Abstract
Alteration of the biological activity among neuronal components of the Mesocorticolimbic (MCL) system has been implicated in the pathophysiology of drug abuse. Changes in the electrophysiological properties of neurons involved in the reward circuit seem to be of utmost importance in addiction. The Hyperpolarization-Activated Cyclic-Nucleotide Current, Ih, is a prominent mixed cation current present in neurons. The biophysical properties of the Ih and its potential modulatory role in cell excitability depend on the expression profile of the Hyperpolarization-activated cyclic nucleotide gated channel (HCN) subunits. We investigated whether cocaine-induced behavioral sensitization, an animal model of drug addiction, elicits region-specific changes in the expression of the HCN2 channel’s subunit in the MCL system. Tissue samples from the ventral tegmental area, prefrontal cortex, nucleus accumbens and hippocampus were analyzed using Western Blot. Our findings demonstrate that cocaine treatment induced a significant increase in the expression profile of the HCN2 subunit in both, its glycosylated and non-glycosylated protein isoforms, in all areas tested. The increase in the glycosylated isoform was only observed in the ventral tegmental area. Together, these data suggest that the observed changes in MCL excitability during cocaine addiction might be associated to alterations in the subunit composition of their HCN channels.
Keywords: addiction, cocaine sensitization, ventral tegmental area, accumbens, Ih current, HCN channels
Introduction
Neuroadaptations within the mesocorticolimbic (MCL) system are hypothesized to trigger drug addiction (Kauer, 2003; Chen et al., 2010). Cocaine blocks cell membrane transporters that usually remove monoamines from the synaptic cleft once these neurotransmitters are released (Reith et al., 1997). This blockade produces a fast elevation of dopamine (DA), norepinephrine (NE) and serotonin (5-HT) levels that, with chronic administration, results in an enduring psychomotor stimulation (Sofuoglu and Sewell, 2009; Filip et al., 2010; Schmitt and Reith, 2010). The enhanced state of excitability produced by an extended cocaine exposure significantly depends on the modulation of voltage-dependent conductances that affect neuronal firing (Ingram et al., 2002). Among these conductances is the hyperpolarization-activated cation current, Ih (Ludwig et al., 1998; Neuhoff et al., 2002).
Ih is known to contribute to neural processes such as resting membrane potential (Lamas, 1998; Doan and Kunze, 1999; Nolan et al., 2007), firing frequency modulation (Neuhoff et al., 2002; Funahashi et al., 2003; Okamoto et al., 2006), synaptic transmission (Beaumont and Zucker, 2000; Chevaleyre and Castillo, 2002; Genlain et al., 2007) and dendritic integration of synaptic inputs (Magee, 1999; Williams and Stuart, 2000; Lorincz et al., 2002). The ion channels underlying Ih current are tetramers made of four distinct channel subunits: HCN1-4. Each monomer consists of six transmembrane domains with a positively charged voltage sensor and a cytoplasmic cyclic nucleotide binding domain that allows a faster channel gating without protein phosphorylation (DiFrancesco and Tortora, 1991; Ludwig et al., 1998; Santoro et al., 2000). This molecular architecture has been shown to strongly determine Ih kinetics, voltage dependency and cyclic nucleotide sensitivity (Wainger et al., 2001; Berrera et al., 2006; Kusch et al., 2012). Therefore, the biophysical properties of the HCN channel are highly dependent on its subunits’ composition.
HCN channels are widely expressed in the central nervous system (CNS), including those areas critical to drug addiction: ventral tegmental area (VTA), nucleus accumbens (NAc), prefrontal cortex (PFC), and hippocampus (HIP) (Santoro et al., 2000; Notomi and Shigemoto, 2004). Despite this fact, the role of HCN channels or Ih in cocaine addiction is basically unknown. A recent publication from our laboratory demonstrates that cocaine sensitization inhibits Ih amplitude and also reduces cell capacitance in VTA DA cells (Arencibia-Albite et al., 2012). These findings suggest that cocaine could affect the physiological and molecular functions of Ih. Due to the fact that the electrophysiological properties of the HCN channels rely on the composition of their subunits, we wanted to know whether the subunit’s expression profile could be altered after chronic cocaine administration. There is a consensus that the HCN2 subunit is the most abundant within these four principal structures of the MCL system (Monteggia et al., 2000; Notomi and Shigemoto, 2004). Thus, the expression of HCN2 subunit in these four areas was analyzed. The current evidence for the presence of HCN channels have come mainly from electrophysiological, immunohistochemical and mRNA studies (Monteggia et al., 2000; Notomi and Shigemoto, 2004). Using Western Blot analysis we tested the specific hypothesis that the HCN2 subunit expression in these regions was altered after cocaine administration. Our work reports that after seven days of repetitive intermittent exposure to cocaine, HCN2 subunit expression is increased in all four major areas of the MCL. These results represent an important molecular discovery in the understanding of neuroadaptations underlying addiction.
Experimental Procedures
Animal Housing
Thirty two male Sprague-Dawley rats (6 weeks, 250–300 g) were used as the experimental subjects (Taconic Farms, Germantown, NY) and housed two per cage. Animals were maintained at constant temperature and humidity with water and food provided ad libitum in a 12:12 h light/dark cycle. The rats were acclimatized in the animal facility for one week and randomly selected for the studies. . All procedures were in accordance with the US Public Health Service publication “Guide for the Care and Use of Laboratory Animals” and were approved by the Animal Care and Use Committee at the University of Puerto Rico Medical Sciences Campus.
Behavioral Sensitization Protocol
Experiments were performed as previously described (Arencibia-Albite et al., 2012). Briefly, animals were randomly divided into two groups: Saline or Cocaine treatment groups. Rats were moved to an isolated acoustic chamber (Whisper Room Inc. Morristown, TN) and placed in clear plastic cages (42 cm × 42 cm × 30 cm) with evenly spaced racks containing infrared beams (2 cm from its floor) to detect locomotion activity (Accuscan Instruments, Columbus, OH). Beams are connected to a detector that identifies stereotypic locomotion as the repeated interruption of the same beam whereas horizontal ambulatory activity was counted as the sequential breaking of two or more different beams. The collected data was stored in a Dell® Personal Computer using Versamax® Software. Two days before the beginning of the experiment, each animal was habituated during 1 hour to the infrared photocell box. On experimental day 1, animals were habituated for 15 minutes before the initiation of the experiment. After that, animals were treated with either 15 mg/kg i.p., cocaine (Sigma, St. Louis, MO) or isovolumetric saline injections. Immediately after the injections, one hour of locomotion activity was recorded, once per day for 7 days.
Tissue Extraction
Animals were deeply anesthetized with an intraperitoneal (i.p.) injection of chloral hydrate (400mg/kg) and immediately decapitated, 24 hours after the last treatment injection. Brains were quickly removed from the skull and placed in ice-cold PBS 1× (0.01 M phosphate buffered solution, pH 7.4, Sigma-Aldrich, St. Louis, MO). Coronal sections of 200-400 μm at the level of the VTA, PFC, NAc and HIP were made following Paxinos and Watson Rat Brain Atlas (2009) as dissection guide, using a Leica® Vibratome VT1000S (Leica, Germany). Brain slices were then mounted onto a glass surface and immediately frozen with dry ice. Micropunches of the selected areas were removed using a 0.8 mm to 1.5 mm stainless steel hypodermic tube (Fine Science Tools), transferred to ice cold microtubes and stored at −20°C.
Western Blotting
Dissected tissue from control and treated animals was homogenized in ice cold Tris lysis buffer (20 mM Tris, 150 mM NaCl, 5 mM NaF, 1 mM EDTA, 1 mM EGTA, pH 8) containing 2 μg/mL antipain, 10 μg/mL aprotinin, 5 mM benzamidine, 1 mM DTT, 10 μg/mL leupeptin, 1 mM sodium orthovanadate, 1 mM PMSF, and 10 μg/mL trypsin inhibitors. Following a centrifugation at 20,000 g for 30 min, the protein concentration of the supernatant fraction was determined using Bio-Rad’s DC Protein assay according to the manufacturer’s instructions (Bio-Rad Laboratories, Hercules, CA). Extracted proteins were resolved in an 8% SDS-PAGE (ran for 1.5 hours at 200 V, 300 mA and 10 W at room temperature). Proteins were then transferred to a nitrocellulose membrane using Trans-Blot wet transfer system (Bio-Rad Laboratories, Hercules, CA) with transfer buffer (25 mM Tris, 192 mM glycine, 20% MeOH, pH 8.3) for 18 hours at 35 V constant voltage. To validate transfer of proteins and expose molecular weight markers, the nitrocellulose membrane was stained with 0.1% Ponceau S made in 0.1% glacial acetic acid for 10 minutes and then rinsed with PBS 1×, three times for 10 minutes each.
The nitrocellulose membrane was incubated in blocking solution (15% skim milk, 20 mM Tris-HCl, 150 mM NaCl, 0.01% Tween-20, pH 7.5) for 2 hours at room temperature. The membrane was probed overnight at 4°C using anti-mouse HCN2 monoclonal antibody (1:500; NeuroMabs Labs/Antibodies Incorporated, UC Davis, CA) in blocking solution. Next day, the membrane was washed 3 times for 10 min each with PBS-T (PBS 1× with 0.01% Tween 20) and then incubated with HRP-conjugated anti-mouse IgG produced in rabbit (1:5,000; Sigma, St. Louis, MO) in blocking solution for 1 hour at room temperature. The membrane was washed twice with PBS-T and twice with Tris-NaCl (10 mM Tris, 100 mM NaCl, and 0.1% Tween-20, pH 7.5) for 10 minutes each. HRP-signal of bands corresponding to 110kDa (HCN2 glycosylated protein) and 97kDa (HCN2 non-glycosylated protein) were enhanced with Super Signal West Dura extended version (Pierce, Rockford, IL) for 1 min according to manufacturer’s instruction before exposure and development. The levels of HCN2 protein were normalized to GAPDH (37kDa) immunoreactivity (1:5,000; Sigma-Aldrich, St.Louis, MO). The development and densitometry analysis of the membrane was made using the Versadoc™ Imaging System and Quantity One Software® (Bio-Rad Laboratories, Hercules, CA).
Deglycosylation Assay
Deglycosylation was performed as described by the PNGase manufacturer (New England, BioLabs). The protein samples were denatured for 5 minutes in a glycoprotein denaturing buffer containing 50 mM β-mercaptoethanol, 5% SDS and 0.4 M dithiothreitol (DTT). The mixture was then transferred to a solution composed of 0.5 M Sodium Phosphate, pH 7.5 and 10% NP-40. Finally 5U of PNGase F (New England, BioLabs) was added to remove carbohydrates molecules. The reaction was incubated at 37°C for 12 hours and the samples analyzed by SDS-PAGE and Western Blot, as described previously.
Statistical Analysis
All statistical analysis was performed using Prism 5® (GraphPad Software Inc., San Diego, CA). Total ambulatory activities were expressed as photocell counts between groups were analyzed using one way ANOVA followed by Newman-Keuls multiple comparison test. Paired sample t-test was used after ANOVA to compare first and last day of injection in order to establish sensitization (numbers are presented as mean +/− standard error). A significant statistical difference of P<0.05 was considered a successful sensitization protocol (Kalivas and Stewart, 1991). The data was expressed as the mean ± S.E.M. and statistical analyses were performed using One-way ANOVA, followed by Newman-Keuls post-hoc test to compare differences among groups. Statistical analysis of protein densitometry between saline and cocaine individual bands was performed using one way ANOVA followed by Newman-Keuls post-hoc test. Differences were considered to be significant when p< 0.05. Each sample tested correspond to one different animal, thus n=x, where x is the number of animals.
Results
HCN2 subunit expression at the MCL system
HCN2 protein expression was analyzed in PFC, NAc, HIP and VTA, using Western Blot analysis. Samples from these four areas were taken from naïve animals, using the micropunch technique (Kim et al., 1993). Rat’s heart lysates were used as positive control tissue because they express the HCN2 subunit (Xia et al., 2010). As a negative control, we used rat liver lysates, where no HCN2 subunit isoforms had been detected (Arroyo et al., 2006). HCN2 immunoreactive bands in the expected position were detected in all four areas tested (Fig. 1A). Also, there are two bands that are immunoreactive with the anti-HCN2 monoclonal antibody (Fig.1A) and detected with an anti-HCN2 polyclonal antibody from Alomone Labs, Israel (data not shown). The lower molecular weight (MW) band corresponds to 97kDa, while the less abundant higher MW band detected was about 110kDa. The relative expression of the 97kDa protein was the following: HIP>PFC>VTA>NAc. However, the expression level of the 110kDa was highest in the NAc>VTA>PFC and lowest in the HIP. To determine whether this MW difference is due to post transcriptional glycosylation, all samples were treated with N-Glycosidase F (PNGase F), an amidase that hydrolyzes N-linked oligosaccharides from glycoproteins (Tarentino et al., 1985). After incubation with this enzyme, the antibody directed against HCN2 only detected a single strong immunoreactive band at 97kDa, in all areas of the MCL system evaluated (Fig. 2A, 2B, 2C and 2D) confirming the glycosylation state of the subunit.
Figure 1. HCN2 subunit expression in areas of the MCL system from naïve rats.
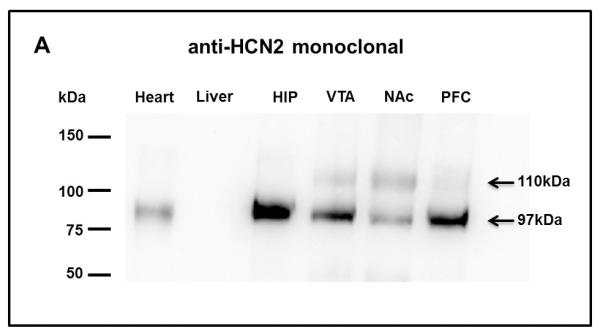
Western Blots show the expression pattern of the HCN2 subunit (97kDa) in all MCL areas, HIP, VTA, NAc, PFC, in naïve animals (n=3). The 97kDa band is in the expected position. There is the presence of a higher molecular weight band (110kDa) in all MCL samples tested. Heart and liver lysates are presented as positive and negative controls, respectively.
Figure 2. HCN2 subunits in MCL structures of naïve rats undergo N-glycosylation.
Treatment with PNGase F (2,500 U) attenuates the high MW band (110kDa) immunoreactive for HCN2 in VTA (A), PFC (B), NAc (C) and HIP (D) tissue samples, demonstrating that this high MW band is a result of N-glycosylation of the HCN2 subunits (n=3). In order to detect the 110kDa band longer time exposures were required for the samples obtained from PFC and HIP. Graphs show a quantitative measure of relative band density expressed as percent change from control. Grey bars show a significant reduction of the glycosylated HCN2 subunit in PNGase treated samples from all areas.
Cocaine Sensitization triggers HCN2 subunit increase in the MCL system
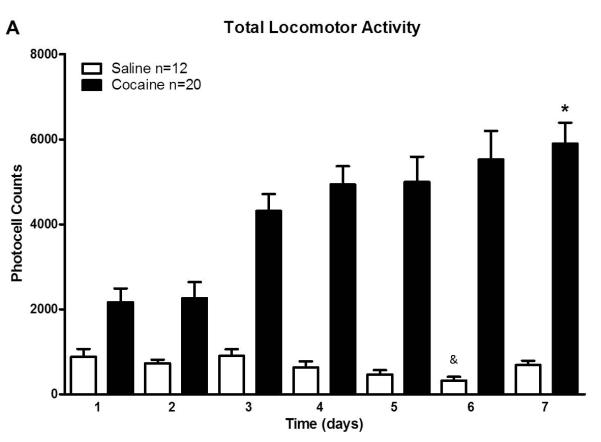
Cocaine sensitization produces neuroadaptations in the MCL. To determine if cocaine produce region specific changes in the expression of HCN2 subunit, first, we subjected animals to a behavioral sensitization protocol. Animals were administered with a single injection of cocaine (15 mg/kg i.p.) during 7 consecutive days. Control animals were treated with isovolumetric saline injections. After injection, total ambulatory locomotor responses were recorded for one hour each day. As expected, cocaine treated animals showed a significant increase in locomotor activity, by day seven of cocaine treatment compared to day one (Fig. 3A, 3B, sample T-test, p<0.05). It is also observed that saline treated animals showed a significant decreased in locomotor activity by day seven of saline treatment compared to day one. This result was expected due to the decrease in novelty and stress after several days of behavioral assessment (Fig. 3B, sample T-test, p<0.05).To investigate whether cocaine sensitization can affect HCN2 protein expression, all animals were sacrificed 24 hours after the last injection. Forty micrograms of protein extract were probed against monoclonal anti-HCN2 in Western Blot analysis after evaluating the linear range of protein detection (data not shown). The HCN2 immunoreactive bands were normalized against the housekeeping protein GADPH (37kDa), used as a loading control (Calvo et al., 2008). VTA samples from cocaine treated animals showed a significant 55% increase in HCN2 expression of the higher MW protein (110kDa) compared to saline control. This increase was not observed in the 97kDa immunoreactive band (n=6, p < 0.05, Fig. 4A, 4B). HCN2 expression was also evaluated in the PFC. In contrast to the previous result, PFC densitometry analysis showed a 35% increase in the 97kDa HCN2 subunit isoforms from cocaine treated rats compared to control, while HCN2
Figure 3. Total locomotor activity for the behavioral sensitization protocol.
Mean total locomotor activity of all experimental subjects. (A) Graph shows a progressive increase in locomotor activity of cocaine treated rats during seven days of cocaine administration. The locomotion of cocaine treated rats on day 7 was significantly higher than on day 1 for both, saline and cocaine injected rats (* p < 0.001, one way ANOVA; F (23) followed by paired-sample T-test, n=32). The time course of locomotor activity (B) demonstrates the amount of photocell beams interrupted during the 60 min recording period. There is an statistically significant difference in locomotion between day 7 of cocaine treated rats vs. day 1, and between day 7 cocaine and day 7 of saline treated rats (* p < 0.05, One way ANOVA; F (28), followed by paired sample T-test, n=32). It can be notice that in day 7, sensitized animals showed more locomotion during the last 15 minutes of the recording session than during the same period of the first day of injection. Also, control animals showed a significant decreased in locomotor activity by days six and seven of saline treatment compared to day one (& p< 0.05, sample T-test).
Figure 4. The HCN2 glycosylated isoform is increased in the VTA after cocaine sensitization.

Rats were treated with cocaine (15 mg/kg) for 7 consecutive days and the levels of HCN2 determined by Western Blot. (A) VTA samples from saline or cocaine treated rats were probed against anti-HCN2 monoclonal antibody. In all Western Blots anti-GAPDH was used as a loading control. (B) Quantitative analysis of the band density (normalized to GAPDH and expressed as percent change) demonstrating a 55% significant increase in the glycosilated-HCN2 subunit (110kDa) in the VTA of cocaine treated rats (*p < 0.05, compared to saline; one way ANOVA; F (41), n=6). Individual comparisons were made with Newman Kuels post-hoc test; numbers on bars signify numbers of animals; graphs represent mean ±SEM.
110kDa band expression remained unaltered (n=6, p < 0.05, Fig. 5A, 5B). In the NAc, there was a 36% increase in the HCN2 97kDa protein in cocaine treated animals compared to control. Also, the 110kDa band density remained unchanged by cocaine administration (n=6, p < 0.05, Fig. 6A, 6B). Similar results were found in the HIP. Samples from the HIP of cocaine treated animals showed a significant increase (22%) in the expression of HCN2 97kDa band density, compared to saline control, but this increase was not observed in the 110kDa immunoreactive band (n=6, p < 0.05, Fig. 7A, 7B). Therefore, the data indicate that HCN2 protein expression (97kDa or 110kDa) in these four areas of the MCL system is increased after chronic cocaine administration.
Figure 5. Non-glycosylated HCN2 isoforms in the Prefrontal Cortex are increased after cocaine sensitization.

(A) PFC samples of saline or cocaine treated rats were probed against anti-HCN2 monoclonal antibody and anti-GAPDH as loading control. (B) Densitometry analysis expressed as percent change of the HCN2 subunit expression shows a 35% up regulation of the HCN2 subunit (97kDa) in the PFC of cocaine treated rats (* p < 0.05, compared to saline using one way ANOVA; F(35), n=6). Individual comparisons were made with Newman Kuels post-hoc test; numbers on bars signify numbers of animals; graphs represent mean ±SEM.
Figure 6. Increase of non-glycosylated HCN2 isoforms in the NAc after cocaine sensitization.

(A) NAc samples from saline or cocaine treated rats were probed against anti-HCN2 monoclonal antibody and anti-GAPDH as loading control. (B) Densitometry analysis graph shows a 36% increment of the HCN2 subunit (97kDa) in the NAc of cocaine treated rats (* p < 0.05, compared to saline using one way ANOVA; F (22), n=6). Comparisons with Newman Kuels post-hoc test; numbers on bars signify the numbers of animals; graphs represent mean ±SEM.
Figure 7. Effect of cocaine sensitization on HCN2 levels in the Hippocampus.

(A) HIP samples from saline or cocaine treated rats were probed against anti-HCN2 monoclonal antibody and anti-GAPDH as loading control. (B) Densitometry analysis expressed as percent change in HCN2 subunit expression in the HIP shows a 22% increase of the HCN2 subunit (97kDa) in the HIP of cocaine treated rats (* p < 0.05, compared to saline using one way ANOVA, F (32), n=6). Comparisons with Newman Kuels post-hoc test; numbers on bars signify the numbers of animals; graphs represent mean ±SEM.
Discussion
In the present study we confirmed the presence of HCN2 channel subunit, at the protein level, in four principal structures of the MCL system: VTA, PFC, NAc and HIP. High relative expression levels of the HCN2 subunit (97kDa) were detected in the HIP, followed by the PFC, VTA and finally NAc, in agreement with a previous mRNA study (Monteggia et al., 2000). We also found that in all areas of both, control and experimental animals, the HCN2 subunit protein could be glycosylated. More importantly, our results demonstrated that the HCN2 subunit expression is increased in all MCL areas after a process of cocaine sensitization. However, the increase in the glycosylated isoform was only observed in the VTA. To our knowledge, this is the first study demonstrating molecular changes in HCN channels in a drug addiction model.
Changes in Ih current properties and HCN channel subunits expression have been reported to occur in several pathologies such as epilepsy, neuropathic pain, inflammation, hypertrophic cardiomyopathy and diabetes (Papp et al., 2010; Takasu et al., 2010; Tu et al., 2010; Emery et al., 2011; Noam et al., 2011; Wei-qing et al., 2011). Such changes are responsible to mediate diverse functional roles of Ih in different areas of the CNS. Studies about the electrophysiological properties of Ih suggest its potential role in the neurological basis underlying addiction disease, especially in areas of the reward system (Okamoto et al., 2006; Hopf et al., 2007; McDaid et al., 2008; Migliore et al., 2008; Chu and Zhen, 2010). Ih has been proposed as an electrophysiological neuronal marker (Neuhoff et al., 2002; Margolis et al., 2006), modulator of pacemaker activity (Mercuri et al., 1995; Seutin et al., 2001; Neuhoff et al., 2002) and spontaneous firing in DA cells of the MCL system (Okamoto et al., 2006; Beckstead and Phillips, 2009), but see (Mercuri et al., 1995).
The VTA is a critical area for the initiation of neural adaptations induced by addictive drugs (Robinson and Berridge, 2003; Chen et al., 2009). Initial studies in addiction dealing with the Ih investigated the effects of ethanol exposure in the VTA. Acute ethanol administration was shown to increase the firing rate of VTA DA neurons (Brodie et al., 1999) while Ih current was enhanced, partially due to cAMP facilitation of voltage gating (Okamoto et al., 2006). On the other hand, a decrease in Ih amplitude in VTA DA cells was observed after one day repeated ethanol administration in vivo (Okamoto et al., 2006). Also, a decrease in the Ih of VTA DA neurons has been reported after a seven days withdrawal from repeated ethanol administration (Hopf et al., 2007). These findings seem to be in conformity with a recent study from our laboratory demonstrating a ~40% decrease in Ih amplitude and ~45% reduction in the number HCN channels in the VTA after cocaine sensitization (Arencibia-Albite et al., 2012).
Our present findings demonstrate a 55% increase in the glycosylated HCN2 subunit (110kDa) in the VTA of cocaine sensitized rats. This outcome results unexpected since an increase in HCN subunits raises the possibility of more channels formation and consequently augmented Ih amplitude. It is important to consider that our study evaluated HCN2 expression in the VTA as a whole while the electrophysiological evidence concentrates only on VTA DA cells. In the VTA area, an increase in HCN2 subunits can be occurring in different cell types, especially in DA neurons, due to the fact that VTA DA cells express a large Ih current (Margolis et al., 2006) and are the most relevant players in the reward system associated with addiction. It is also possible that the increase in HCN2 expression is taking place in GABAergic neurons of the VTA, since a recent work established the presence of Ih in these neurons (Margolis et al., 2012). Also, previous work proposes that HCN2 is expressed in other CNS cells such as oligodendrocytes (Notomi and Shigemoto, 2004) and astrocytes (Yu et al., Society for Neuroscience, 2009), but there is no evidence confirming or denying the presence of HCN2 subunits or Ih in VTA glial cells. Therefore, it is important to further establish the cell type that undergoes such HCN2 increment.
Our previous data (Arencibia-Albite et al., 2012) also established that concurrently with cocaine sensitization there was a significant reduction in cell size given by a decrease in DA cell capacitance. In the present study, there was an increase in the VTA glycosylated HCN2 subunit isoform. N-glycosylation of HCN2 subunit had been described as a trafficking promoter and essential for protein surface expression (Much et al., 2003; Hegle et al., 2010). It is possible that with decreased capacitance, there is less surface area for HCN channels to be expressed in the surface. Although, some evidence indicates that HCN2 surface expression is not dependent on N-glycosylation but it is highly important for the stable formation of heteromeric channels (Much et al., 2003; Zha et al., 2008). In addition, N-glycosylation of a single subunit can rescue the surface expression of a non-glycosylated subunit when both are co-assembled (Much et al., 2003; Zha et al., 2008). This type of formation of heteromeric HCN channels had been found to be altered, for example, after seizure-like events in the hippocampus (Bender and Baram, 2008; Zha et al., 2008). In addition, there is data demonstrating that N-glycosylation is not required for HCN2 function (Hegle et al., 2010). Nevertheless, the increased expression of glycosylated HCN2 channels in the VTA after cocaine sensitization could be promoting the formation of functional channels with a potential to be inserted into the membrane. Although glycosylation promotes trafficking and surface expression, there might be also a cocaine-induced disruption in the membrane expression of HCN2 glycosylated subunits.
HCN2 seems to be the most abundant subunit in the VTA but not the only one expressed in this area. To date, few investigations describe the expression of the HCN2 subunit in the VTA. A study suggests that only the HCN2 subunit is expressed in the VTA (Notomi and Shigemoto, 2004). In contrast, another work demonstrated the presence of all HCN subunits’ mRNAs in the VTA (Monteggia et al., 2000). Similarly to HCN4, and in contrast with HCN1 or HCN3, HCN2 subunit produces a channel with slower activation kinetics and confers higher affinity to second messenger cascades involving cAMP (Wainger et al., 2001; Ulens and Siegelbaum, 2003; Berrera et al., 2006; Surges et al., 2006; Kusch et al., 2012). When co-expressed, HCN2 can form functional heteromeric channels with other HCN subunits that exhibit an Ih current with combined biophysical characteristics (Ulens and Tytgat, 2001; Wainger et al., 2001). Therefore, it is important to investigate whether glycosylation and HCN2 increased expression in the VTA can be affecting its interaction with other HCN’s subunits. Ultimately, higher HCN2 subunit expression can produce an heteromeric channel with slower activation kinetics but better facilitation of voltage gating, changing Ih functional roles in the VTA and contributing to the initiation of cocaine sensitization. Also, it is important to consider that the samples evaluated in this study were total protein extractions representing subunits in the plasma and inside the cell attached to organelles and not necessarily expressed in the membrane (Zabel and Klose, 2009). Therefore, additional measurements will be needed to accurately establish the surface expression of these glycosylated channels. Further experiments need to elucidate whether the increased glycosylated HCN2 subunit expression is a contributor to functional HCN2-containing channel formation.
The increased HCN2 expression was observed in other MCL areas besides the VTA. In the PFC, we found a 35% increase in the expression of non-glycosylated HCN2 (97kDa) protein. The PFC area provides glutamatergic projections to NAc and VTA (Carr and Sesack, 2000; Torregrossa et al., 2008). Stimulation of this area increases DA levels in NAc (Taber and Fibiger, 1995; Karreman and Moghaddam, 1996; You et al., 1998) while it modulates the firing pattern of VTA DA neurons projecting to NAc cells (Gao et al., 2007). A deficiency in inhibitory inputs that control PFC excitatory transmission to the NAc and VTA can facilitate the sensitization process (Steketee, 2005). Thus, the enhanced HCN subunits expression in the PFC should promote excitability in this area, which will facilitate the progression of cocaine sensitization.
Cocaine administration alters dendritic spine morphology, and plasticity (Robinson and Berridge, 2003; Shen et al., 2009). Ih has been identified as a modulator factor in the initiation of dendritic spikes and pyramidal cells’ synaptic integration (Day et al., 2005; Barth et al., 2008). Ih inhibition results in an enhanced temporal summation of synaptic inputs in the cortex (Carr et al., 2007). In addition, HCN2 subunits and alpha-2 adrenoreceptors are co-expressed in dendritic spines of PFC cells. After NE activation of alpha-2 adrenoreceptors, the intracellular cascade inhibits cAMP production, diminishing HCN2 channels’ open probability which results in a better synaptic input integration and network activity during working memory (Wang et al., 2007). Likewise, in VTA neurons, alpha-2 adrenoreceptors activation reduces Ih amplitude (Inyushin et al., 2010). Therefore, enhancement of noradrenergic transmission during the development of cocaine sensitization can be activating intracellular cascades that affect Ih gating in dendritic spines which then alter integration of synaptic inputs. If alpha-2 adrenoreceptors are activated, HCN2 channel gating will be diminished, leading to a decreased shunting of synaptic inputs that result in better integration. This improved integration can result in more excitation of the PFC. More specific studies should be carried out to test this hypothesis during cocaine sensitization.
The NAc is an important area related to the expression of sensitization. Substantial evidence establishes that disruption of neuronal activity in this area support drug addiction (Hyman et al., 2006; Volkow et al., 2011). However, the physiological role of Ih in the NAc is basically unknown. Using intracellular recordings investigators demonstrated the presence of Ih in the NAc (Uchimura et al., 1990). However, it is not clear which type of NAc cells express HCN channels. Our findings demonstrate the presence of HCN2 protein in this area in agreement with mRNA and immunohistochemical analysis demonstrating the expression of HCN subunits (Monteggia et al., 2000; Notomi and Shigemoto, 2004). Moreover, cocaine sensitization increased (36%) the expression profile of HCN2 channel subunits. Although to date there are no studies demonstrating a significant physiological role of Ih in this area, the HCN2 altered expression after cocaine treatment suggest a possible involvement of this subunit in addiction processes.
In the NAc, the medium spiny neurons (MSNs) are the major cell type (Wolf, 2010; Kim et al., 2011). They receive and integrate glutamatergic inputs from cortical and limbic regions influencing goal-directed behaviors (Wolf, 2010). During early withdrawal days (1 to 4) of passive cocaine administration there is a decrease in MSNs intrinsic excitability that persists for several weeks (Ishikawa et al., 2009; Mu et al., 2010). Preliminary data identified the presence of Ih in the NAc (Kim, et al. Society for Neuroscience, 2009). This study revealed that bath applications of the Ih blocker, ZD7288, enhance the excitability of MSNs, increasing input resistance and action potential firing. It has been proposed that in the NAc, MSNs undergo long term depression (LTD) during cocaine administration (Thomas et al., 2000; Kourrich et al., 2007; Mameli et al., 2009). LTD is characterized by an activity-dependent decrease in neuronal synaptic efficiency that last for a considerable time, making NAc MSNs less excitable (Kauer and Malenka, 2007). Therefore, it is possible that the augmented expression in HCN2 found in the NAc can lead to an increase in Ih current that will promote a reduction in MSNs excitability. This reduction in excitability can be an essential part of the LTD that occurs in the NAc during cocaine sensitization. In addition, an up-regulation of the cAMP pathway takes place in the nucleus accumbens after chronic drug exposure (Self and Nestler, 1998; Self et al., 1998). This neuroadaptation can enhance HCN2 mediated effects in the NAc, due to the high cAMP sensitivity of the HCN2 subunit.
The NAc contains two sub-regions, the core and the shell, both with different anatomical connectivity and apparent function (Luscher and Malenka, 2011; Fischer-Smith et al., 2012). Our study used samples from all NAc areas. For that reason, further studies should address HCN channels and Ih distinct functional properties in these two sub-regions during cocaine administration.
The hippocampus, as a limbic structure, participates in learning and memory processes during drug addiction (Chauvet et al., 2011). This area provides glutamatergic projections to other limbic structures, mainly, the NAc (Groenewegen et al., 1987). The HIP plays an important role in context dependent processes, such as drug sensitization (Perez et al., 2010). The initiation of the expression of sensitization can be studied by drug-induced locomotion and stereotype enhancement but it also be demonstrated to the incentive-motivational properties of drugs and the cues paired with their administration (Wolf, 2010). Additionally, the context in which drugs are administered is fundamental for the expression of cocaine sensitization (Robinson and Berridge, 2008) and for humans it seems to be even of higher importance (Leyton, 2007). Studies have shown that separate inactivation of specific anatomical areas within the HIP decreases cocaine and cue-induced reinstatement (Wells et al., 2011; Belujon and Grace, 2011). It has been shown that cocaine administration produces changes in hippocampal synaptic plasticity (Stramiello and Wagner, 2010). Indeed, in the HIP as in other areas of the MCL system, cocaine induces long-term potentiation (LTP). LTP is a persistent increase in synaptic strength known to participate in learning and memory processes (Stramiello and Wagner, 2010)). Ih activation constrains LTP formation in CA1 pyramidal neurons and perforant path (Nolan et al., 2004; Matt et al., 2011). It was found that LTP is enhanced in mice lacking the HCN2 subunit (Matt et al., 2011). In contrast, presynaptic HCN channels are necessary for LTP induction in hippocampal mossy fibers (Chevaleyre and Castillo, 2002). Due to these conflictive findings, it is premature to assess a possible role of Ih current modulation of the hippocampal LTP that occurs during cocaine exposure.
The HIP expresses high levels of HCN1 subunit, although HCN2 is also present (Franz et al., 2000; Notomi and Shigemoto, 2004). The expression of hippocampal heteromeric HCN1/HCN2 complexes is altered after seizure like events (Zha et al., 2008). Our results demonstrate an increase (22%) in the HCN2 subunit in this area after cocaine sensitization. This finding raises the possibility of a co-assembly process between HCN1 and HCN2 channels, with more HCN2 subunit expression. This co-assembly progression can greatly regulate the activation kinetics of the Ih in the HIP, slowing the kinetics and allowing the opportunity for cAMP regulation (Wainger et al., 2001). Therefore, strengthening of hippocampal-limbic circuits’ interactions can facilitate the induction of cocaine sensitization, demonstrating the importance of hippocampal afferents reinforcing drug seeking behaviors.
Altered expression of HCN channels can either increase or decrease excitability, depending on the animal model, cell type and areas expressing them (Bean, 2009; Dyhrfjeld-Johnsen et al., 2009; Noam et al., 2011). Our data demonstrated an increase in HCN2 subunit expression in several structures of the MCL system after cocaine administration. Thus, changes in the expression of HCN subunits could be important neurobiological mediators in the CNS adaptations that occur during drug addiction. Such alterations can be relevant in the development of new strategies for the treatment of addiction disorders.
Acknowledgements
The authors thank Ivelisse Cruz, Rodney M. Rosenbaum and María C. Velásquez, MS for their technical assistance during animal experiments. Also, we want to recognize Melissa Serrano, MS and José Agosto, Ph.D. for their technical support and advice during the molecular experiments. Special thanks to the personnel of the Animal Resources Center at University of Puerto Rico, Medical Science Campus. This work was in partial fulfillment of Bermary Santos-Vera doctoral thesis dissertation and was supported by MBRS-SCORE (GM-08224 and GM 084854), MBRS RISE Program (R25GM061838) and the Associate Deanship of Biomedical Sciences at the University of Puerto Rico, School of Medicine. María E. Vélez-Hernández and Rodney Rosenbaum were supported by MBRS RISE Program. The content of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
References
- 1.Arencibia-Albite F, Vazquez R, Velasquez-Martinez MC, Jimenez-Rivera CA. Cocaine sensitization inhibits the hyperpolarization-activated cation current Ih and reduces cell size in dopamine neurons of the ventral tegmental area. J Neurophysiol. 2012;107:2271–2282. doi: 10.1152/jn.00818.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo A, Kim B, Rasmusson RL, Bett G, Yeh J. Hyperpolarization-activated cation channels are expressed in rat hypothalamic gonadotropin-releasing hormone (GnRH) neurons and immortalized GnRH neurons. J Soc Gynecol Investig. 2006;13:442–450. doi: 10.1016/j.jsgi.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Barth AM, Vizi ES, Zelles T, Lendvai B. Alpha2-adrenergic receptors modify dendritic spike generation via HCN channels in the prefrontal cortex. J Neurophysiol. 2008;99:394–401. doi: 10.1152/jn.00943.2007. [DOI] [PubMed] [Google Scholar]
- 4.Bean BP. Inhibition by an excitatory conductance: a paradox explained. Nat Neurosci. 2009;12:530–532. doi: 10.1038/nn0509-530. [DOI] [PubMed] [Google Scholar]
- 5.Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- 6.Beckstead MJ, Phillips TJ. Mice selectively bred for high- or low-alcohol-induced locomotion exhibit differences in dopamine neuron function. J Pharmacol Exp Ther. 2009;329:342–349. doi: 10.1124/jpet.108.146316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belujon P, Grace AA. Hippocampus, amygdala, and stress: interacting systems that affect susceptibility to addiction. Ann N Y Acad Sci. 2011;1216:114–121. doi: 10.1111/j.1749-6632.2010.05896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bender RA, Baram TZ. Hyperpolarization activated cyclic-nucleotide gated (HCN) channels in developing neuronal networks. Prog Neurobiol. 2008;86:129–140. doi: 10.1016/j.pneurobio.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrera M, Pantano S, Carloni P. cAMP Modulation of the cytoplasmic domain in the HCN2 channel investigated by molecular simulations. Biophys J. 2006;90:3428–3433. doi: 10.1529/biophysj.105.071621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- 11.Calvo AC, Moreno-Igoa M, Manzano R, Ordovas L, Yague G, Olivan S, Munoz MJ, Zaragoza P, Osta R. Determination of protein and RNA expression levels of common housekeeping genes in a mouse model of neurodegeneration. Proteomics. 2008;8:4338–4343. doi: 10.1002/pmic.200701091. [DOI] [PubMed] [Google Scholar]
- 12.Carr DB, Andrews GD, Glen WB, Lavin A. alpha2-Noradrenergic receptors activation enhances excitability and synaptic integration in rat prefrontal cortex pyramidal neurons via inhibition of HCN currents. J Physiol. 2007;584:437–450. doi: 10.1113/jphysiol.2007.141671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chauvet C, Lardeux V, Jaber M, Solinas M. Brain regions associated with the reversal of cocaine conditioned place preference by environmental enrichment. Neuroscience. 2011;184:88–96. doi: 10.1016/j.neuroscience.2011.03.068. [DOI] [PubMed] [Google Scholar]
- 15.Chen BT, Hopf FW, Bonci A. Synaptic plasticity in the mesolimbic system: therapeutic implications for substance abuse. Ann N Y Acad Sci. 2010;1187:129–139. doi: 10.1111/j.1749-6632.2009.05154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JC, Chen PC, Chiang YC. Molecular mechanisms of psychostimulant addiction. Chang Gung Med J. 2009;32:148–154. [PubMed] [Google Scholar]
- 17.Chevaleyre V, Castillo PE. Assessing the role of Ih channels in synaptic transmission and mossy fiber LTP. Proc Natl Acad Sci U S A. 2002;99:9538–9543. doi: 10.1073/pnas.142213199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chu HY, Zhen X. Hyperpolarization-activated, cyclic nucleotide-gated (HCN) channels in the regulation of midbrain dopamine systems. Acta Pharmacol Sin. 2010;31:1036–1043. doi: 10.1038/aps.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Day M, Carr DB, Ulrich S, Ilijic E, Tkatch T, Surmeier DJ. Dendritic excitability of mouse frontal cortex pyramidal neurons is shaped by the interaction among HCN, Kir2, and Kleak channels. J Neurosci. 2005;25:8776–8787. doi: 10.1523/JNEUROSCI.2650-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiFrancesco D, Tortora P. Direct activation of cardiac pacemaker channels by intracellular cyclic AMP. Nature. 1991;351:145–147. doi: 10.1038/351145a0. [DOI] [PubMed] [Google Scholar]
- 21.Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol. 1999;514(Pt 1):125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dyhrfjeld-Johnsen J, Morgan RJ, Soltesz I. Double Trouble? Potential for Hyperexcitability Following Both Channelopathic up- and Downregulation of I(h) in Epilepsy. Front Neurosci. 2009;3:25–33. doi: 10.3389/neuro.01.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery EC, Young GT, Berrocoso EM, Chen L, McNaughton PA. HCN2 ion channels play a central role in inflammatory and neuropathic pain. Science. 2011;333:1462–1466. doi: 10.1126/science.1206243. [DOI] [PubMed] [Google Scholar]
- 24.Filip M, Alenina N, Bader M, Przegalinski E. Behavioral evidence for the significance of serotoninergic (5-HT) receptors in cocaine addiction. Addict Biol. 2010;15:227–249. doi: 10.1111/j.1369-1600.2010.00214.x. [DOI] [PubMed] [Google Scholar]
- 25.Fischer-Smith KD, Houston AC, Rebec GV. Differential effects of cocaine access and withdrawal on glutamate type 1 transporter expression in rat nucleus accumbens core and shell. Neuroscience. 2012;210:333–339. doi: 10.1016/j.neuroscience.2012.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franz O, Liss B, Neu A, Roeper J. Single-cell mRNA expression of HCN1 correlates with a fast gating phenotype of hyperpolarization-activated cyclic nucleotide-gated ion channels (Ih) in central neurons. Eur J Neurosci. 2000;12:2685–2693. doi: 10.1046/j.1460-9568.2000.00151.x. [DOI] [PubMed] [Google Scholar]
- 27.Funahashi M, Mitoh Y, Kohjitani A, Matsuo R. Role of the hyperpolarization-activated cation current (Ih) in pacemaker activity in area postrema neurons of rat brain slices. J Physiol. 2003;552:135–148. doi: 10.1113/jphysiol.2003.047191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao M, Liu CL, Yang S, Jin GZ, Bunney BS, Shi WX. Functional coupling between the prefrontal cortex and dopamine neurons in the ventral tegmental area. J Neurosci. 2007;27:5414–5421. doi: 10.1523/JNEUROSCI.5347-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Genlain M, Godaux E, Ris L. Involvement of hyperpolarization-activated cation channels in synaptic modulation. Neuroreport. 2007;18:1231–1235. doi: 10.1097/WNR.0b013e32821c538f. [DOI] [PubMed] [Google Scholar]
- 30.Groenewegen HJ, Vermeulen-Van der Zee E, te KA, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–120. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- 31.Hegle AP, Nazzari H, Roth A, Angoli D, Accili EA. Evolutionary emergence of N-glycosylation as a variable promoter of HCN channel surface expression. Am J Physiol Cell Physiol. 2010;298:C1066–C1076. doi: 10.1152/ajpcell.00389.2009. [DOI] [PubMed] [Google Scholar]
- 32.Hopf FW, Martin M, Chen BT, Bowers MS, Mohamedi MM, Bonci A. Withdrawal from intermittent ethanol exposure increases probability of burst firing in VTA neurons in vitro. J Neurophysiol. 2007;98:2297–2310. doi: 10.1152/jn.00824.2007. [DOI] [PubMed] [Google Scholar]
- 33.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 34.Ingram SL, Prasad BM, Amara SG. Dopamine transporter-mediated conductances increase excitability of midbrain dopamine neurons. Nat Neurosci. 2002;5:971–978. doi: 10.1038/nn920. [DOI] [PubMed] [Google Scholar]
- 35.Inyushin MU, Arencibia-Albite F, Vazquez-Torres R, Velez-Hernandez ME, Jimenez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience. 2010;167:287–297. doi: 10.1016/j.neuroscience.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schluter OM, Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- 38.Karreman M, Moghaddam B. The prefrontal cortex regulates the basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- 39.Kauer JA. Addictive drugs and stress trigger a common change at VTA synapses. Neuron. 2003;37:549–550. doi: 10.1016/s0896-6273(03)00087-4. [DOI] [PubMed] [Google Scholar]
- 40.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 41.Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 42.Kim K, Jarry H, Knoke I, Seong JY, Leonhardt S, Wuttke W. Competitive PCR for quantitation of gonadotropin-releasing hormone mRNA level in a single micropunch of the rat preoptic area. Mol Cell Endocrinol. 1993;97:153–158. doi: 10.1016/0303-7207(93)90222-6. [DOI] [PubMed] [Google Scholar]
- 43.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusch J, Thon S, Schulz E, Biskup C, Nache V, Zimmer T, Seifert R, Schwede F, Benndorf K. How subunits cooperate in cAMP-induced activation of homotetrameric HCN2 channels. Nat Chem Biol. 2012;8:162–169. doi: 10.1038/nchembio.747. [DOI] [PubMed] [Google Scholar]
- 45.Lamas JA. A hyperpolarization-activated cation current (Ih) contributes to resting membrane potential in rat superior cervical sympathetic neurones. Pflugers Arch. 1998;436:429–435. doi: 10.1007/s004240050653. [DOI] [PubMed] [Google Scholar]
- 46.Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci. 2002;5:1185–1193. doi: 10.1038/nn962. [DOI] [PubMed] [Google Scholar]
- 48.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- 49.Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Magee JC. Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci. 1999;2:508–514. doi: 10.1038/9158. [DOI] [PubMed] [Google Scholar]
- 51.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Luscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 52.Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Margolis EB, Toy B, Himmels P, Morales M, Fields HL. Identification of rat ventral tegmental area GABAergic neurons. PLoS One. 2012;7:e42365. doi: 10.1371/journal.pone.0042365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matt L, Michalakis S, Hofmann F, Hammelmann V, Ludwig A, Biel M, Kleppisch T. HCN2 channels in local inhibitory interneurons constrain LTP in the hippocampal direct perforant path. Cell Mol Life Sci. 2011;68:125–137. doi: 10.1007/s00018-010-0446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100:1202–1210. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mercuri NB, Bonci A, Calabresi P, Stefani A, Bernardi G. Properties of the hyperpolarization-activated cation current Ih in rat midbrain dopaminergic neurons. Eur J Neurosci. 1995;7:462–469. doi: 10.1111/j.1460-9568.1995.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 57.Migliore M, Cannia C, Canavier CC. A modeling study suggesting a possible pharmacological target to mitigate the effects of ethanol on reward-related dopaminergic signaling. J Neurophysiol. 2008;99:2703–2707. doi: 10.1152/jn.00024.2008. [DOI] [PubMed] [Google Scholar]
- 58.Monteggia LM, Eisch AJ, Tang MD, Kaczmarek LK, Nestler EJ. Cloning and localization of the hyperpolarization-activated cyclic nucleotide-gated channel family in rat brain. Brain Res Mol Brain Res. 2000;81:129–139. doi: 10.1016/s0169-328x(00)00155-8. [DOI] [PubMed] [Google Scholar]
- 59.Mu P, Moyer JT, Ishikawa M, Zhang Y, Panksepp J, Sorg BA, Schluter OM, Dong Y. Exposure to cocaine dynamically regulates the intrinsic membrane excitability of nucleus accumbens neurons. J Neurosci. 2010;30:3689–3699. doi: 10.1523/JNEUROSCI.4063-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Much B, Wahl-Schott C, Zong X, Schneider A, Baumann L, Moosmang S, Ludwig A, Biel M. Role of subunit heteromerization and N-linked glycosylation in the formation of functional hyperpolarization-activated cyclic nucleotide-gated channels. J Biol Chem. 2003;278:43781–43786. doi: 10.1074/jbc.M306958200. [DOI] [PubMed] [Google Scholar]
- 61.Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002;22:1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noam Y, Bernard C, Baram TZ. Towards an integrated view of HCN channel role in epilepsy. Curr Opin Neurobiol. 2011;21:873–879. doi: 10.1016/j.conb.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nolan MF, Dudman JT, Dodson PD, Santoro B. HCN1 channels control resting and active integrative properties of stellate cells from layer II of the entorhinal cortex. J Neurosci. 2007;27:12440–12451. doi: 10.1523/JNEUROSCI.2358-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nolan MF, Malleret G, Dudman JT, Buhl DL, Santoro B, Gibbs E, Vronskaya S, Buzsaki G, Siegelbaum SA, Kandel ER, Morozov A. A behavioral role for dendritic integration: HCN1 channels constrain spatial memory and plasticity at inputs to distal dendrites of CA1 pyramidal neurons. Cell. 2004;119:719–732. doi: 10.1016/j.cell.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 65.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol. 2004;471:241–276. doi: 10.1002/cne.11039. [DOI] [PubMed] [Google Scholar]
- 66.Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Papp I, Hollo K, Antal M. Plasticity of hyperpolarization-activated and cyclic nucleotid-gated cation channel subunit 2 expression in the spinal dorsal horn in inflammatory pain. Eur J Neurosci. 2010;32:1193–1201. doi: 10.1111/j.1460-9568.2010.07370.x. [DOI] [PubMed] [Google Scholar]
- 68.Perez MF, Gabach LA, Almiron RS, Carlini VP, de Barioglio SR, Ramirez OA. Different chronic cocaine administration protocols induce changes on dentate gyrus plasticity and hippocampal dependent behavior. Synapse. 2010;64:742–753. doi: 10.1002/syn.20788. [DOI] [PubMed] [Google Scholar]
- 69.Reith ME, Li MY, Yan QS. Extracellular dopamine, norepinephrine, and serotonin in the ventral tegmental area and nucleus accumbens of freely moving rats during intracerebral dialysis following systemic administration of cocaine and other uptake blockers. Psychopharmacology (Berl) 1997;134:309–317. doi: 10.1007/s002130050454. [DOI] [PubMed] [Google Scholar]
- 70.Robinson TE, Berridge KC. Addiction. Annu Rev Psychol. 2003;54:25–53. doi: 10.1146/annurev.psych.54.101601.145237. [DOI] [PubMed] [Google Scholar]
- 71.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Ann N Y Acad Sci. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 74.Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. J Neurosci. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 76.Seutin V, Massotte L, Renette MF, Dresse A. Evidence for a modulatory role of Ih on the firing of a subgroup of midbrain dopamine neurons. Neuroreport. 2001;12:255–258. doi: 10.1097/00001756-200102120-00015. [DOI] [PubMed] [Google Scholar]
- 77.Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sofuoglu M, Sewell RA. Norepinephrine and stimulant addiction. Addict Biol. 2009;14:119–129. doi: 10.1111/j.1369-1600.2008.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steketee JD. Cortical mechanisms of cocaine sensitization. Crit Rev Neurobiol. 2005;17:69–86. doi: 10.1615/critrevneurobiol.v17.i2.20. [DOI] [PubMed] [Google Scholar]
- 80.Stramiello M, Wagner JJ. Cocaine enhancement of long-term potentiation in the CA1 region of rat hippocampus: lamina-specific mechanisms of action. Synapse. 2010;64:644–648. doi: 10.1002/syn.20764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Surges R, Brewster AL, Bender RA, Beck H, Feuerstein TJ, Baram TZ. Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus. Eur J Neurosci. 2006;24:94–104. doi: 10.1111/j.1460-9568.2006.04880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taber MT, Fibiger HC. Electrical stimulation of the prefrontal cortex increases dopamine release in the nucleus accumbens of the rat: modulation by metabotropic glutamate receptors. J Neurosci. 1995;15:3896–3904. doi: 10.1523/JNEUROSCI.15-05-03896.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takasu K, Ono H, Tanabe M. Spinal hyperpolarization-activated cyclic nucleotide-gated cation channels at primary afferent terminals contribute to chronic pain. Pain. 2010;151:87–96. doi: 10.1016/j.pain.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 84.Tarentino AL, Gomez CM, Plummer TH., Jr. Deglycosylation of asparagine-linked glycans by peptide:N-glycosidase F. Biochemistry. 1985;24:4665–4671. doi: 10.1021/bi00338a028. [DOI] [PubMed] [Google Scholar]
- 85.Thomas MJ, Malenka RC, Bonci A. Modulation of long-term depression by dopamine in the mesolimbic system. J Neurosci. 2000;20:5581–5586. doi: 10.1523/JNEUROSCI.20-15-05581.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Torregrossa MM, Tang XC, Kalivas PW. The glutamatergic projection from the prefrontal cortex to the nucleus accumbens core is required for cocaine-induced decreases in ventral pallidal GABA. Neurosci Lett. 2008;438:142–145. doi: 10.1016/j.neulet.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tu H, Zhang L, Tran TP, Muelleman RL, Li YL. Diabetes alters protein expression of hyperpolarization-activated cyclic nucleotide-gated channel subunits in rat nodose ganglion cells. Neuroscience. 2010;165:39–52. doi: 10.1016/j.neuroscience.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 88.Uchimura N, Cherubini E, North RA. Cation current activated by hyperpolarization in a subset of rat nucleus accumbens neurons. J Neurophysiol. 1990;64:1847–1850. doi: 10.1152/jn.1990.64.6.1847. [DOI] [PubMed] [Google Scholar]
- 89.Ulens C, Siegelbaum SA. Regulation of hyperpolarization-activated HCN channels by cAMP through a gating switch in binding domain symmetry. Neuron. 2003;40:959–970. doi: 10.1016/s0896-6273(03)00753-0. [DOI] [PubMed] [Google Scholar]
- 90.Ulens C, Tytgat J. Functional heteromerization of HCN1 and HCN2 pacemaker channels. J Biol Chem. 2001;276:6069–6072. doi: 10.1074/jbc.C000738200. [DOI] [PubMed] [Google Scholar]
- 91.Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F. Addiction: beyond dopamine reward circuitry. Proc Natl Acad Sci U S A. 2011;108:15037–15042. doi: 10.1073/pnas.1010654108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wainger BJ, DeGennaro M, Santoro B, Siegelbaum SA, Tibbs GR. Molecular mechanism of cAMP modulation of HCN pacemaker channels. Nature. 2001;411:805–810. doi: 10.1038/35081088. [DOI] [PubMed] [Google Scholar]
- 93.Wang M, Ramos BP, Paspalas CD, Shu Y, Simen A, Duque A, Vijayraghavan S, Brennan A, Dudley A, Nou E, Mazer JA, McCormick DA, Arnsten AF. Alpha2A-adrenoceptors strengthen working memory networks by inhibiting cAMP-HCN channel signaling in prefrontal cortex. Cell. 2007;129:397–410. doi: 10.1016/j.cell.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 94.Wei-qing H, Qing-nuan K, Lin X, Cheng-hao G, Qi-yi Z. Expression of hyperpolarization-activated cyclic nucleotide-gated cation channel (HCN4) is increased in hypertrophic cardiomyopathy. Cardiovasc Pathol. 2011;20:110–113. doi: 10.1016/j.carpath.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 95.Wells AM, Lasseter HC, Xie X, Cowhey KE, Reittinger AM, Fuchs RA. Interaction between the basolateral amygdala and dorsal hippocampus is critical for cocaine memory reconsolidation and subsequent drug context-induced cocaine-seeking behavior in rats. Learn Mem. 2011;18:693–702. doi: 10.1101/lm.2273111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol. 2000;83:3177–3182. doi: 10.1152/jn.2000.83.5.3177. [DOI] [PubMed] [Google Scholar]
- 97.Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia S, Wang Y, Zhang Y, Deng SB, Du JL, Wang XC, She Q. Dynamic changes in HCN2, HCN4, KCNE1, and KCNE2 expression in ventricular cells from acute myocardial infarction rat hearts. Biochem Biophys Res Commun. 2010;395:330–335. doi: 10.1016/j.bbrc.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 99.You ZB, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an in vivo microdialysis study in freely moving rats. J Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zabel C, Klose J. Protein extraction for 2DE. Methods Mol Biol. 2009;519:171–196. doi: 10.1007/978-1-59745-281-6_11. [DOI] [PubMed] [Google Scholar]
- 101.Zha Q, Brewster AL, Richichi C, Bender RA, Baram TZ. Activity-dependent heteromerization of the hyperpolarization-activated, cyclic-nucleotide gated (HCN) channels: role of N-linked glycosylation. J Neurochem. 2008;105:68–77. doi: 10.1111/j.1471-4159.2007.05110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]