Abstract
Objectives
We investigated the effect of reducing mitochondrial oxidative stress by the mitochondrial-targeted antioxidant peptide SS-31 in hypertensive cardiomyopathy.
Background
Oxidative stress has been implicated in hypertensive cardiovascular diseases. Mitochondria and NADPH oxidase have been proposed as primary sites of reactive oxygen species (ROS) generation.
Methods
The mitochondrial targeted antioxidant peptide SS-31 was used to determine the role of mitochondrial oxidative stress in Angiotensin II (Ang)-induced cardiomyopathy, as well as in Gαq overexpressing mice with heart failure.
Results
Angiotensin II induces mitochondrial ROS in neonatal cardiomyocytes, which is prevented by SS-31, but not the non-targeted antioxidant N-acetyl cysteine (NAC). Continuous administration of Ang for 4 weeks in mice significantly increased both systolic and diastolic blood pressure, and this was not affected by SS-31 treatment. Ang was associated with upregulation of NADPH oxidase 4 (NOX4) expression, increased cardiac mitochondrial protein oxidative damage and induced the signaling for mitochondrial biogenesis. Reducing mitochondrial ROS by SS-31 substantially attenuated Ang-induced NOX4 upregulation, mitochondrial oxidative damage, upregulation of mitochondrial biogenesis, phosphorylation of p38 MAP kinase, and prevented apoptosis, concomitant with amelioration of Ang induced cardiac hypertrophy, diastolic dysfunction, and fibrosis, despite the absence of blood pressure lowering effect. NAC did not show any beneficial effect. SS-31 administration for 4 weeks also partially rescued the heart failure phenotype of Gαq overexpressing mice.
Conclusions
Mitochondrial targeted peptide SS-31 ameliorates cardiomyopathy resulting from prolonged Ang stimulation as well as Gαq overexpression, suggesting its potential clinical application for target organ protection in hypertensive cardiovascular diseases.
Keywords: mitochondria, hypertension, cardiomyopathy
Hypertension is a major global health issue, accounting for approximately half of the cases of stroke and ischemic heart disease, and about 13% of total death worldwide(1). Systemic hypertension induces left ventricular hypertrophy (LVH), fibrosis and diastolic dysfunction, increases the risk of coronary artery disease, which leads to congestive heart failure (2). The Renin-Angiotensin Aldosterone System is the central regulator of hypertensive cardiovascular diseases. Angiotensin II (Ang) induces LVH, cardiac fibrosis and diastolic dysfunction. At the molecular level, Ang binds to the angiotensin receptor-1 (ATR1), a Gαq coupled receptor, then stimulates NADPH oxidases to produce reactive oxygen species (ROS) (3). Recent studies have reported that the NOX4 isoform of NADPH oxidase is present in mitochondria. ROS generated by NADPH oxidase was shown to stimulate mitochondrial ROS production and induce mitochondrial dysfunction (4–7). Furthermore, we previously demonstrated that mitochondrial ROS plays a key role in aging and lifespan regulation, as shown by ~20% extension of lifespan in mice overexpressing catalase targeted to mitochondria (mCAT)(8). We further showed that mCAT attenuated age-dependent left ventricular hypertrophy and diastolic dysfunction, concomitant with attenuation of age-dependent increases in cardiac mitochondrial oxidative damage, without any effect on the increase of cardiac Ang observed in aged mice (9). We therefore hypothesized that Ang might induce mitochondrial ROS in cardiomyocytes and that scavenging mitochondrial ROS by a mitochondrial targeted antioxidant peptide might be beneficial in the setting of hypertensive cardiomyopathy.
The Szeto-Schiller (SS)-31 peptide (D-Arg-2`,6`-dimethyltyrosine-Lys-Phe-NH2) belongs to a family of aromatic cationic peptides that selectively target to mitochondrial inner membrane and can scavenge superoxide, hydrogen peroxide, peroxynitrite and hydroxyl radicals (10,11). SS-31 has been shown to reduce mitochondrial ROS in epithelial, endothelial and neuronal cells exposed to electron-transport-chain inhibitors as well as pro-oxidants including t-butylhydroperoxide and hypochlorous acid (12)(13). SS-31 also inhibits apoptosis mediated by mitochondrial release of cytochrome c elicited by mitochondrial permeability transition (11). In vivo administration of SS-31 has demonstrated efficacy in several animal models associated with mitochondrial oxidative stress, including reduction of ischemia-reperfusion injury (14), protection against neurodegeneration (15), and prevention of insulin resistance induced by high-fat diet (16). In this study, we demonstrated that Ang induced mitochondrial ROS in cardiomyocytes and increased cardiac mitochondrial protein oxidative damage. Reduction of mitochondrial ROS by SS-31 ameliorated Ang induced cardiac hypertrophy, fibrosis and apoptosis, concomitant with reduced activation of p38 MAP kinase. SS-31 administration also partially rescued the heart failure phenotype of mice with Gαq overexpression as a model of chronic catecholamine/Ang stimulation (17).
Methods
Detailed methods are provided in the online supplement. Neonatal mouse cardiomyocytes were stimulated with Ang (1 µM), with or without simultaneous treatment of either of the following: SS-31 (1 nM, kindly provided by Stealth Peptides Inc.), N-acetyl cysteine (NAC, 0.5 mM), 4-chlorodiazepam (25 µM), diazoxide (200 µM), cyclosporine (0.5 µM) or 5-hydroxydecanoate (100µM). Cardiomyocytes were then loaded with MitoSOX (5 µM) and CM-DCFDA (5 µM) to measure mitochondrial superoxide and total cellular ROS, respectively.
C57BL/6J mice (6–10/group, aged ~12 weeks) were treated with saline, Ang (1.1mg/kg/d), Ang + SS-31 (3mg/kg/d), Ang + NAC (approximately 500mg/kg/d in drinking water)(18), Gαq (FVBxC57BL/6 F2), Gαq +SS-31 (3mg/kg/d) and WT littermates. Ang and SS-31 were continuously administered for 4 weeks using subcutaneous Alzet 1004 osmotic minipumps. Echocardiography was performed at baseline and 4 weeks after pump. As a reference for SS-31 effects, we included a genetic mouse model of Rosa-26 inducible-mCAT (C57BL/6), in which mitochondrial catalase was expressed two weeks before Ang treatment. Blood pressure was measured in a separate group of mice by telemetry using an intravascular catheter PA-C10 (DSI, MN). After 4 weeks of treatment mouse ventricles were harvested. Quantitative image analysis of trichrome staining was performed to evaluate the severity of fibrosis. Cardiac mitochondrial protein carbonyl content was analyzed using OxiSelect™ Protein Carbonyl ELISA Kit (Cell Biolabs). Quantitative PCR (qPCR) was performed using Applied Biosystem 7900 thermocycler with Taqman Gene Expression Assays on Demand. Western blotting was used to analyze NOX4, cleaved caspase-3, phosphorylated and total p38 MAPK.
Statistical Analysis
All data are presented as mean±SEM. Comparisons between two groups are performed using Student t-tests. One-way ANOVA was used to compare differences among multiple groups, followed by Tukey post-hoc test for significance. p<0.05 were considered significant.
Results
Ang increased mitochondrial and total cellular ROS in neonatal cardiomyoctyes, which was alleviated by SS-31
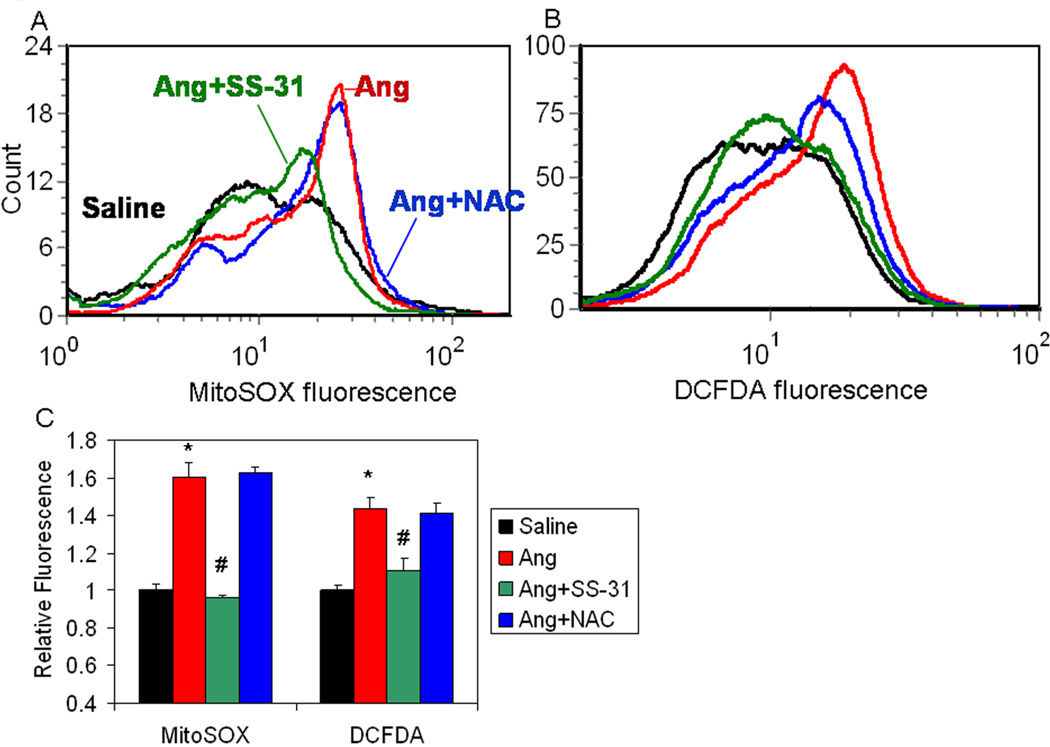
Flow cytometric analysis demonstrated that Ang increased MitoSOX fluorescence (an indicator of mitochondrial superoxide, Figure 1A) and DCFDA fluorescence (an indicator of total cellular ROS, Figure 1B) in neonatal cardiomyocytes by approximately 60 and 45%, respectively (p<0.01 for both, Figure 1C). Treatment with NAC, a non-targeted antioxidant drug, did not show any significant effect on mitochondrial or total cellular ROS after Ang. In contrast, SS-31 significantly reduced Ang-induced MitoSOX and DCFDA fluorescence to near control levels (Figure 1). Ang induced MitoSOX and DCFDA fluorescence could also be attenuated by 4-Cl-benzodiazepam, an inhibitor of the inner mitochondrial anion channel, or cyclosporine A, an inhibitor of the mitochondrial permeability transition pore or diazoxide, an activator of the mitochondrial K-ATP channel, (SFig.1A–D,G), further supporting mitochondrial mechanisms of ROS induction (see Discussion). In contrast, 5-hydroxydecanoate, an inhibitor of the mitochondrial K-ATP channel, did not significantly affect Ang-induced ROS (SFig 1E, F).
Figure 1. Ang increased mitochondrial and total cellular oxidative stress in neonatal cardiomyocytes is prevented by SS-31 but not N-acetyl cysteine.
Flow cytometry of neonatal cardiomyocytes stimulated with Ang (1µM) and loaded with MitoSOX (A) or CM-DCFDA (B). (C) Quantitative analysis (presented as mean±SEM of histogram medians, n=3–5) revealed a significant increase in MitoSOX and DCFDA fluorescence in Ang-treated cardiomyotyes (red), compared with saline treatment (black). The non-targeted antioxidant NAC (0.5mM, blue line) had no significant effect on Ang induced mitochondrial ROS (A, C) and total cellular ROS (B, C). Simultaneous treatment with SS-31 (1nM, green line) substantially reduced Ang induced mitochondrial ROS (A, C) and total cellular ROS (B, C).*p<0.01 vs. saline; #p<0.01 vs. Ang.
SS-31 ameliorates Ang-induced cardiomyopathy despite the absence of pressor effects
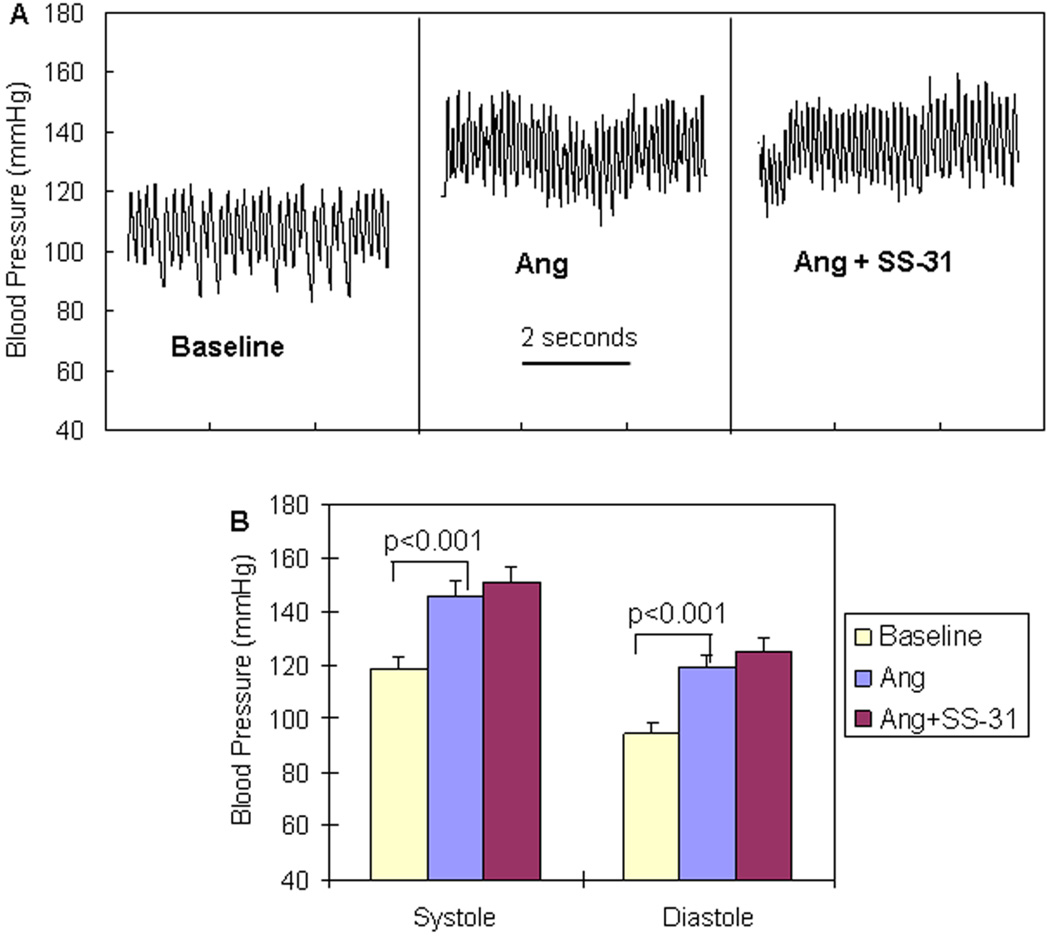
To recapitulate hypertensive cardiomyopathy, we administered a pressor dose of Ang (1.1mg/kg/d) for 4 weeks via subcutaneous pumps. Intravascular telemetry (Figure 2) revealed Ang-induced systolic and diastolic blood pressure increases of 25–28 mmHg above baseline (BP: 118.8 ± 4.0 / 94.5 ± 3.5 mmHg at baseline vs. 146.0 ± 5.6 / 119.3 ± 4.0 mmHg after Ang, p<0.001). Simultaneous administration of SS-31 did not show any effect on BP.
Figure 2. No effect of SS-31 on blood pressure increase after pressor dose of Ang.
(A) Representative blood pressure tracings of mice at baseline and after Ang (1.1mg/kg/d) administered with a subcutaneous pump. (B) Ang significantly increased systolic and diastolic BP. Administration of SS-31 (3mg/kg/d) did not show any significant effect on BP when added to Ang treatment. (n=3)
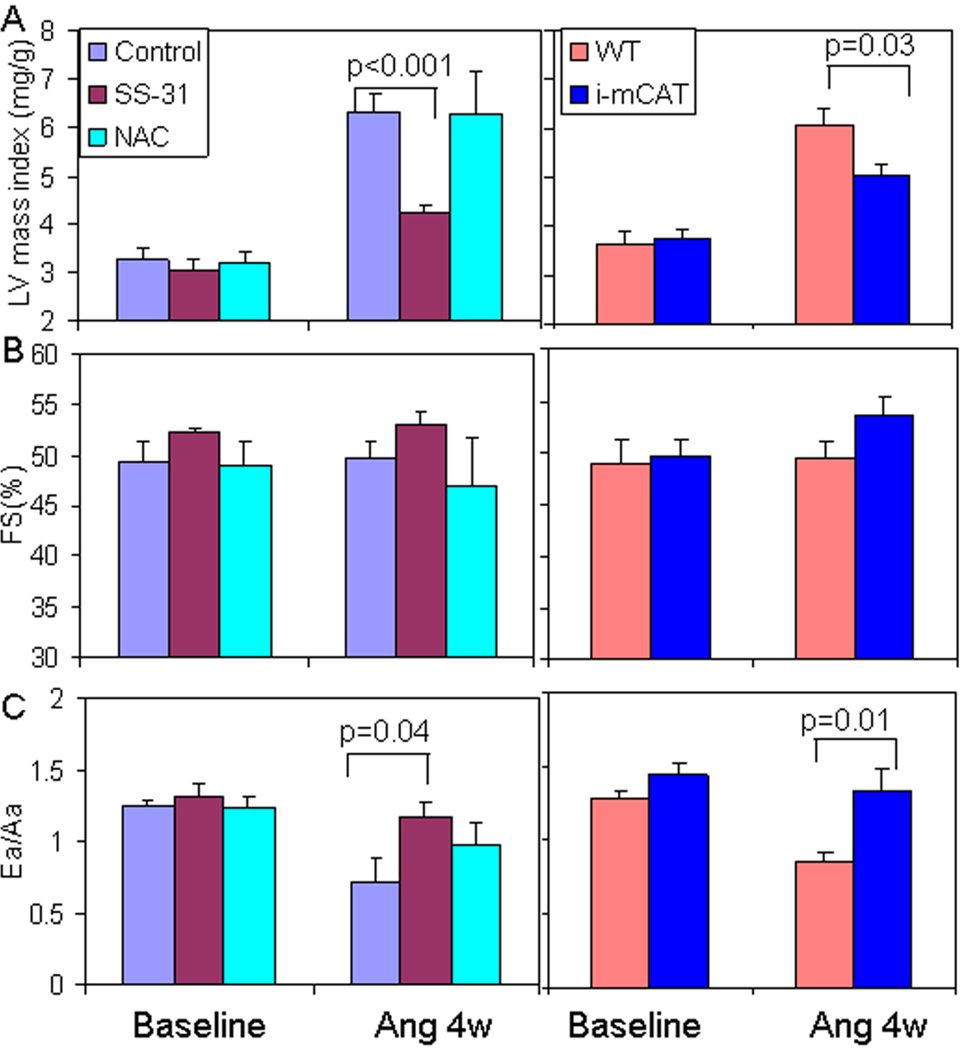
After 4 weeks of Ang, echocardiography revealed an approximately 2-fold increase in left ventricular mass index (LVMI) compared with baseline (Figure 3A), no change in LV end diastolic diameter (data not shown) or systolic function as measured by fractional shortening (FS, Figure 3B), and a ~35% decline in Ea/Aa, an indicator of diastolic function (Figure 3C). Simultaneous administration of SS-31 significantly ameliorated Ang-induced cardiac hypertrophy and diastolic dysfunction, with a 33% reduction of LVMI (Ang: 6.32 ± 0.39 vs. Ang + SS-31: 4.21 ± 0.17 mg/g, p=0.001, Figure 3A left panel) and better preservation of Ea/Aa (Ang: 0.723 ± 0.15 vs. Ang+SS-31: 1.17 ± 0.11, p=0.04, Figure 3C left panel). These effects were comparable to those of catalase targeted to mitochondria (i-mCAT), in which induction of mitochondrial catalase two weeks before Ang also conferred protection against Ang induced cardiac hypertrophy and diastolic dysfunction (Figure 3A–D, right panels). To investigate whether the protective effect is specific to a mitochondrial targeted antioxidant, we administered the non-targeted antioxidant NAC in drinking water for 4 weeks simultaneously with the Ang pump. This treatment has been shown to ameliorate cardiomyopathy after acute aortic banding in mice (18), however, it did not show any beneficial effect on Ang-induced cardiomyopathy (Figure 3A–C).
Figure 3. SS-31 ameliorates Ang-induced cardiac hypertrophy and diastolic dysfunction.
(A) Ang for 4 weeks substantially increased LV mass index in control mice. Simultaneous administration of SS-31 significantly attenuated this increase in LVMI (left panel). This was similar extent to that observed in mice with inducible overexpression of mitochondrial catalase (i-mCAT, right panel). (B) Fractional shortening (FS,%) was not significantly changed after 4 weeks of Ang in the presence or absence of mitochondrial antioxidants. (C) Diastolic function measured by tissue Doppler imaging of Ea/Aa was significantly reduced after Ang, but was significantly ameliorated by SS-31 or genetic overexpression of mCAT. Administration of NAC for 4 weeks did not confer any significant protection for Ang-induced hypertrophy and diastolic dysfunction (light blue bar on left panel, A–C). n=6–7
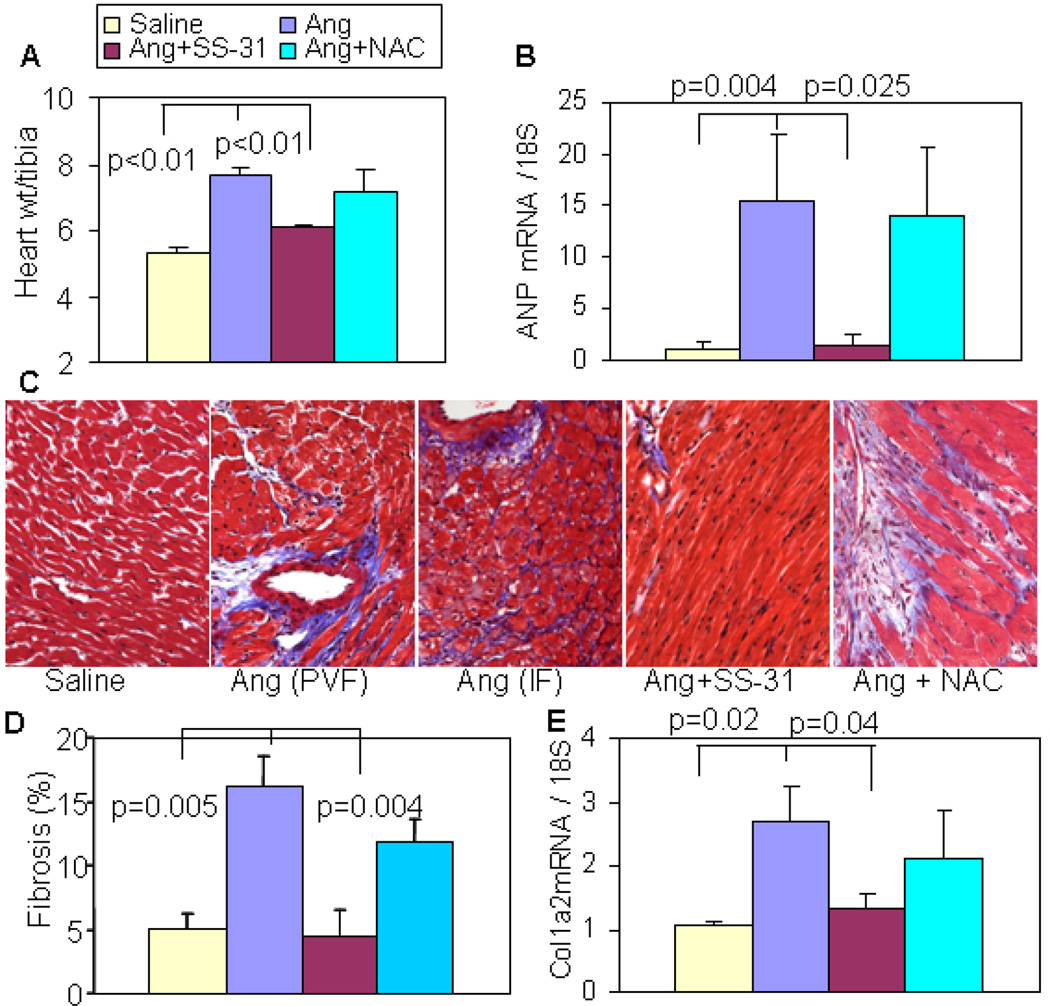
Ang increased heart weights by 45% above those of saline treated controls (Figure 4A; 5.3 ± 0.18 in saline vs. 7.69 ± 0.20 in Ang, p<0.001) and SS-31 protected heart weights (6.05 ± 0.14 mg/mm, p<0.01 vs. Ang alone), while oral NAC did not show any significant protection of heart weights (7.13 ± 0.7 in Ang+ NAC vs. 7.69 ± 0.20 in Ang, p=0.47). The cardiac hypertrophy phenotype was confirmed by qPCR for atrial natriuretic peptide (ANP), a fetal gene reactivated during hypertrophy. Ang induced a ~15 fold increased ANP gene expression, which was almost completely protected by SS-31, with no protection observed in the NAC-treated hearts (Figure 4B). Masson trichrome staining demonstrated perivascular fibrosis and interstitial fibrosis after Ang (Figure 4C). Ang significantly increased ventricular fibrosis, which was attenuated by SS-31 (Figure 4D). This was confirmed by qPCR of the procollagen 1a2 gene, the main component of cardiac fibrosis. As shown in Figure 4E, Ang-induced Coll1a2 gene expression was almost fully attenuated by SS-31. Oral NAC showed a non-significant reduction of ventricular fibrosis and Coll1a2 gene expression (Figure 4D–E).
Figure 4. Ang-induced cardiac hypertrophy and fibrosis were attenuated by SS-31 but not oral NAC.
(A) Ang significantly increased heart weight (normalized to tibia length), and this was significantly attenuated by SS-31 but not by NAC. (B) qPCR showed a dramatic increase in ANP gene expression, which was significantly prevented by SS-31, but not by NAC. (C) Representative histopathology showing that Ang induced substantial perivascular fibrosis (PVF) and interstitial fibrosis (IF), which was protected by SS-31 but not NAC. (D) Analysis of blue trichrome staining demonstrated a significant increase in ventricular fibrosis after Ang, and this was substantially attenuated by SS-31 but not NAC. (E). qPCR showed upregulation of pro-collagen1a2 mRNA after Ang, which was significantly reduced in SS-31 hearts but not in NAC hearts. n=4–7.
Ang induced mitochondrial protein oxidative damage and signaling for mitochondrial biogenesis
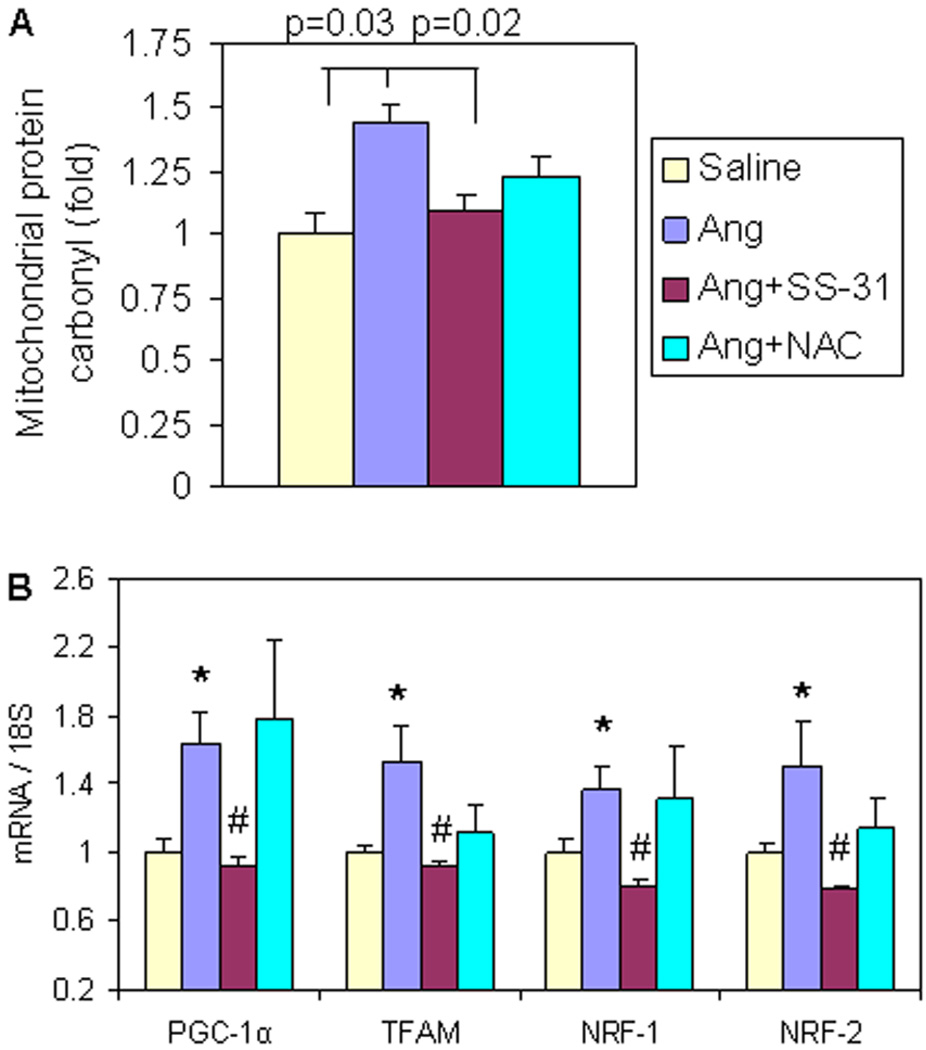
Consistent with studies of ROS in cardiomyocytes (Figure 1), chronic administration of Ang for 4 weeks significantly increased ventricular mitochondrial protein carbonyl content, an indicator of protein oxidative damage (p=0.03, Figure 5A). This was significantly reduced by SS-31 (p=0.02, Figure 5A), while non-targeted NAC did not show any significant reduction of mitochondrial protein oxidative damage (1.27 ± 0.1 in Ang + NAC vs. 1.44 ± 0.1 in Ang, p=0.19) (Figure 5A)
Figure 5. Mitochondrial protein carbonyl and signaling for mitochondrial biogenesis increased after 4 weeks of Ang treatment, which was prevented by SS-31.
(A) Ang significantly increased cardiac mitochondrial protein carbonyl content, and was significantly ameliorated by SS-31, but not by NAC. (B) qPCR revealed significant upregulation of genes in mitochondrial biogenesis, all of which were attenuated by SS-31. NAC treatment did not significantly change any genes in mitochondrial biogenesis signaling. *p–0.05 compared with saline group, #p–0.05 compared with Ang treated group, n=4–7.
Peroxisome proliferator-activated receptor gamma co-activator (PGC-1α) is known as a master regulator of mitochondrial biogenesis, regulating nuclear respiratory factors (NRFs) and mitochondrial transcription factor A (TFAM), which transcribe nuclear DNA and mitochondrial DNA encoded mitochondrial proteins, respectively (19). Ang induced the expression of PGC-1α and its downstream target genes, including TFAM, NRF-1 and NRF-2 (Figure 5B). SS-31 fully prevented this upregulation of PGC-1α and target genes (p<0.05 for all, Figure 5B), while NAC did not show significant attenuation of any of these transcription factors.
SS-31 attenuates Ang induced upregulation of NOX4 and reduces activation of p38 MAPK and apoptosis
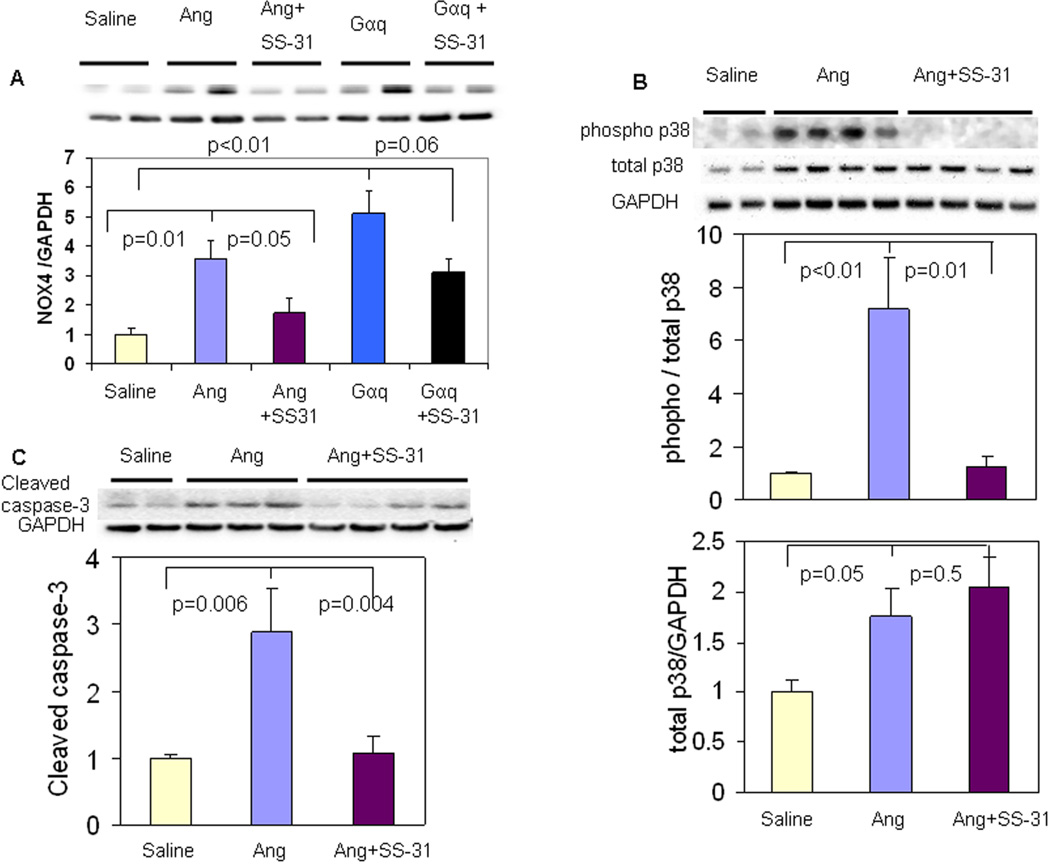
Consistent with a recent report by Ago, et al (6), cardiac NOX4 protein expression was significantly increased in Ang-treated and Gαq overexpressing hearts (p=0.01, Figure 6A). SS-31 significantly reduced NOX4 upregulation in Ang-treated hearts (p=0.05) and trended toward reduction of NOX4 upregulation in Gαq hearts (p=0.06, Figure 6A), while NOX4 upregulation was not attenuated by NAC (p=0.19, SFig. 2A).
Figure 6. SS-31 reduces Ang-induced upregulation of NOX4, activation of p38 MAPK and apoptosis.
(A) NADPH oxidase-4 (NOX4) protein was significantly increased after 4 weeks of Ang or by Gαq overexpression, and this was significantly attenuated by SS-31. (B) Ang for 4 weeks substantially induced apoptosis, as shown by increases cleaved caspase 3 message, and this was significantly attenuated by SS-31. (C) Phosphorylation of p38 MAP kinase was significantly increased after Ang, but was substantially lower in SS-31 treated hearts (upper panel). Protein levels of p38 MAP kinase also increased after Ang, n=3–6.
Excessive mitochondrial ROS is well known to induce apoptosis. Activated (cleaved) caspase 3, a marker of apoptosis, was increased in Ang-treated ventricular tissue (p=0.006), and this was almost completely attenuated by SS-31 (p=0.004, Figure 6C). In contrast, NAC treatment did not reduce Ang-induced cleaved caspase-3 (SFig.2B). Ang treatment increased phosphorylation of p38 mitogen-activated protein kinase (MAPK), and this was significantly attenuated by both SS-31 and NAC (Figure 6B and SFig 2C), suggesting that p38 phosphorylation depends on total ROS, regardless of the location in mitochondria or cytosol.
SS-31 partially rescues Gαq overexpression-induced heart failure
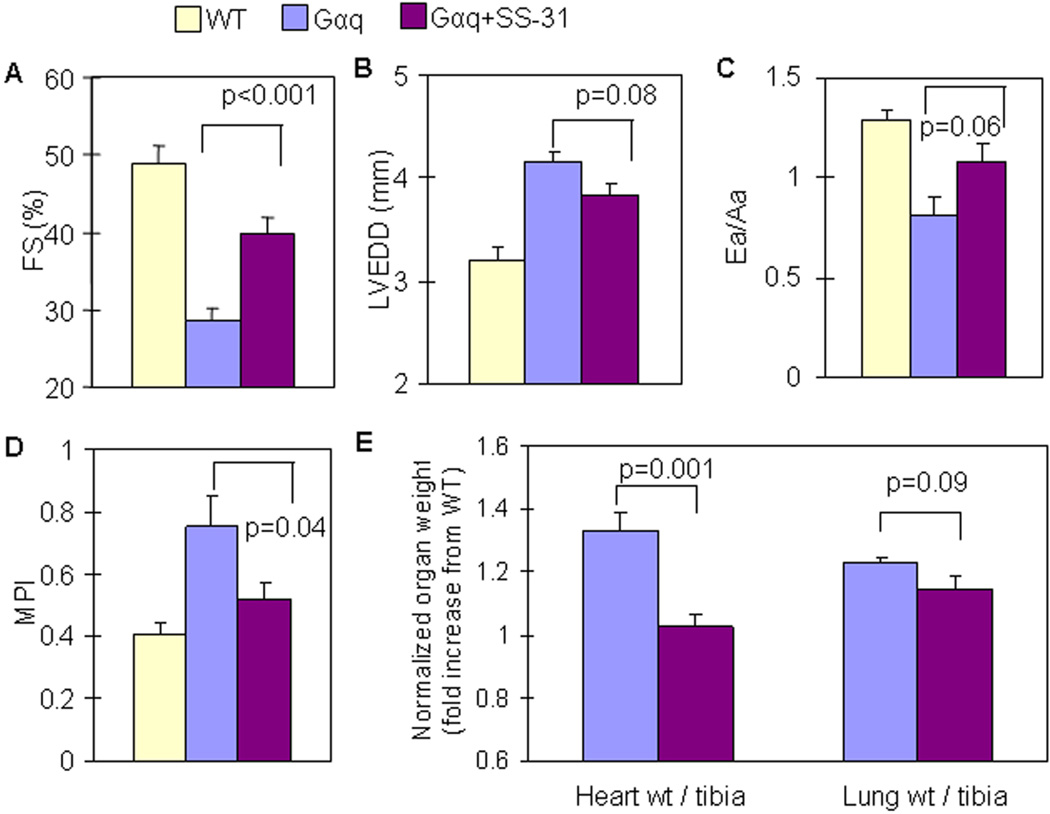
To extend our observations to a model of chronic catecholamine/Ang stimulation, we applied the genetic mouse model of cardiac-specific overexpression of Gαq (a coupling protein to catecholamine and Ang receptors), which causes heart failure in mice by 14–16 weeks of age, despite the absence of increased blood pressure (17). We confirmed that Gαq mice had impaired systolic function at 16 weeks old, as shown by a substantial decline in FS (Figure 7A), with LV chamber enlargement (Figure 7B), diastolic dysfunction, indicated by Ea/Aa < 1 (Figure 7C), and worsening of the myocardial performance index (MPI, Figure 7D). SS-31 administered from 12 to 16 weeks of age significantly reduced cardiac enlargement (p=0.001, Figure 7E), ameliorated systolic function (p<0.001 vs. untreated Gαq, Figure 7A) and improved myocardial performance (p=0.04, Figure 7D). There were trends toward amelioration of LV chamber enlargement (p=0.08, Figure 7B) and preservation of Ea/Aa (p=0.06, Figure 7C) in SS-31 treated Gαq mice. There was trend toward partial attenuation of increased lung weights by SS-31 (p=0.09, Figure 7E). Ventricular fibrosis increased by approximately 2 fold in Gαq mice, which was not changed in SS-31 treated mice (SFig. 3A), and this was confirmed by procollagen 1a2 qPCR (SFig.3B). Mitochondrial protein oxidative damage was evident in Gαq hearts (p=0.01, SFig.3C), and SS-31 treated mice displayed significant reduction of cardiac mitochondrial protein carbonyls (p=0.05, SFig.3C). There was no evidence of increased cleaved-caspase 3 in Gαq mouse hearts (data not shown).
Figure 7. SS-31 ameliorated cardiac hypertrophy and failure in Gαq overexpressing mice.
Echocardiography of Gαq mice 16 weeks of age. (A) SS-31 (3mg/kg/d) for 4 weeks (from age 12–16 weeks) significantly ameliorated the decline in systolic function, as indicated by FS in Gαq mice. (B,C) Chamber enlargement and diastolic dysfunction in Gαq mice were slightly attenuated by SS-31 with a statistical trend, p=0.08 and 0.06, respectively. (D) Worsening of myocardial performance index (MPI) in Gαq mice was significantly ameliorated by SS-31. (E) An increase in normalized heart weight in Gαq mice was substantially protected by SS-31, while there was a trend towards protection from increased normalized lung weight by SS-31 (p=0.09), n=3–6.
Discussion
We found that mitochondrial ROS plays an essential role in hypertensive cardiomyopathies downstream of Ang, and protection from mitochondrial ROS by a mitochondrial-targeted antioxidant confers resistance to cardiomyopathy, while nontargeted antioxidant NAC did not. Our study provides direct evidence that Ang induces mitochondrial ROS, which is prevented by SS-31, but not by NAC (Figure 1). We suggest that ROS amplified in mitochondria subsequently activates downstream ROS-sensitive signaling pathways implicated in pathological cardiac hypertrophy, including p38 MAPK (Figure 6C), apoptosis signal-regulating kinase (20), other MAPKs (21), NF-κB (22) and calcineurin-NFAT (23).
Exposure to Ang for 4 weeks increased cardiac mitochondrial protein oxidative damage and induced the signaling for mitochondrial biogenesis (Figure 5), consistent with the previous report that H2O2 directly activates transcription of PGC-1α, the master regulator of mitochondrial biogenesis (24). SS-31 significantly attenuated Ang-induced mitochondrial oxidative stress, reduced upregulation of mitochondrial biogenesis, and reduced ROS mediated p38 MAPK signaling (Figure 8). As inhibition of p38 has been shown to improve cardiac remodeling and inflammation and preserve cardiac function in heart failure (25,26), the attenuation of Ang-induced p38 phosphorylation by SS-31 may be one of the downstream mechanisms underlying protection by SS-31. Furthermore, mitochondrial ROS can lead to apoptosis and our results confirm that SS-31 prevented caspase-3 activation (Figure 6), concomitant with amelioration of Ang induced cardiomyopathy (Figures 3 and 4).
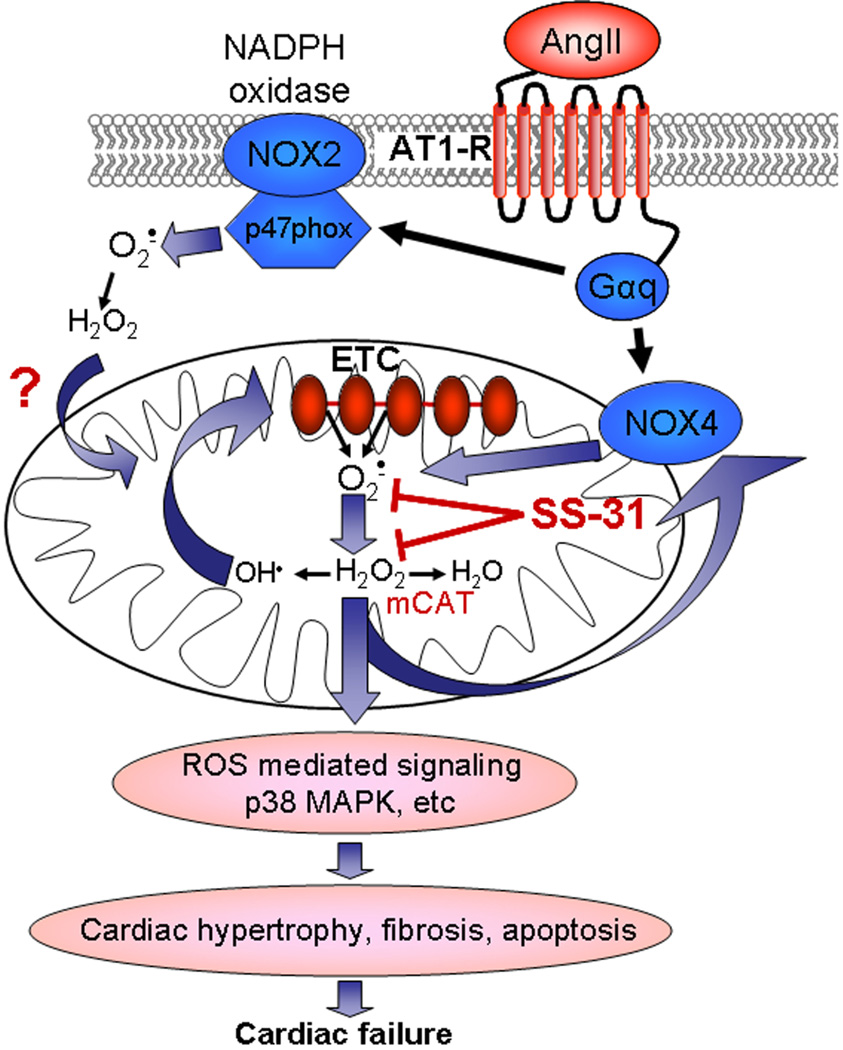
Figure 8. Diagrammatic illustration of the proposed mechanisms of SS-31 protection in Ang- and Gαq-induced cardiomyopathy.
Ang and Gαq activate NOX isoforms that may directly (NOX4) or indirectly (NOX2) elevate mitochondrial ROS. Amplification by ROS induced ROS mechanisms may facilitate ROS-mediated activation of signaling in hypertrophy and failure.
It has been proposed that Ang mediates its effects via Gαq. However, Gαq transgenic overexpression involves a persistent lifelong stimulation of Gαq, starting from developmental stage, and is likely a stronger stimulus than obtained with Ang. Thus, transgenic overexpression of Gαq displayed a much stronger phenotype than 4-week Ang, including systolic heart failure in mice by 14–16 weeks of age (17). SS-31 for 4 weeks partially rescued the heart failure phenotype in mice overexpressing Gαq, including a significant amelioration of systolic dysfunction, cardiac hypertrophy, and myocardial performance (Figure 7A,D,E).
Central to the scheme hypothesized in Figure 8 is the amplification of mitochondrial ROS, which can occur by multiple mechanisms. Ang is a key mediator of hypertension that binds to ATR1, a Gαq coupled-receptor, then activates NADPH oxidase through a PKC-dependent mechanism. While the NOX2 isoform of NADPH oxidase has been shown to mediate Ang effects, the NOX4 isoform has recently been reported to increase in response to various hypertrophic stimuli (6), is localized in mitochondria (7) and activation of this isoform increases mitochondrial ROS (6). Although our hypothesis indicates that ROS produced directly from NOX4 and/or indirectly by NOX2 isoform is amplified within mitochondria, the relative contribution of these isoforms in elevating ROS in mitochondria is not yet clear, and it is possible that NOX independent mechanism(s) may also contribute to the Ang and Gαq phenotypes.
As escape of electrons from the mitochondrial electron transport complexes is a principal source of cellular ROS, oxidative damage to these complexes has been proposed to lead to vicious cycle (27). Another potential mechanisms of Ang-induced mitochondrial ROS could be the previously reported ROS-induced ROS release from mitochondria, which might be mediated by activation of the inner mitochondrial anion channel (inhibited by 4-chlorodiazepam)(28) or the mitochondrial permeability transition pore (inhibited by cyclosporine)(29) or mitochondrial KATP channels (activated by diazoxide)(4). As confirmed by observations that Ang-induced mt-ROS was reduced by simultaneous treatment with diazoxide, 4-chlorodiazepam or cyclosporine A (Figure 1), the involvement of ROS-induced ROS release from mitochondria should be considered as a primary mechanism of Ang-induced ROS signaling (30). This places mitochondria in a central position for signal amplification, and conversely, for therapeutic targeting. While most antihypertensive medications act upstream at the receptor level (β-blockers, calcium channel blockers, angiotensin receptor blockers) or even at remote sites of action (diuretics, ACE inhibitor), antioxidants provide an alternate intervention strategy in cardiac hypertrophy and failure. Several antioxidant studies have demonstrated some protective effect in cardiovascular diseases. For instance, Euk-8, a superoxide dismutase and catalase mimetic, ameliorated heart failure in ROS-sensitive mouse models subjected to pressure overload (31). Zhou et al. reported that overexpression of metallothionein suppressed oxidative and nitrosative stress, apoptosis and pathological remodeling in response to a short-term subpressor dose of Ang(32). Our study, however, points to the rationale of targeting antioxidants to mitochondria. The mitochondrial antioxidant MitoQ has been shown to reduce BP in the spontaneous hypertensive rat, concomitant with improvement of endothelial function and reduction of heart weight; however, the mechanism of cardioprotection was unclear (33). Our study found no effect of SS-31 on BP, but showed evidence of direct cardioprotective mechanisms involving reduction of cardiac mitochondrial damage, amelioration of apoptosis and fibrosis and attenuation of p38 MAP kinase activation.
Hypertension is a highly prevalent disease that imposes a major risk for the development of atherosclerosis, cardiomyopathy, stroke, sudden cardiac death and heart failure. Hypertension induced heart failure may be manifested as systolic heart failure or heart failure with preserved ejection fraction (HFpEF); the latter accounts for nearly half of the patients with heart failure and the prognosis of HFpEF is only marginally better than that of systolic heart failure (34). Although antihypertensive treatment reduces mortality and improves quality of life in patients with established systolic heart failure(35), there is no convincing evidence for any effective treatment of HFpEF. These underscore the urgent need to develop new prevention and treatment strategies for hypertensive cardiovascular diseases. Although clinical trials applying antioxidants to disease prevention in general and cardioprotection in particular (36) have been disappointing, these studies have generally utilized non-targeted cellular antioxidants. Our study demonstrates that a mitochondrial-targeted antioxidant drug is beneficial in preventing hypertension induced target organ damage, and provides a strong rationale for investigating its clinical application for the treatment or prevention of hypertensive cardiovascular diseases.
Supplementary Material
Acknowledgments
Grant support: National Institute of Health Grants R01 HL101186, P30 AG013280 and P01 AG001751.
Financial Disclosure:
H.H. Szeto is the inventor of SS-31 and the Cornell Research Foundation (CRF) holds several patents covering the SS peptides and a patent application has been filed for the findings described in this article, with H.H. Szeto and P.S. Rabinovitch as inventors. CRF, has licensed the SS peptide technology for further research and development to a commercial enterprise in which CRF and H.H. Szeto have financial interests.
Abbreviations List
- Ang
Angiotensin II
- ATR1
Angiotensin II receptor-1
- BP
Blood pressure
- HFpEF
Heart failure with preserved ejection fraction
- LVH
Left ventricular hypertrophy
- MAPK
Mitogen-activated protein kinase
- mCAT
Catalase targeted to mitochondria
- MPI
Myocardial performance index
- NAC
N-acetyl cysteine
- ROS
Reactive oxygen species
- qPCR
Quantitative PCR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lawes CM, Vander Hoorn S, Rodgers A. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 2.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? Jama. 2004;292:2396–2398. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 3.Mollnau H, Wendt M, Szocs K, et al. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 4.Kimura S, Zhang GX, Nishiyama A, et al. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 5.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin IImediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 6.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Block K, Gorin Y, Abboud HE. Subcellular localization of Nox4 and regulation in diabetes. Proc Natl Acad Sci U S A. 2009;106:14385–14390. doi: 10.1073/pnas.0906805106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schriner SE, Linford NJ, Martin GM, et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 9.Dai DF, Santana LF, Vermulst M, et al. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szeto HH. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 11.Zhao K, Zhao GM, Wu D, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 12.Whiteman M, Spencer JP, Szeto HH, Armstrong JS. Do mitochondriotropic antioxidants prevent chlorinative stress-induced mitochondrial and cellular injury? Antioxid Redox Signal. 2008;10:641–650. doi: 10.1089/ars.2007.1879. [DOI] [PubMed] [Google Scholar]
- 13.Zhao K, Luo G, Giannelli S, Szeto HH. Mitochondria-targeted peptide prevents mitochondrial depolarization and apoptosis induced by tert-butyl hydroperoxide in neuronal cell lines. Biochem Pharmacol. 2005;70:1796–1806. doi: 10.1016/j.bcp.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Cho S, Szeto HH, Kim E, Kim H, Tolhurst AT, Pinto JT. A novel cell-permeable antioxidant peptide, SS31, attenuates ischemic brain injury by down-regulating CD36. J Biol Chem. 2007;282:4634–4642. doi: 10.1074/jbc.M609388200. [DOI] [PubMed] [Google Scholar]
- 15.Yang L, Zhao K, Calingasan NY, Luo G, Szeto HH, Beal MF. Mitochondria targeted peptides protect against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity. Antioxid Redox Signal. 2009;11:2095–2104. doi: 10.1089/ars.2009.2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson EJ, Lustig ME, Boyle KE, et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009 doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Angelo DD, Sakata Y, Lorenz JN, et al. Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Byrne JA, Grieve DJ, Bendall JK, et al. Contrasting roles of NADPH oxidase isoforms in pressure-overload versus angiotensin II-induced cardiac hypertrophy. Circ Res. 2003;93:802–805. doi: 10.1161/01.RES.0000099504.30207.F5. [DOI] [PubMed] [Google Scholar]
- 19.Wu Z, Puigserver P, Andersson U, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 20.Izumiya Y, Kim S, Izumi Y, et al. Apoptosis signal-regulating kinase 1 plays a pivotal role in angiotensin II-induced cardiac hypertrophy and remodeling. Circ Res. 2003;93:874–883. doi: 10.1161/01.RES.0000100665.67510.F5. [DOI] [PubMed] [Google Scholar]
- 21.Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of "stress-regulated" mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 22.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 23.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St-Pierre J, Drori S, Uldry M, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 25.Li M, Georgakopoulos D, Lu G, et al. p38 MAP kinase mediates inflammatory cytokine induction in cardiomyocytes and extracellular matrix remodeling in heart. Circulation. 2005;111:2494–2502. doi: 10.1161/01.CIR.0000165117.71483.0C. [DOI] [PubMed] [Google Scholar]
- 26.See F, Thomas W, Way K, et al. p38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 27.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Aon MA, Cortassa S, Akar FG, Brown DA, Zhou L, O'Rourke B. From mitochondrial dynamics to arrhythmias. Int J Biochem Cell Biol. 2009;41:1940–1948. doi: 10.1016/j.biocel.2009.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zorov DB, Filburn CR, Klotz LO, Zweier JL, Sollott SJ. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J Exp Med. 2000;192:1001–114. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown DA, Aon MA, Akar FG, Liu T, Sorarrain N, O'Rourke B. Effects of 4'-chlorodiazepam on cellular excitation-contraction coupling and ischaemia-reperfusion injury in rabbit heart. Cardiovasc Res. 2008;79:141–149. doi: 10.1093/cvr/cvn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Empel VP, Bertrand AT, van Oort RJ, et al. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:824–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 32.Zhou G, Li X, Hein DW, et al. Metallothionein suppresses angiotensin II-induced nicotinamide adenine dinucleotide phosphate oxidase activation, nitrosative stress, apoptosis, and pathological remodeling in the diabetic heart. J Am Coll Cardiol. 2008;52:655–666. doi: 10.1016/j.jacc.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Graham D, Huynh NN, Hamilton CA, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 34.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 35.Dzau VJ, Antman EM, Black HR, et al. The cardiovascular disease continuum validated: clinical evidence of improved patient outcomes: part I: Pathophysiology and clinical trial evidence (risk factors through stable coronary artery disease) Circulation. 2006;114:2850–2870. doi: 10.1161/CIRCULATIONAHA.106.655688. [DOI] [PubMed] [Google Scholar]
- 36.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.