, perhaps in part because of differences in dietary habits during pregnancy, as well as increases over time. FAO data suggest that there have been substantial increases in fruit intakes in Norway over this period [a 35% increase, from 274 to 369 g/d, between 1994 and 2006, compared with a 22% increase in France, from 249 to 305 g/d, for the same period (16)].
The process also provided insights on the types of limitations to intake data standardization that may arise. We were unable to assess intakes of red and processed meats separately because of disparities in how foods were typically prepared and processed in different food cultures. Similar challenges may arise in analyses in which relevant health effects may be more strongly related to specific subgroups of foods, rather than broader subgroups. We were, for example, unable to assess subgroups of dairy foods with higher and lower fat content, or intakes of oily compared with lean fish. For the estimation of mean intakes of broader food groups, however, we did not detect evidence of systematic differences in estimates related to factors such as the number of items included in questionnaires, or to differences in the trimester when intakes were assessed. Nonetheless, it cannot be ascertained whether or how such factors might have influenced the reporting of intakes by individual study participants. For example, the use of brief FFQ instruments may not influence mean intakes, but may reduce variability and the ability to detect associations.
Building on lessons learned in previous efforts to harmonize dietary data (5, 14, 15), this project focused on selected food groups rather than on nutrients, for which standardization is much more complex (17). It is important to note, however, that the food groups analyzed here were amenable to post hoc standardization. Other foods, notably cereal products, may be more challenging to standardize adequately. Levels of intake in terms of grams per day may appear to be very disparate across food cultures in which bread consumption predominates rather than pasta or rice, given the large differences in the weights of typical portions. Although the use of servings might suggest smaller disparities, few partner studies had readily available intake data calculated in terms of servings rather than grams, and definitions of standard servings varied widely across food cultures.
The project supports the feasibility of post hoc harmonization of intakes of selected food groups, a methodologic issue on which there has been substantial debate (5, 14, 15). An indirect added value of this process will be the strengthening of collaboration between pregnancy cohorts in Europe and the promotion of further analyses to examine the association between nutrition and health effects for women and their offspring. This work can serve as a basis for the development of a harmonized database that may facilitate future pooled analyses of prospective relationships between dietary intakes in pregnancy and health outcomes with the use of eligible data from multiple cohorts. Importantly, limitations in the direct comparability of levels of intake may not hamper future work that involves the pooling of data for analyses of diet–outcome relationships. Analyses conducted as part of the Pooling Project of Prospective Studies on Diet and Cancer (18), as well as analyses that involve the pooling of biomarker data (19), suggest that similar, valid results may be obtained through the categorization of study-specific levels (eg, into high, medium, low) or with the use of quantiles based on universal cutoffs applied in each of the pooled studies.
Although it had been suggested previously that in the context of industrialized countries, where marked dietary deficiencies are rare, maternal diet during pregnancy is unlikely to influence either offspring or maternal health, recent studies suggest that this may not be the case (20, 21). For example, dietary patterns characterized by factors such as higher fruit and vegetable intakes have been associated with lower risk of preterm birth (22, 23) and preeclampsia (24), whereas some types of seafood have been associated with increased risk of small size for gestational age (25–27). Other data suggest that maternal diet during pregnancy may be related to other aspects of child health, which include respiratory outcomes (28, 29), neurodevelopment (30), and bone health (31). Given the growing evidence that diet during pregnancy is relevant in the context of industrialized countries, and the need for regional data that describe prevailing patterns of intake as a basis for the development of appropriate dietary guidelines in pregnancy, the proposed project can make an important contribution to both the scientific and public health literature.
Acknowledgments
We acknowledge the essential contributions of each of the collaborating study partners: TI Halldorsson and SF Olsen from the Danish National Birth Cohort in Denmark; M Haugen and HM Meltzer from the Norwegian Mother and Child Birth Cohort in Norway; C Atkinson and A Ness from the Avon Longitudinal Study of Parents and Children in England; P Amiano, F Ballester, M Rebagliato, A Tardón, and J Vioque from the different centers of the Infancy and Environment study–Spain; H Freisling and P Rust from Austria; MA Charles from France-EDEN; J Heinrich and S Sausenthaler from LISA-Germany; L Chatzi and T Roumeliotaki from Rhea-Greece; D Porta and F Forastiere from GEPSI-Italy; V Jaddoe, E Steegers, and S Timmermans from Generation R–Netherlands; W Jedrychowski and A Pac from Krakow, Poland; I Bojar from Lublin, Poland; E Pinto and I Santos Silva from Generation XXI–Portugal; L Craig and G Devereux from Seaton–Scotland; and H Brekke and J Ludviggson from ABIS–Sweden.
The authors’ responsibilities were as follows—MM and MK: development of methods; MM: coordination of the study and drafting of the manuscript; and MK: study conception and provision of input and revisions to the manuscript. The authors declared no conflicts of interest.
REFERENCES
- 1.Jackson AA, Robinson SM. Dietary guidelines for pregnancy: a review of current evidence. Public Health Nutr 2001;4:625–30 [DOI] [PubMed] [Google Scholar]
- 2.Moore VM, Davies MJ. Diet during pregnancy, neonatal outcomes and later health. Reprod Fertil Dev 2005;17:341–8 [DOI] [PubMed] [Google Scholar]
- 3.Godfrey KM, Barker DJ. Fetal nutrition and adult disease. Am J Clin Nutr 2000;71:1344S–52S [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Food-based dietary guidelines in the WHO European Region. Geneva, Switzerland: WHO, 2003. (Publication no. EUR/03/5045414 E79832.) [Google Scholar]
- 5.Verger P, Ireland J, Moller A, Abravicius JA, De Henauw S, Naska A. Improvement of comparability of dietary intake assessment using currently available individual food consumption surveys. Eur J Clin Nutr 2002;56(suppl 2):S18–24 [DOI] [PubMed] [Google Scholar]
- 6.Olsen J, Melbye M, Olsen SF, et al. The Danish National Birth Cohort–its background, structure and aim. Scand J Public Health 2001;29:300–7 [DOI] [PubMed] [Google Scholar]
- 7.Magnus P, Irgens LM, Haug K, Nystad W, Skjaerven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa). Int J Epidemiol 2006;35:1146–50 [DOI] [PubMed] [Google Scholar]
- 8.Ness AR. The Avon Longitudinal Study of Parents and Children (ALSPAC)–a resource for the study of the environmental determinants of childhood obesity. Eur J Endocrinol 2004;151(suppl 3):U141–9 [DOI] [PubMed] [Google Scholar]
- 9.Ribas-Fito N, Ramon R, Ballester F, et al. Child health and the environment: the INMA Spanish Study. Paediatr Perinat Epidemiol 2006;20:403–10 [DOI] [PubMed] [Google Scholar]
- 10.Agudo A, Slimani N, Ocké MC, et al. Consumption of vegetables, fruit and other plant foods in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohorts from 10 European countries. Public Health Nutr 2002;5(6B):1179–96 [DOI] [PubMed] [Google Scholar]
- 11.Pala V, Krogh V, Berrino F, et al. Meat, eggs, dairy products, and risk of breast cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. Am J Clin Nutr 2009;90:602–12 [DOI] [PubMed] [Google Scholar]
- 12.Agudo A, Pera G. Vegetable and fruit consumption associated with anthropometric, dietary and lifestyle factors in Spain. EPIC Group of Spain. European Prospective Investigation into Cancer. Public Health Nutr 1999;2:263–71 [DOI] [PubMed] [Google Scholar]
- 13.Welch AA, Lund E, Amiano P, Dorronsoro M. Variability in fish consumption in 10 European countries. IARC Sci Publ 2002;156:221–2 [PubMed] [Google Scholar]
- 14.Beer-Borst S, Hercberg S, Morabia A, et al. Dietary patterns in six european populations: results from EURALIM, a collaborative European data harmonization and information campaign. Eur J Clin Nutr 2000;54:253–62 [DOI] [PubMed] [Google Scholar]
- 15.Irala-Estevez JD, Groth M, Johansson L, Oltersdorf U, Prattala R, Martinez-Gonzalez MA. A systematic review of socio-economic differences in food habits in Europe: consumption of fruit and vegetables. Eur J Clin Nutr 2000;54:706–14 [DOI] [PubMed] [Google Scholar]
- 16.FAO FAOSTAT Food and Agriculture Organization of the United Nations Food Supply Statistics. Available from: http://faostat.fao.org/site/609/default.aspx~ancor (cited May 2010) [Google Scholar]
- 17.Slimani N, Deharveng G, Unwin I, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr 2007;61:1037–56 [DOI] [PubMed] [Google Scholar]
- 18.Smith-Warner SA, Spiegelman D, Ritz J, et al. Methods for pooling results of epidemiologic studies: the Pooling Project of Prospective Studies of Diet and Cancer. Am J Epidemiol 2006;163:1053–64 [DOI] [PubMed] [Google Scholar]
- 19.Taioli E, Bonassi S. Pooled analysis of epidemiological studies involving biological markers. Int J Hyg Environ Health 2003;206:109–15 [DOI] [PubMed] [Google Scholar]
- 20.Mathews F, Yudkin P, Neil A. Influence of maternal nutrition on outcome of pregnancy: prospective cohort study. BMJ 1999;319:339–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lagiou P, Mucci L, Tamimi R, et al. Micronutrient intake during pregnancy in relation to birth size. Eur J Nutr 2005;44:52–9 [DOI] [PubMed] [Google Scholar]
- 22.Haugen M, Meltzer HM, Brantsaeter AL, et al. Mediterranean-type diet and risk of preterm birth among women in the Norwegian Mother and Child Cohort Study (MoBa): a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:319–24 [DOI] [PubMed] [Google Scholar]
- 23.Mikkelsen TB, Osterdal ML, Knudsen VK, et al. Association between a Mediterranean-type diet and risk of preterm birth among Danish women: a prospective cohort study. Acta Obstet Gynecol Scand 2008;87:325–30 [DOI] [PubMed] [Google Scholar]
- 24.Brantsaeter AL, Haugen M, Samuelsen SO, et al. A dietary pattern characterized by high intake of vegetables, fruits, and vegetable oils is associated with reduced risk of preeclampsia in nulliparous pregnant Norwegian women. J Nutr 2009;139:1162–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mendez MA, Torrent M, Julvez J, Ribas-Fito N, Kogevinas M, Sunyer J. Maternal fish and other seafood intakes during pregnancy and child neurodevelopment at age 4 years. Public Health Nutr 2009;12:1702–10 [DOI] [PubMed] [Google Scholar]
- 26.Halldorsson TI, Meltzer HM, Thorsdottir I, Knudsen V, Olsen SF. Is high consumption of fatty fish during pregnancy a risk factor for fetal growth retardation? A study of 44,824 Danish pregnant women. Am J Epidemiol 2007;166:687–96 [DOI] [PubMed] [Google Scholar]
- 27.Guldner L, Monfort C, Rouget F, Garlantezec R, Cordier S. Maternal fish and shellfish intake and pregnancy outcomes: a prospective cohort study in Brittany, France. Environ Health 2007;6:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chatzi L, Kogevinas M. Prenatal and childhood Mediterranean diet and the development of asthma and allergies in children. Public Health Nutr 2009;12:1629–34 [DOI] [PubMed] [Google Scholar]
- 29.Romieu I, Torrent M, Garcia-Esteban R, et al. Maternal fish intake during pregnancy and atopy and asthma in infancy. Clin Exp Allergy 2007;37:518–25 [DOI] [PubMed] [Google Scholar]
- 30.Oken E, Bellinger DC. Fish consumption, methylmercury and child neurodevelopment. Curr Opin Pediatr 2008;20:178–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yin J, Dwyer T, Riley M, Cochrane J, Jones G. The association between maternal diet during pregnancy and bone mass of the children at age 16. Eur J Clin Nutr 2010;64:131–7 [DOI] [PubMed] [Google Scholar]