Abstract
Object
Primary malignant spinal glioma represents a significant clinical challenge due to the devastating effect on patient clinical outcomes seen in the majority of cases. As they are infrequently encountered in any one center, there has been little population-based data analysis on the incidence patterns of these aggressive tumors. The objective of this study was to use publically available Surveillance, Epidemiology and End Results (SEER) program data to examine overall incidence and incidence patterns over time with regard to patient age at diagnosis, gender, race, primary site of tumor and histological subtype for patients diagnosed with primary malignant spinal cord gliomas between 1973 and 2006.
Methods
The study population of interest was limited to primary, malignant, pathologically confirmed spinal cord gliomas using data from the SEER 9 standard registries for patients diagnosed between 1973 and 2006. Variables of interest included age at diagnosis, gender, race, primary site of tumor, and histological subtype of tumor. The SEER*Stat 6.5.2. program was used to calculate frequencies, age-adjusted incidence rates with 95% confidence intervals and annual percentage change (APC) statistics with a 2-sided p-values. In addition, linear correlation coefficients (R2) were calculated for the time association stratified by variables of interest.
Results
The overall age-adjusted incidence rate for primary malignant spinal gliomas was 0.12 per 100,000 and increased significantly over the study time period (APC= 1.74; p-value=0.0004; R2=0.36). Incidence was highest for patients diagnosed at ages 35–49 (0.17 per 100,000), males (0.14 per 100,000), Whites (0.13 per 100,000) and those who had epdenymomas (0.07 per 100,000). Over the study period, the incidence of ependymomas increased significantly (APC = 3.17; p-value<0.0001; R2=0.58) as did the incidence of these tumors in Whites (APC = 2.13; p-value=0.001) and for both males (APC=1.90, p-value<0.0001) and females (APC=1.60, p-value<0.0001). No significant changes in incidence over time by age of diagnosis were found.
Conclusions
This study demonstrates an increasing overall incidence of primary, malignant spinal cord glioma over the past three decades. Notably, for ependymoma the incidence has increased, while the incidence of most other glioma subtypes remained stable. This may be due to improved diagnostic and surgical techniques, changes in histological classification criteria, and changes in neuro-pathology diagnostic criteria. Although rare, an improved understanding of the incidence of these rare tumors will assist investigators and clinicians in planning potential studies and preparing for allocation of resources to care for these challenging patients.
Keywords: Spinal cord glioma, incidence, patterns over time, population-based, SEER
Introduction
Primary spinal cord tumors are considered rare, accounting for only 4–8% of all primary central nervous system (CNS) lesions5,6,8,14. To date, there have been no large population based epidemiological studies analyzing incidence patterns over time for this tumor type.
The overall incidence of primary spinal cord tumors (malignant and non-malignant combined) was recently reported as 0.74 per 100,000 person-years, with the lowest incidence rates in children 0 – 19 years of age at diagnosis and the highest in adults 65 – 74 years of age at diagnosis, with a median age at diagnosis of 51 years. Incidence varies by gender and race: 0.77 per 100,000 in females and 0.70 per 100,000 in males; 0.45 per 100,000 in non-Hispanic Blacks, 0.61 per 100,000 in Hispanics, and 0.79 per 100,000 in non-Hispanic Whites. In a recent study of 3,226 patients with primary spinal tumors diagnosed between 1998 and 2002, 69% were non-malignant (56% benign, 13% uncertain) and 31% were malignant19. Therefore, malignant spinal cord tumors represent the minority of this tumor type.
The incidence of spinal cord gliomas has been reported as approximately 0.22 per 100,00019. Astrocytomas and ependymomas are the most common gliomas of the spinal cord with ependymomas comprising 60–80% of all spinal gliomas11,10,22. Additionally, overall incidence of ependymomas in children appears to be increasing in all locations 9,24.
Because of the rarity of primary malignant spinal cord gliomas, there has been little analysis of population-based data on the incidence patterns of these tumors. The objective of this study was to use publically available Surveillance, Epidemiology and End Results (SEER) program data to examine overall incidence and incidence patterns over time of pathologically confirmed primary spinal cord gliomas with regard to patient age at diagnosis, gender, race, primary site of tumor and histological subtype over time for patients diagnosed between 1973 and 2006.
Methods
Description of SEER data
SEER is the United States, National Cancer Institute supported, national cancer surveillance program that collects cancer statistics from 17 geographic areas representing 26% of the US population. SEER collects information on demographics, clinical information at the time of diagnosis, first course of treatment, and active follow up for vital status18. These data are updated yearly and are publically available for use in incidence and survival studies. This analysis used these data for patients diagnosed between 1973 and 2006.
Study Population/Case Selection
Analysis was limited to primary, malignant, pathologically confirmed spinal cord tumors using data from the SEER 9 standard registries, which include data from 1973 to 2006. These 9 registries include Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. The International Classification of Diseases for Oncology, Third Edition (ICDO-3) topography codes for primary sites of interest were: spinal cord (C72.0), spinal meninges (C70.1), and cauda equina (C72.1). Individuals were excluded if they were diagnosed at autopsy or by death certificate only and if their race or sex was coded as unknown.
Variables of interest
Variables of interest included age at diagnosis, gender, race, primary site of tumor, and histological subtype of tumor. Analysis was restricted to the racial categories of White, Black, or Other (American Indian/Alaskan Native and Asian/Pacific Islander). Age at diagnosis was divided into six groups: 0–9 years, 10–19 years, 20–34 years, 35–49 years, 50–64 years, and over 65 years.
The histological subtype of tumor categories were coded using the 4-digit ICDO-3 morphology codes into 7 groups according to updated World Health Organization (WHO) histological classifications: Ependymoma (9391–9394), Astrocytoma Grade I (9421), Astrocytoma Grade II (9410,9420,9430), Astrocytoma Grade II–III (9382,9411,9450), Astrocytoma Grade III (9401), Astrocytoma Grade IV (Glioblastoma – GBM) (9440–9441) and Astrocytoma, not otherwise specified (NOS) (9400)15.
SEER*Stat 6.5.2.20 was used to calculate frequencies of the variables of interest and age-adjusted incidence rates with adjustment to the 2000 US Standard population. The incidence rates were calculated overall and by sex, race, age category, histological subtype, and primary location. An annual percentage change (APC) statistic to assess incidence patterns over time with a 2-sided p-value was calculated using the weighted least squares method. If sample sizes within categories of variables of interest were too small, the APC was not calculable (NC). In addition, linear correlation coefficients (R2) were calculated for the time association stratified by variables of interest (ranging between −1 and 1, where 1 symbolizes a perfect increasing linear trend).
This study was approved by the Case Cancer Institutional Review Board as an exempt study.
Results
Overall Incidence
There were 988 pathologically confirmed primary malignant spinal gliomas diagnosed between 1973 and 2006 that were used for analysis (Table 1). The overall age-adjusted incidence for all primary, malignant spinal cord gliomas combined was 0.12 per 100,000. These tumors were most common in males (age-adjusted incidence rate = 0.14 per 100,000) and Whites (age-adjusted incidence rate = 0.13 per 100,000). The vast majority originated in the spinal cord (97.57%), as opposed to the spinal meninges or cauda equina. The most common histological subtypes were ependymomas (age-adjusted incidence rate = 0.07 per 100,000) and astrocytoma, NOS (age-adjusted incidence rate = 0.03 per 100,000). The other gliomas subtypes were all rare with age-adjusted incidence rates varying between 0.003–0.009 per 100,000.
Table 1.
Age-adjusted incidence rates of primary malignant spinal cord tumor patients by variables of interest diagnosed in 1973 to 2006.
| Variable of Interest | No. (%) | Incidence ratex | 95% CIxx |
|---|---|---|---|
| OVERALL | 988 (100) | 0.12 | (0.12,0.13) |
| Age at diagnosis | |||
| 0–9 | 91 (9.21) | 0.08 | (0.06, 0.09) |
| 10–19 | 110 (11.13) | 0.09 | (0.07,0.11) |
| 20–34 | 238 (24.09) | 0.12 | (0.11,0.14) |
| 35–49 | 278 (28.14) | 0.17 | (0.15,0.19) |
| 50–64 | 180 (18.22) | 0.16 | (0.13,0.18) |
| 65+ | 91 (9.21) | 0.10 | (0.08,0.12) |
| Sex | |||
| Male | 557 (56.38) | 0.14 | (0.13,0.16) |
| Female | 431 (43.62) | 0.11 | (0.10,0.12) |
| Race | |||
| White | 850 (86.03) | 0.13 | (0.12,0.14) |
| Black | 86 (8.70) | 0.09 | (0.07,0.12) |
| Other* | 52 (5.26) | 0.07 | (0.06,0.10) |
| Histological type | |||
| Astrocytoma Grade I | 77 (7.79) | 0.009 | (0.007,0.01) |
| Astrocytoma Grade II | 31 (3.14) | 0.004 | (0.003,0.005) |
| Astrocytoma Grade II–III | 26 (2.63) | 0.003 | (0.002,0.005) |
| Astrocytoma Grade III | 38 (3.85) | 0.005 | (0.003,0.007) |
| GBM | 39 (3.95) | 0.005 | (0.003,0.007) |
| Astrocytoma, NOS | 228 (23.08) | 0.03 | (0.02,0.03) |
| Ependymoma | 549 (55.57) | 0.07 | (0.06,0.08) |
| Primary Site | |||
| C70.1-Spinal meninges | 6 (0.61) | 0.0008 | (0.0003,0.002) |
| C72.0-Spinal cord | 964 (97.57) | 0.12 | (0.11,0.13) |
| C72.1-Cauda equina | 18 (1.82) | 0.002 | (0.001,0.004) |
Incidence rates are per 100,000 and age-adjusted to the 2000 US Standard Population
95% Confidence interval for incidence rate.
American Indian/AK Native, Asian/Pacific Islander
Incidence Patterns Over Time
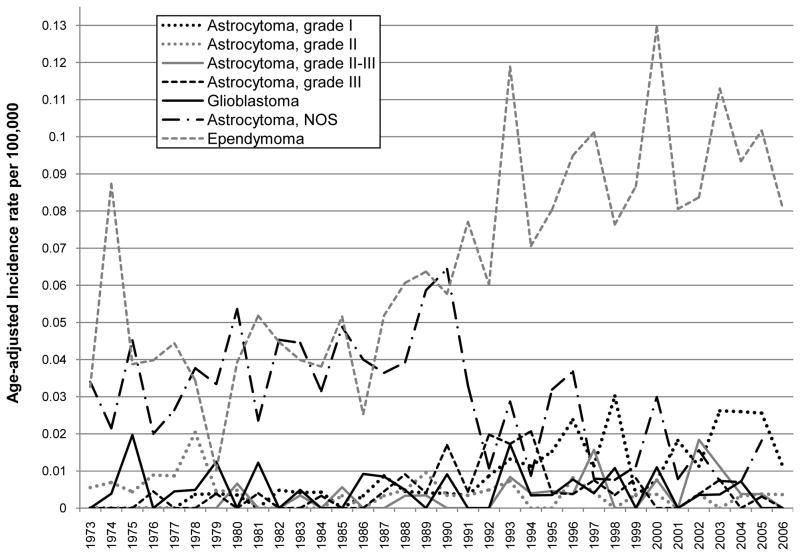
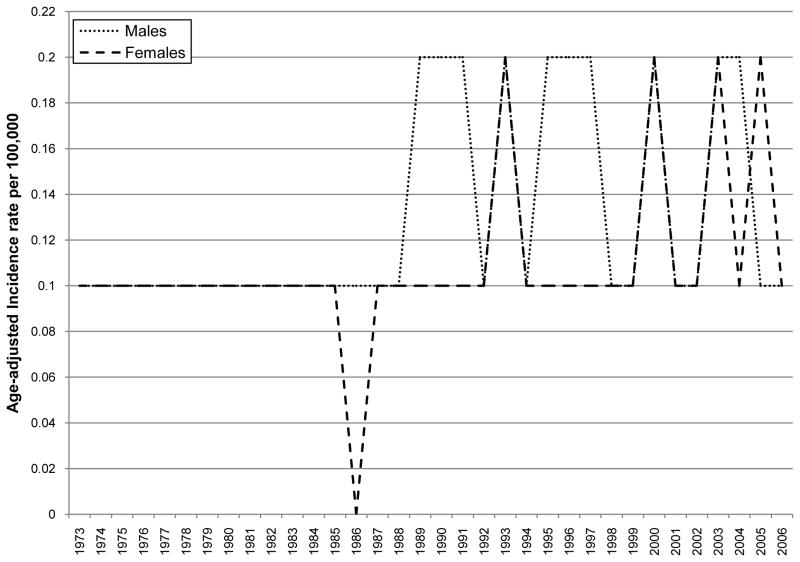
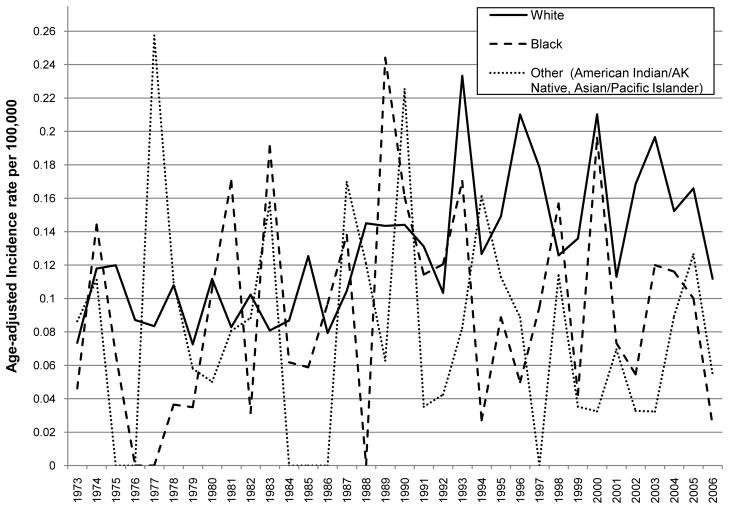
The overall age-adjusted incidence of primary malignant spinal gliomas showed a statistically significant increase over time between 1973 and 2006 (APC = 1.74, p-value = 0.00038; Table 2). When stratifying by histological subtype of tumor, the incidence of ependymoma demonstrated a statistically significant increasing pattern over time (APC = 3.17, p-value <0.0001; Table 2 and Figure 1). While no APC statistic could be calculated for the other histological subtypes because of small sample sizes, Figure 1 & Table 2 show an increase in grade I astrocytoma (R2 for a positive linear trend=0.59) and a decrease in astrocytoma, NOS (R2 for a positive linear trend=0.31) over time. No clear incidence pattern by age at diagnosis category was seen, although it was noted that indivdiuals in the following age categories: 35–49, 50–64 and 65 and over had the highest incidence rates over time as compared to the other age groups (Table 2 and Figure 2). For both males and females, incidence increased significantly over time (male APC=1.90, p-value<0.0001 and female APC=1.60, p-value<0.0001) (Table 2 and Figure 3). There was also a statistically significant increase in incidence for Whites with an APC of 2.13 (p-value=0.001; Table 2 and Figure 4) while little change was seen for Blacks or those of Other races (no APC statistics could be calculated for these catergories because of small sample sizes).
Table 2.
Age-adjusted incidence trend statistics and linear correlation coefficients for primary malignant spinal cord glioma by variables of interest.
| APC statistic | APC p-value* | R2 value^ | |
|---|---|---|---|
| Overall | 1.74 | 0.00038# | 0.3572 |
| Age at diagnosis | |||
| 0–9 | NC | NC | 0.13 |
| 10–19 | NC | NC | 0.003 |
| 20–34 | 0.69 | 0.26 | 0.05 |
| 35–49 | 1.16 | 0.15 | 0.14 |
| 50–64 | NC | NC | 0.28 |
| 65+ | NC | NC | 0.26 |
| Sex | |||
| Male | 1.9 | <0.0001# | 0.17 |
| Female | 1.6 | <0.0001# | 0.14 |
| Race | |||
| White | 2.13 | 0.0001# | 0.4 |
| Black | NC | NC | 0.03 |
| Other | NC | NC | 0.007 |
| Histological Type | |||
| Astrocytoma Grade I | NC | NC | 0.59 |
| Astrocytoma Grade II | NC | NC | 0.11 |
| Astrocytoma Grade II–III | NC | NC | 0.19 |
| Astrocytoma Grade III | NC | NC | 0.05 |
| GBM | NC | NC | 0.01 |
| Astrocytoma, NOS | NC | NC | 0.31 |
| Ependymoma | 3.17 | <0.0001 | 0.58 |
APC - Annual Percentage Change statistic for trend
R2 value - measure of strength of linear trend (ranges between −1 and 1)
NC - Not calculable due to small sample sizes
increasing trend
Figure 1.
Age adjusted incidence by year of diagnosis and histological type of tumor of primary malignant spinal cord tumor patients diagnosed in 1973 to 2006 (per 100,000 and age-adjusted to the 2000 US Standard Population)
Figure 2.
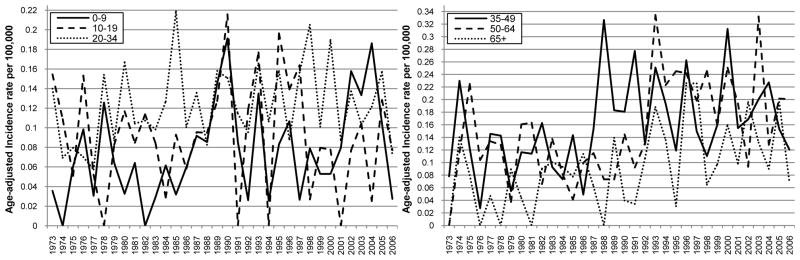
Age adjusted incidence by year of diagnosis and age at diagnosis of primary malignant spinal cord tumor patients diagnosed in 1973 to 2006 (per 100,000 and age-adjusted to the 2000 US Standard Population)
Figure 3.
Age adjusted incidence by year of diagnosis and sex of primary malignant spinal cord tumor patients diagnosed in 1973 to 2006 (per 100,000 and age-adjusted to the 2000 US Standard Population)
Figure 4.
Age adjusted incidence by year of diagnosis and race of primary malignant spinal cord tumor patients diagnosed in 1973 to 2006 (per 100,000 and age-adjusted to the 2000 US Standard Population)
Discussion
Primary malignant spinal gliomas are a rare malignancy with limited published population-based incidence data. This study, based on the SEER data, provides a large population-based study cohort that facilitated the study of these neoplasms over several decades with long-term follow-up. The incidence rates of primary malignant spinal gliomas described here are consistent with other recent incidence studies. The most common histological subtypes of spinal cord glioma is ependymoma1,15,23, with the highest incidence among white males between ages 35–65 16,19.
Our analysis of incidence patterns demonstrates that pathologically confirmed primary malignant spinal gliomas have been increasing over the last 30 years, primarily due to the significant increased incidence of ependymomas. The incidence of grade I astrocytomas also appeared to trend upwards, while the incidence of astrocytoma NOS trended downward and the incidence of other subtypes did not change appreciably during this time period (Figure 1). While no percentage change statistics could be calculated because of small sample sizes, an increase in the incidence of astrocytomas has been observed by others16. Decreases in the “not otherwise specified” histological categories are not uncommon in SEER studies, and most likely reflects improved diagnosis and classification over time2.
The biological explanation for the observed increased incidence of primary malignant spinal gliomas, specifically ependymomas, remains unclear. MRI of the spine is currently the diagnostic study of choice and almost all intrinsic malignant spinal cord tumors enhance with gadolinium21. The advent of MRI imaging may have led to increased detection and it is possible that the observed increased incidence we have identified merely reflects the increased sensitivity of our diagnostic technologies resulting in increased frequency of identification of these neoplasms and a related increased tendency towards surgical diagnosis and intervention rather than a true increased incidence. However, there were no sharp inflections in incidence with our analysis, which would be expected with an acute advancement in diagnostic technology. Histologic criteria, which are somewhat qualitative and subjective, have also evolved over time and are dependent on pathologist experience and preference, which could also have influenced incidence rates4,7.
The explanation for increasing incidence among Whites and by gender has been observed previously as well, but is poorly understood10, 27. Some investigators have suggested that gender specific oncological mutations, environmental and lifestyle factors may be related to gender differences in incidence13 for multiple different malignancies, and subsequently this may also be true for race3,12,17. We have previously made similar observations on the increased incidence of malignant brain gliomas in whites vs blacks2.
Because of the overall rarity of primary malignant spinal cord gliomas, even within the population-based studies such as this, small sample sizes result when the data are stratified into categories by race, age, histological subtype, etc which can limit interpretation. It is also possible that specific variables that are not available in these data impact incidence patterns, such as socio-economic status, access to care, supportive services or data on biological factors that confer differences in glial tumors. These analyses, based on intention to treat, represent the changes in primary spinal cord tumor histopathological diagnosis throughout the United States over the past thirty years.
Balancing these limitations is a number of strengths. The SEER data are known to be of very high quality. The information collected on each individual case is meticulously monitored for accuracy. As a population-based study, SEER data are more likely to apply to the greater United States population than data from a single-institution study. Unlike most of the previous studies, which were primarily single-institutional reviews, population-based registries such as SEER are not limited by small overall sample sizes and are not likely to be influenced by selection bias that can be associated with treatment practices and referral patterns, thereby allowing this study to investigate incidence patterns for ~1000 individuals diagnosed with primary malignant spinal cord gliomas. In addition, this study utilized current SEER Program data, with follow-up on each individual through 2006.
Conclusions
Age-adjusted incidence of primary malignant spinal cord gliomas has been increasing in the United States over the last 30 years. The most common primary malignant spinal cord glioma is ependymoma. These tumors occur most frequently in whites and in males. Notably, ependymoma incidence has increased over the past three decades, while most astrocytic subtypes did not. This may be due to improved diagnostic and surgical technique, changes in classification criteria, histopathological tools, and diagnosis. An improved understanding of the patterns in incidence of these rare tumors assists researchers, clinicians, and public health officials in planning studies and allocating resources to assist in the care of patients with these often devastating tumors.
Acknowledgments
Financial Support:
This work was supported in part by Case Comprehensive Cancer Center Core Grant (P30-CA043703) (SH, MQ, JSB-S).
The first authors, Steven Hsu, BS, and Marisa Quattrone, BSE, shared the work equally. We thank Yingli Wolinsky, PhD, MBA for her technical assistance.
Footnotes
Disclosure/Disclaimer
None
References
- 1.Alter M. Tumours of the spine and spinal cord. Amsterdam: American Elsevier; 1975. [Google Scholar]
- 2.Barnholtz-Sloan JS, Sloan AE, Schwartz AG. Relative survival rates and patterns of diagnosis analyzed by time period for individuals with primary malignant brain tumor, 1973–1997. J Neurosurg. 2003;99:458–466. doi: 10.3171/jns.2003.99.3.0458. [DOI] [PubMed] [Google Scholar]
- 3.Burchard EG, Ziv E, Coyle N, Gomez SL, Tang H, Karter AJ, et al. The importance of race and ethnic background in biomedical research and clinical practice. N Engl J Med. 2003;348:1170–1175. doi: 10.1056/NEJMsb025007. [DOI] [PubMed] [Google Scholar]
- 4.Castillo MS, Davis FG, Surawicz T, Bruner JM, Bigner S, Coons S, et al. Consistency of primary brain tumor diagnoses and codes in cancer surveillance systems. Neuroepidemiology. 2004;23:85–93. doi: 10.1159/000073980. [DOI] [PubMed] [Google Scholar]
- 5.CBTRUS. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004–2006. Hinsdale, IL: Central Brain Tumor Registry of the United States; 2010. [Google Scholar]
- 6.Chi JG, Khang SK, Koo HS, Roh JK. Acardiac twins--an analysis of 10 cases central nervous system tumors among Koreans--a statistical study on 697 cases histopathologic study on muscle diseases among Koreans (274 muscle biopsy analysis) J Korean Med Sci. 1989;4:203–216. [Google Scholar]
- 7.Coons SW, Johnson PC, Scheithauer BW, Yates AJ, Pearl DK. Improving diagnostic accuracy and interobserver concordance in the classification and grading of primary gliomas. Cancer. 1997;79:1381–1393. doi: 10.1002/(sici)1097-0142(19970401)79:7<1381::aid-cncr16>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 8.Elia-Pasquet S, Provost D, Jaffre A, Loiseau H, Vital A, Kantor G, et al. Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology. 2004;23:110–117. doi: 10.1159/000075953. [DOI] [PubMed] [Google Scholar]
- 9.Farwell JR, Dohrmann GJ, Flannery JT. Central nervous system tumors in children. Cancer. 1977;40:3123–3132. doi: 10.1002/1097-0142(197712)40:6<3123::aid-cncr2820400656>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Helseth A, Mork SJ. Primary intraspinal neoplasms in Norway, 1955 to 1986. A population-based survey of 467 patients. J Neurosurg. 1989;71:842–845. doi: 10.3171/jns.1989.71.6.0842. [DOI] [PubMed] [Google Scholar]
- 11.Henson JW. Spinal cord gliomas. Curr Opin Neurol. 2001;14:679–682. doi: 10.1097/00019052-200112000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Jorde L. Human Genetic Variation and Disease. In: Meyers R, editor. Encyclopedia of Molecular Biology and Molecular Medicine. 2. Vol. 6. Weinheim: Wiley-VCH Publishers; 2005. pp. 323–337. [Google Scholar]
- 13.Kirsch-Volders M, Bonassi S, Herceg Z, Hirvonen A, Moller L, Phillips DH. Gender-related differences in response to mutagens and carcinogens. Mutagenesis. doi: 10.1093/mutage/geq008. [epub ahead of print March 3, 2010. geq008 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Liigant A, Asser T, Kulla A, Kaasik AE. Epidemiology of primary central nervous system tumors in Estonia. Neuroepidemiology. 2000;19:300–311. doi: 10.1159/000026269. [DOI] [PubMed] [Google Scholar]
- 15.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Milano MT, Johnson MD, Sul J, Mohile NA, Korones DN, Okunieff P, et al. Primary spinal cord glioma: a Surveillance, Epidemiology, and End Results database study. J Neurooncol. 2009 doi: 10.1007/s11060-009-0054-7. [epub ahead of print November 10, 2009. [DOI] [PubMed] [Google Scholar]
- 17.Mountain JL, Risch N. Assessing genetic contributions to phenotypic differences among ‘racial’ and ‘ethnic’ groups. Nat Genet. 2004;36:S48–53. doi: 10.1038/ng1456. [DOI] [PubMed] [Google Scholar]
- 18.NCI. Surveillance, Epidemiology and End Results (SEER) Bethesda, MD: NCI; 2010. http://www.seer.cancer.gov/ [Google Scholar]
- 19.Schellinger KA, Propp JM, Villano JL, McCarthy BJ. Descriptive epidemiology of primary spinal cord tumors. J Neurooncol. 2008;87:173–179. doi: 10.1007/s11060-007-9507-z. [DOI] [PubMed] [Google Scholar]
- 20.SEER*Stat: Version 6.5.2. Bethesda, MD: National Cancer Institute; 2009. [Google Scholar]
- 21.Sevick RJ, Wallace CJ. MR imaging of neoplasms of the lumbar spine. Magn Reson Imaging Clin N Am. 1999;7:539–553. ix. [PubMed] [Google Scholar]
- 22.Shrivastava RK, Epstein FJ, Perin NI, Post KD, Jallo GI. Intramedullary spinal cord tumors in patients older than 50 years of age: management and outcome analysis. J Neurosurg Spine. 2005;2:249–255. doi: 10.3171/spi.2005.2.3.0249. [DOI] [PubMed] [Google Scholar]
- 23.Slooff JLKJ, MacCarty CS. Primary Intramedullary Tumours of the Spinal Cord and Filum Terminale. London: WB Saunders; 1964. [Google Scholar]
- 24.Smith MA, Freidlin B, Ries LA, Simon R. Trends in reported incidence of primary malignant brain tumors in children in the United States. J Natl Cancer Inst. 1998;90:1269–1277. doi: 10.1093/jnci/90.17.1269. [DOI] [PubMed] [Google Scholar]