Abstract
Objective
A growing body of literature suggests ethnic differences in experimental pain. However, these studies largely focus on adults and the comparison between Caucasians and African-Americans. The primary aim of this study is to determine ethnic differences in laboratory induced pain in a multi-ethnic child sample.
Methods
Participants were 214 healthy children (mean age = 12.7, SD= 3.0 yrs). Ninety-eight Caucasian, 58 Hispanic, 34 African-American, and 24 Asian children were exposed to four trials of pressure and radiant heat pain stimuli. Pain responses were assessed with self-report measures (i.e., pain intensity and unpleasantness) and behavioral observation (i.e., pain tolerance).
Results
Asians demonstrated more pain sensitivity than Caucasians, who evidenced more pain sensitivity than African-Americans and Hispanics. The results hold even after controlling for age, sex, SES, and experimenter’s ethnicity. Asians also showed higher anticipatory anxiety compared with other ethnic groups. Anticipatory anxiety accounted for some ethnic differences in pain between Asians, Hispanics, and African Americans.
Conclusions
By examining response to laboratory pain stimuli in children representing multiple ethnicities, an understudied sample, the study reveals unique findings compared to the existing literature. These findings have implications for clinicians who manage acute pain in children from diverse ethnic backgrounds. Future investigations should examine mechanisms that account for ethnic differences in pain during various developmental stages.
Keywords: ethnicity, laboratory pain, children, health disparity, anticipatory anxiety
Pain management among children is challenging because pain is a highly complex phenomenon that involves biological, psychological, and social variables such as culture and ethnicity (Fortier, Anderson, & Kain, 2009). Ethnicity is defined as a group of people with distinguishing behaviors, culture, history, experience, ancestry, and beliefs (C. L. Edwards, Fillingim, & Keefe, 2001). Ethnic minorities account for 44% of children in the U.S. today, and are expected to account for 50% of children in 2023, and 62% in 2050 (U.S. Census Bureau., 2008). The growing diverse populations in the U.S. make it imperative to understand ethnic differences in pain among children. Understanding factors that influence pain responses in children is important, because the neurobiological processes involved in the perception and experience of pain are still developing (Fitzgerald, 2005), and early pain experiences in childhood influence pain and disability in adulthood (Davis, Luecken, & Zautra, 2005). Data on ethnic differences in childhood pain responses may assist in the formulation of interventions tailored to specific racial/ethnic groups that may help prevent the development of chronic pain in adulthood.
Accumulating evidence suggests ethnic disparities in pain among adults (C. L. Edwards, et al., 2001; R. R. Edwards, Doleys, Fillingim, & Lowery, 2001; Green et al., 2003; Zatzick & Dimsdale, 1990). Despite the drastic rise in studies examining ethnic differences among adults, ethnic differences in pain among children are largely unknown. We were only able to identify three studies that examined ethnic differences in pain among children in the U.S. (Lewis, Ramsay, & Kawakami, 1993; Pfefferbaum, Adams, & Aceves, 1990; Widmalm SE, Christiansen RL, & Gunn, 1995). The first study found a higher rate of oral/facial pain symptoms related to temporomandibular disorders (TMD) in African-American compared with Caucasian children ages 4-6 (Widmalm SE, et al., 1995). The second study found no differences in pain between Hispanic and Caucasian children undergoing oncology procedures (Pfefferbaum, et al., 1990). A third study found a less intense affective response to inoculation but a greater cortisol stress response among Japanese infants compared with Caucasian infants (Lewis, et al., 1993). Although these studies only compared one minority group with Caucasians and the results are mixed, the studies suggest the existence of ethnic differences in pain among children.
Traditionally, ethnic differences in pain are studied using survey methods in a clinical context; lately, the paradigm of laboratory induced pain has attracted attention. Survey studies of clinical pain can help further the understanding of health disparities in pain among patients. However, clinical pain is greatly influenced by factors such as disease severity and type of treatment. Thus, the exact source of pain is typically unknown and the amount of noxious pain stimuli varies significantly across and within patients. In contrast, laboratory induced pain standardizes the amount and nature of noxious pain stimuli, and thus offers advantages relative to studies of clinical pain. Laboratory pain affords the ability to test pain stimuli with different temporal sensory characteristics, which can provide more specific information regarding the nature of ethnic differences in pain (Green, et al., 2003).
A growing number of studies have reported ethnic differences in laboratory pain responses. However, the vast majority of laboratory studies compared Caucasians with African Americans (Campbell, Edwards, & Fillingim, 2005; Campbell et al., 2008; R. R. Edwards, et al., 2001; R. R. Edwards & Fillingim, 1999; Mechlin, Morrow, Maixner, & Girdler, 2007), thus providing limited data for other groups. In addition, no study has investigated ethnic differences in children’s laboratory-induced pain responses in a multi-ethnic sample. The goal of the current study is to examine ethnic differences in laboratory pain responses among non-clinical children in a sample including Caucasians, Asians, Hispanics (Latinos), and African Americans.
Among adults, findings of differences in laboratory pain responses between African Americans and Caucasians are fairly consistent. African-Americans have reported higher pain intensity and unpleasantness for heat pain compared with Caucasians (Campbell, et al., 2005; Sheffield, Biles, Orom, Maixner, & Sheps, 2000). African Americans also demonstrated lower pain tolerance and threshold for heat, cold, and tourniquet ischemic pain relative to Caucasians (Campbell, et al., 2005; Campbell, et al., 2008; R. R. Edwards, et al., 2001; R. R. Edwards & Fillingim, 1999; Mechlin, et al., 2007). Other minorities are understudied in adult laboratory pain investigations. One early study found that Asians had lower pressure pain tolerance compared with Caucasians (Woodrow, Friedman, Siegelaub, & Collen, 1972). A recent study found that Hispanics were less tolerant of heat and cold pressor pain than were Caucasians (Rahim-Williams et al., 2007). In summary, existing literature provides some evidence suggesting that ethnic minorities demonstrate higher pain sensitivity in response to laboratory pain compared with Caucasians in adult samples.
According to the model proposed by Price and colleagues, pain has multiple dimensions including sensory-discriminative, affective, and behavioral responses (Wade, Dougherty, Archer, & Price, 1996). These dimensions are viewed as unique subcomponents and as representing four different stages of pain processing. The first stage is the sensory-discriminative dimension which includes the spatial, temporal, and intensive features of the painful sensation. The second stage is the immediate unpleasantness that is associated with the perceived intrusiveness of the painful sensation. This stage reflects the individual’s immediate affective response to the sensations evoked by nociceptive stimuli. The third stage characterizes the quality of the magnitude of suffering and secondary affective response (e.g., depression, frustration, etc). The fourth stage is pain behavior (e.g., grimacing, withdrawal, etc). The third stage, suffering or secondary affective response, is not highly applicable to laboratory pain, because participants may voluntarily terminate exposure to the pain stimulus. Thus the current laboratory study assessed laboratory pain response across three dimensions: sensory, affect, and pain behavior. These three dimensions are measured by pain intensity (i.e., how much pain is experienced by the individual), pain unpleasantness (i.e., how much a person is bothered by pain), and pain tolerance behavior (i.e., how long a person endures pain), respectively. Although these dimensions are associated, it is important to assess all three dimensions, as brain imaging studies suggest a segregation of function and underlining neuronal pathways between pain affect and sensation (Rainville, Duncan, Price, Carrier, & Bushnell, 1997).
In the current study, we tested the hypotheses that the three minority groups (African Americans, Asians, and Hispanics) would exhibit more pain intensity and unpleasantness, as well as less pain tolerance, compared with Caucasians. How these three minority groups differed from each other on laboratory pain responses would also be explored. It has been shown that laboratory pain responses in children are associated with anticipatory anxiety, which has been conceptualized as an index of the perceived threat of impending pain (Tsao et al., 2006). Thus, anticipatory anxiety is a specific, proximal measure of the perceived threat value of pain that is related to, but partially distinct from more distal trait constructs associated with anxiety (e.g., negative affect) that may also influence pain responses (Tsao et al., 2004). Anxiety is also an indicator of autonomic arousal with important links to pain perception and modulation (Price, 2002). Autonomic arousal during pain anticipation may activate brain regions involved in ‘priming’ pain sensitivity and thus may influence processes related to ethnic differences in pain response in children. Therefore, we would also test whether anticipatory anxiety may account for ethnic differences in pain responses.
Methods
Participants
The current sample was drawn from a larger sample of 244 children who participated in a study on the effects of gender and puberty on laboratory pain responses described previously (Lu et al., 2005). The larger sample was composed of 98 Caucasians, 58 Hispanics, 34 African-Americans, 24 Asians, and 30 other (mixed ethnicities). The final sample for this paper excludes the “other” category, and includes 214 participants with a mean age of 12.7 years (SD=3.0, range 8 to 18 years). Demographic characteristics of participants are presented in Table 1. Power analysis revealed a power of 87% to detect a moderate effect size of group differences in pain responses.
Table 1.
Demographic Characteristics of the Four Ethnic Groups
| Variable | African- American (N=34) |
Hispanic (N=58) |
Caucasian (N=98) |
Asian (N=24) |
Total sample (N=214) |
P values |
|---|---|---|---|---|---|---|
| Age (Mean, SD) | 14(3.2) | 13.1(3) | 12.5(2.9) | 12.6(2.8) | 12.7(3) | >0.05 |
| SES (Mean, SD)1 | 41.5(7.4) | 38.3 (14.1) | 55.6(8.2) | 54.4(7.5) | 48.7(12.3) | <0.001 |
| Sex (%male) | 47.1 | 58.6 | 51 | 37.5 | 49.2 | >0.05 |
| Experimenter (%) | >0.05 | |||||
| Asian | 51.5 | 48.2 | 49.5 | 59.1 | 50.5 | |
| Hispanic | 33.3 | 35.7 | 28.4 | 22.7 | 30.6 | |
| Caucasian | 3 | 3.6 | 3.2 | 4.5 | 3.4 | |
| Mixed | 12.1 | 12.5 | 18.9 | 13.6 | 15.5 |
Note: Abbreviation: SES, socioeconomic status. SES was obtained with Hollingsheads score, with lower scores indicative of lower SES.
Participants aged from 8 to 18 years were recruited from the Los Angeles metropolitan area through mass mailing, posted advertisements, and classroom presentations. The wide age range in the present sample was designed to include participants at all stages of pubertal development. We made every effort to increase the sample representativeness by recruiting participants from communities. The mailings and advertisements were widely targeted across sites with varying ethnicities and income levels, because one of the goals of recruitment was to enhance the enrollment of children from low-income and minority neighborhoods. The racial and ethnic composition of the study was similar to the LA County racial/ethnic composition in 2000 when the study was conducted. Potential participants and their parents were told that the study was designed to learn more about how healthy children and adolescents responded to pain. Eligibility was confirmed by telephone. Individuals reporting an ongoing acute or chronic illness, or use of potentially pain-altering prescription medications (e.g. opioids, anticonvulsants, tricyclic antidepressants, or SSNRI’s) that might affect study measures were excluded. Parents and children signed consent and assent forms, respectively. Participants received a $30 video store gift certificate and a T-shirt for their participation. The University of California, Los Angeles (UCLA) Institutional Review Board (IRB) as well as the IRBs for recruitment sites approved all study procedures.
Procedure and Laboratory Tasks
On the day of the laboratory session an experimenter greeted participants and their parents, and escorted them into separate rooms where there was no contact between them until the session was completed. The parents completed demographic information including the mother’s and father’s occupation and education, child’s ethnicity, age, and sex. Pain tasks were then administered to children. Participants were informed that the tasks may “cause discomfort”. Participants were instructed to continue with the task for as long as they could and to remove their finger or arm from the apparatus at any time during the procedures if it became too uncomfortable or painful. All tasks were extensively piloted on volunteers in the targeted age range to determine the lowest level of stimulation that would allow sufficient variation in response.
Pressure task
The Ugo Basile Analgesy-Meter 37215 (Ugo Basile Biological Research Apparatus, Comerio, Italy) was used to administer focal pressure through a Lucite point approximately 1.5 mm in diameter to the second dorsal phalanx of the middle and index finger of each hand. Four trials at two levels of pressure (two at 322.5 g and two at 465 g) were completed with an uninformed ceiling of 3 minutes. A comparable device has been used in healthy and clinical pediatric samples (aged 5-17 years) without adverse effects (Gil et al., 1997).
Thermal heat task
The Ugo Basile 7360 Unit (Ugo Basile Biological Research Apparatus) was used to administer a total of four trials of two infrared stimulus intensities (15, 20) of radiant heat 2 inches proximal to the wrist and 3 inches distal to the elbow on both volar forearms with an uninformed ceiling of 20 seconds (s). Thermal pain tolerance was electronically measured with an accuracy of 0.1 s. A similar task has been used in a sample aged 6-17 years without adverse effects (Meier, Berde, DiCanzio, Zurakowski, & Sethna, 2001).
Measures
Pain ratings and anticipatory anxiety
Pain intensity, pain unpleasantness, and anticipatory anxiety were measured by a vertical sliding visual analogue scale (VAS). The 10 cm VAS is anchored on the bottom with 0 indicating the lowest value and 10 at the top indicating the greatest value. The scale also had color cues, graded from white at the bottom to dark red at the top, as well as a neutral face at the bottom and a negative facial expression at the top. The VAS is brief, easily understood, and sensitive to changes in pain. Pain sensation intensity and pain unpleasantness visual analog scales have been validated among adults and were shown to measure the two constructs separately (Wade, et al., 1996). Previous research has used the VAS for quick and accurate laboratory-induced pain ratings in children (Miller, Barr, & Young, 1994). The VAS has excellent psychometric properties; patient, parent, and physician ratings of pain intensity using the VAS have been found to be positively correlated with each other and with measures of disease activity (Gragg et al., 1996). The VAS can be used by children in the age range included in the current study (8-18 years of age) (McGrath & Gillespie, 2001)
Before the trial, participants were instructed on the use of the VAS and gave three practice ratings. The practice trials asked: (1) “How afraid or nervous would you be right before taking an important exam or test?” (2) “How much would it bother you to eat your favorite dessert?” (3) “How afraid, nervous, or worried do you feel right now?” The instructions and practice trials were repeated until participants fully understood the VAS.
Using the VAS, participants answered the following questions. For Anticipatory Anxiety, immediately prior to each trial participants were asked “how nervous, afraid, or worried” they were about the upcoming pain task. For Pain Intensity, immediately after each trial, participants were asked, “At its worst, how much pain did you feel during the task?” For Pain Unpleasantness, immediately after each trial and after rating the pain intensity, participants were also asked, “At its worst, how much did the task bother you?” The scores for anticipatory anxiety, pain intensity, and pain unpleasantness during heat or pressure pain task were computed by averaging the four trials of each task. The internal reliabilities for all the measures were good (Cronbach’s Alpha=0.928, 0.939, and 0.937, for anticipatory anxiety, pain intensity, and pain unpleasantness for heat task, and Cronbach’s Alpha=0.936, 0.942, and 0.939 for anticipatory anxiety, pain intensity, and pain unpleasantness for pressure task).
Pain tolerance
Pain tolerance is defined as the highest level of noxious stimulation subjects can endure (Gelfand, 1964). In this study, tolerance was determined as the time in seconds elapsed from the onset of the heat or pressure pain stimulus to the participant’s withdrawal from the stimulus. Tolerance for heat or pressure was computed by averaging the endurance time of the four trials of each task. The internal reliabilities for both heat and pressure tasks were good (Cronbach’s Alpha =0.887, 0.885, respectively).
Ethnicity and Socioeconomic status (SES)
Parents identified child’s ethnicity as belonging to one of the following five different ethnic categories (‘African-American,’ ‘Asian,’ ‘Caucasian,’ ‘Hispanic,’ and ‘other’). This parent categorization was used as a proxy measure of child’s ethnicity. As previously mentioned, children categorized as belonging to the “other” (mixed ethnicity) category were excluded from the analysis. SES was indicated by the Hollingshead score (Hollingshead, 1975), a composite score of parents’ education and occupation. Computed scores ranged from a high of 66 to a low of 6, with higher numbers indicating higher SES.
Statistical Analysis Plan
Preliminary analyses were conducted to examine assumptions for statistical analysis, outliers, and the distribution of variables. Pressure tolerance was not normally distributed, and logarithm transformation was used to achieve normal distribution for further analysis. Levene’s Test of Equality of Error Variances and Brown & Forsythe’s test of homogeneity of variance which is more robust for unequal group sizes suggested no violation of the equal variance assumption. Box’s test of equality of covariance matrices suggested the observed covariance matrices of the dependent variables were equal across groups. Demographic characteristics of participants (age, sex, and socioeconomic status) and experimenters’ ethnicity were compared among the four ethnic groups with chi-square tests for categorical variables and Analysis of Variance (ANOVA) for continuous variables.
Planned comparisons were conducted using MANCOVA to test the differences between the three minority groups and Caucasians, with ethnicity as the independent variable; pain responses as dependent variables, and age and sex as covariates (alpha level was set to 0.05 for two-tailed tests). Three sets of analyses were run with pain intensity, unpleasantness, and tolerance as dependent variables. We also explored how the three minority groups differed from each other on laboratory pain responses in post hoc analysis. Bonferroni-Holm step down test (Holm, 1979) was used to reduce the likelihood of Type I error associated with post hoc multiple comparisons1.
To further investigate whether ethnic differences in pain were independent of SES, a second set of analyses were conducted with SES as an additional covariate. We also compared group differences in anticipatory anxiety and tested ethnic differences in pain responses after controlling for anticipatory anxiety. Furthermore, the potential effect of experimenter’s ethnicity and the interaction effect between participants’ and experimenters’ ethnicities were examined. Finally, potential interaction effects between ethnicity and sex of participants, and ethnicity and age of participants were examined. Effect sizes (ES) were estimated using partial eta squared and were judged using Cohen’s criteria (Cohen, 1988) for small, medium, and large effects with 0.01, 0.059, and 0.138, respectively (corresponding to Cohen’s d = 0.2, 0.5, 0.8).
Results
Preliminary Analyses
As shown in Table 1, the four ethnic groups did not differ in age or sex (p>0.05), but sex differences emerged for pressure pain tolerance such that boys exhibited greater pressure tolerance than girls (p<0.05). In addition, bivariate correlations revealed that age was positively associated with pressure and heat tolerance, and negatively associated with pain intensity, unpleasantness, and anticipatory anxiety for both pressure and heat tasks (ps<0.05). Thus, sex and age were used as covariates in all analyses. ANOVA revealed that the four groups differed significantly (p<0.001) on SES. Specifically, Hispanics and African- Americans had lower SES compared with Caucasians and Asians (p <0.001). Furthermore, SES was positively associated with heat intensity, pressure unpleasantness, and heat unpleasantness (p<0.05). Therefore, SES was included as an additional covariate; results with and without SES as a covariate were reported, so that the current results may be easily compared with studies which have largely not controlled for SES.
Ethnic Differences in Pain
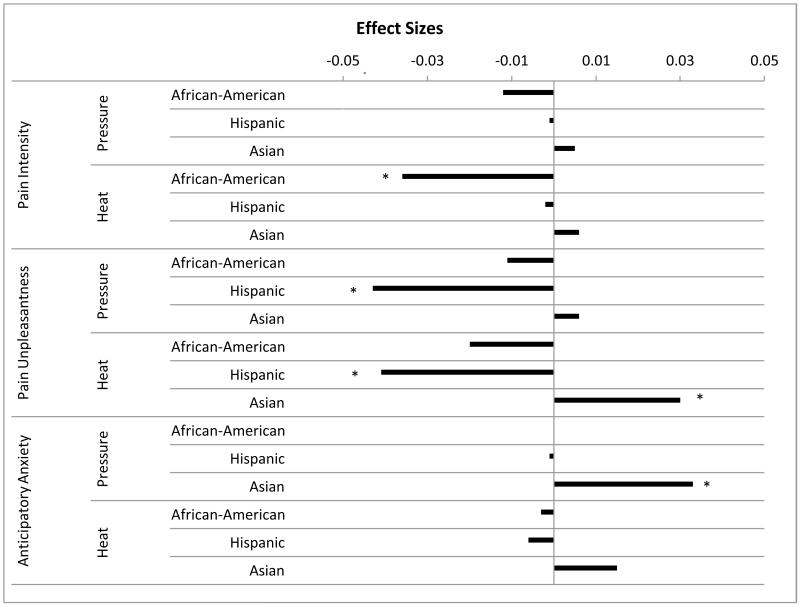
Table 2 presents descriptive statistics of pain responses. Tables 3 shows the results of planned comparisons of group differences in pain responses and post hoc analysis controlling for sex and age. Asians reported more heat pain unpleasantness than did Caucasians (p=0.013), Hispanics (p<0.001), and African Americans (p=0.01). Asians also reported significantly more pressure pain unpleasantness compared with Hispanics (p=0.007), and greater heat pain intensity compared with African Americans (p=0.002). Hispanics reported less unpleasantness for both pressure and heat pain than did Caucasians (p=0.01 and 0.008 respectively). African-Americans reported less heat pain intensity than did Caucasians (p=0.006). No differences emerged for pressure or heat pain tolerance. Ethnic differences in pain responses remained significant, even after controlling for SES. Overall, calculation of effect sizes revealed small to medium effect sizes in group differences (Figure 1), suggesting higher pain sensitivity among Asians and lower pain sensitivity among African-Americans and Hispanics, compared with that among Caucasians.
Table 2.
Descriptive Statistics for Pain Responses
| Pain Measure | African-American | Hispanic | Caucasian | Asian | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | N | Mean | (SD) | |
| Intensity | |||||||||||||||
| Pressure | 33 | 3.89 | 2.67 | 56 | 4.63 | 2.36 | 96 | 4.99 | 2.71 | 24 | 5.60 | 2.62 | 209 | 4.79 | 2.63 |
| Heat | 33 | 3.39 | 2.65 | 57 | 4.73 | 2.87 | 96 | 5.19 | 2.71 | 24 | 5.83 | 2.44 | 210 | 4.86 | 2.79 |
| Unpleasantness | |||||||||||||||
| Pressure | 30 | 2.98 | 2.74 | 45 | 2.50 | 2.07 | 72 | 3.85 | 2.70 | 16 | 4.63 | 2.71 | 163 | 3.39 | 2.62 |
| Heat | 30 | 2.66 | 2.45 | 46 | 2.43 | 2.22 | 72 | 3.76 | 2.65 | 16 | 5.35 | 2.51 | 164 | 3.34 | 2.62 |
| Tolerance (seconds) | |||||||||||||||
| Pressure | 33 | 38.96 | 38.44 | 56 | 35.80 | 40.12 | 96 | 46.40 | 49.35 | 24 | 43.52 | 59.22 | 209 | 42.05 | 46.63 |
| Heat | 33 | 11.63 | 4.73 | 57 | 10.77 | 4.58 | 96 | 11.17 | 5.48 | 24 | 9.78 | 5.82 | 210 | 10.97 | 5.16 |
| Anticipatory Anxiety | |||||||||||||||
| Pressure | 33 | 2.74 | 2.69 | 56 | 2.80 | 2.15 | 96 | 3.11 | 2.47 | 24 | 4.52 | 2.62 | 209 | 3.13 | 2.48 |
| Heat | 33 | 3.19 | 2.68 | 57 | 3.30 | 2.45 | 96 | 3.99 | 2.85 | 24 | 4.95 | 2.60 | 210 | 3.79 | 2.73 |
Note: Unpleasantness VAS was not administered until a number of participants (N=36) had completed the study and thus, the n for the pain unpleasantness ratings was smaller than the other measures.
Table 3.
Planned Comparison on Pain Response: [Estimated Marginal Means and Standard Error (SE)]
| Dependent Variables |
African- American Mean |
Hispanic Mean |
Caucasian Mean |
Asian Mean |
Ethnic differences |
Controlling for anticipatory Anxiety |
|---|---|---|---|---|---|---|
| Intensity | ||||||
| Pressure pain | ||||||
| Mean | 4.10 | 4.67 | 4.92 | 5.67 | AA<C | |
| SE | 0.45 | 0.34 | 0.26 | 0.54 | ||
| Heat pain* | ||||||
| Mean | 3.61a | 4.79a,b | 5.11b | 5.92b | AA<C=A | AA<C=H |
| SE | 0.46 | 0.36 | 0.27 | 0.56 | ||
| Unpleasantness | ||||||
| Pressure pain* | ||||||
| Mean | 3.07a,b | 2.53a | 3.82b | 4.64b | H<C=A | H<C |
| SE | 0.47 | 0.39 | 0.30 | 0.67 | AA<C | |
| Heat pain* | ||||||
| Mean | 2.76a,b | 2.43a | 3.72b | 5.51c | H <C<A | H<C=A |
| SE | 0.46 | 0.38 | 0.29 | 0.65 | AA<A | AA<C=A |
| Tolerance | ||||||
| Pressure pain | ||||||
| Mean | 1.33 | 1.30 | 1.44 | 1.30 | H<C+ | |
| SE | 0.08 | 0.06 | 0.04 | 0.09 | ||
| Heat pain | ||||||
| Mean | 10.69 | 10.34 | 11.49 | 9.95 | H<C | |
| SE | 0.77 | 0.59 | 0.45 | 0.92 |
a,b,c Notate similarities and differences between group means. Superscripts with the different letter are statistically different. - - Notate p<0.05.
+ p=0.067 the Covariates are age and sex.
AA= African-American, H= Hispanics, C=Caucasian, A=Asian
Figure 1.
Effect Sizes (Partial Eta Squared) Associated with Ethnic Differences in Laboratory Pain Responses
Note: * indicate significant differences between the minority group and the Caucasian group. Effect sizes are indicated by partial eta squared. Effect size in the negative direction indicates a lower value in the pain variables for the minority group compared with Caucasians. For example, for heat intensity, the negative direction of the effect size associated with the bar of African-American indicates that African-American reported lower pain intensity.
Similar group differences also emerged in anticipatory anxiety. Asians reported higher anticipatory anxiety for pressure pain than did Caucasians (p=0.0092), Hispanics (p=0.006), and African Americans (p=0.024) (see Table 4). Asians also reported higher anticipatory anxiety for heat pain (p=0.015) compared with Hispanics. After anticipatory anxiety was accounted for, Asians still had significantly greater heat pain unpleasantness than did Hispanics (p=0.025) and African Americans (p=0.026), but the other differences in pain responses between Asians and other groups disappeared. To examine the possibility that anticipatory anxiety might mediate group differences in pain, we followed up with Sobel tests (Sobel, 1982). Sobel tests revealed that anticipatory anxiety fully mediated the difference in pressure pain unpleasantness between Asian and Hispanics (z=2.79, p=0.005), and in heat pain intensity between Asians and African Americans (z=1.98, p=0.05). Anticipatory anxiety also partially mediated the difference in heat pain unpleasantness between Asians and Hispanics (z=2.52, p=0.01), and between Asians and African Americans (z=1.97, p=0.05). However, Sobel test did not suggest that anxiety mediated the difference in heat pain unpleasantness between Asians and Caucasians (z= 1.59, p=0.11).
Table 4.
Planned Comparisons on Anticipatory Anxiety [Estimated Marginal Means and Standard Errors (SE)]
| Dependent Variables |
African- American mean |
Hispanic Mean |
Caucasian mean |
Asian mean |
Ethnic differences |
|---|---|---|---|---|---|
| Anticipatory anxiety | |||||
| Pressure pain | |||||
| Mean | 2.99a | 2.83a | 3.02a | 4.48b | H=AA=C<A |
| SE | 0.42 | 0.32 | 0.24 | 0.50 | |
| Heat pain | |||||
| Mean | 3.42a,b | 3.35a | 3.86a,b | 4.97b | H<A |
| SE | 0.46 | 0.36 | 0.27 | 0.55 |
Note:a,b,c Notate similarities and differences between group means. Superscripts with the same letter are statistically equivalent. - - Notate p>0.05.
Covariates are age and sex.
After anticipatory anxiety was accounted for, the group differences between African Americans and Caucasians become more pronounced and consistent across the two pain modalities. Specifically, African Americans had less pain intensity (p=0.03 for heat, and 0.044 for pressure) and less pain unpleasantness compared with Caucasians (p=0.044 for heat, and 0.036 for pressure). Similarly, after anticipatory anxiety was controlled for, the differences between Hispanics and Caucasians in pain unpleasantness remained; new results also emerged, such that Hispanics exhibited significantly less pressure pain tolerance (p=0.038) and marginally less heat pain tolerance (p=0.067) compared with Caucasians. Table 3 also summarizes group differences in pain responses after anticipatory anxiety was controlled for.
In order to rule out the possibility that pain responses across ethnic groups may have differed based on the ethnicity of experimenters, additional MANCOVAs were conducted to examine the influence of experimenters and potential interaction effects between the ethnicity of participants and the ethnicity of experimenters. Across the laboratory sessions, 50.5% were run by Asian experimenters, 30.6 % were run by Hispanics experimenters, 3.4% were run by Caucasian experimenters, and 15.5% were run by experimenters with mixed ethnicity. Chi-square tests revealed that the ethnicity of the experimenters was equally distributed across the four ethnic groups of participants. Due to the small number of sessions run by Caucasian experimenters and difficulties in interpretation for the mixed ethnicity experimenters, the analyses were conducted only among the sessions run by Asian and Hispanic experimenters. No significant main effect of the ethnicity of experimenters or the interaction between ethnicity of participants and ethnicity of experimenters emerged. Furthermore, we also explored the potential interaction effect of participants’ ethnicity and age, and participant’s ethnicity and sex on pain responses, and we did not find significant interaction effects.
Discussion
Circumventing many potential confounders in clinical pain studies, laboratory pain investigations control for parameters of noxious stimuli, and thus have particular utility in illuminating ethnic disparities in pain (Green, et al., 2003). As the first attempt to investigate ethnic differences in laboratory pain responses in a multi-ethnic sample of children, we clearly demonstrated differences between ethnic groups. However, many of the differences that emerged contradicted our priori hypotheses. The hypothesis that ethnic minorities had higher pain sensitivities compared with Caucasians was only supported in Asians, and the opposite was found for Hispanics and African Americans. Asians had greater pain sensitivity compared with Caucasians, who had greater pain sensitivity compared with African Americans and Hispanics. The results hold even after controlling for age, sex, and SES. By examining laboratory pain responses to multiple pain modalities in children representing multiple ethnicities, an understudied sample, this study contributes to the literature in several important ways.
First, Asians and Hispanics are understudied in the literature, as previous laboratory pain studies largely focused on the comparison between African-Americans and Caucasians (Rahim-Williams, Riley, Williams, & Fillingim, 2012). By including Asians and Hispanics, this study revealed important ethnic differences that have not been previously reported. Asians experienced heightened pain sensitivity compared with the other three groups. They reported greater heat pain unpleasantness compared with Caucasians, Hispanics, and African Americans. They also reported greater pressure pain unpleasantness compared with Hispanics, and more heat pain intensity compared with African-Americans. Although previous studies have not compared Asians with other minorities, studies that compared Asians with Caucasians were in line with our findings. For example, one adult study in Europe found that south Asian immigrants had lower heat pain threshold compared with British Whites (Watson, Latif, & Rowbotham, 2005). A recent study found that Asians had lower cold pain threshold and tolerance compared with non-Hispanic Whites (Rowell, Mechlin, Ji, Addamo, & Girdler, 2011). Another study found a greater cortisol response following routine inoculation among Japanese infants compared with Caucasian-American infants (Lewis, et al., 1993). The greater cortisol responses suggested greater pain sensitivity among Asians, as greater cortisol response has been associated with higher pain sensitivity among children (Allen, Lu, Tsao, Worthman, & Zeltzer, 2009). Together, these studies highlight increased pain sensitivity among Asians compared with Caucasians.
Despite greater pain ratings among Asians, they did not differ in pain tolerance compared with other groups. Empirical evidence suggests that despite their desire to express, Asian adults showed less behavioral expression of emotion than did Caucasians (Lu & Stanton, 2010). Similarly, Japanese infants showed less behavioral expression of pain than did Caucasian-American infants during and following routine inoculation, despite their greater cortisol stress response (Lewis, et al., 1993). Therefore, the heightened pain sensitivity among Asians may be manifested in pain threshold or pain ratings, but not in observable behaviors.
We also found that Asian children experienced higher pain anticipatory anxiety compared with the other groups. Anticipatory anxiety is conceptualized as a specific, proximal index of the perceived threat of imminent pain which is partially distinct from more distal, trait measures associated with anxiety such as negative affect (Tsao, et al., 2004). Anticipatory anxiety is also an indication of arousal (Price, 2002), which may increase the threat value of pain, and thus increase its perceived unpleasantness. Although anticipatory anxiety did not account for differences in pain responses between Asians and Caucasians, it explained some of the ethnic differences in pain intensity and unpleasantness between Asians and the other minority groups (Hispanics and African Americans). These findings suggest that anticipatory anxiety may play a large role in influencing pain responses among Asian children. Few studies have examined ethnic differences in anticipatory anxiety among Children, but previous studies have found that Asians living in North America experienced higher social anxiety than Caucasians(Hsu et al., 2012), which may be due to fear of losing face (Lau, Fung, Wang, & Kang, 2009) and lower self-efficacy and social status (Hsu, et al., 2012). Asian children may develop more fear of pain or injury compared with other children as Asian parents tend to be protective and shelter their children from pain, injury, or challenging situations. Such speculation requires further study.
In contrast to the results for Asians, Hispanics reported lower heat and pressure pain unpleasantness than Caucasians, and these results held even after anticipatory anxiety was controlled for. Moreover, after controlling for anticipatory anxiety, Hispanics also exhibited significantly lower heat and marginally lower pressure pain tolerance compared with Caucasians. These results were partially consistent with a previous study among adults which found lower heat pain tolerance but not pressure pain tolerance among Hispanics compared with Caucasians; the previous study did not report differences for heat or pressure pain unpleasantness (Rahim-Williams, et al., 2007). Therefore, our study provides new findings to fill a knowledge gap about pain unpleasantness among Hispanics. Perhaps Hispanics appraise these pain tasks to be less intrusive; this possibility is worth investigating in future studies. Our results show that pain unpleasantness and tolerance, as they respectively represent affective and behavioral dimensions of pain, may diverge in their pattern of responses across different ethnic groups. As discussed in more detail below, the various dimensions of pain do not always show a consistent pattern since pain perception and unpleasantness are mediated by distinct brain regions (Rainville, et al., 1997). It is conceivable that the development of central pain processing differs in children of various ethnic groups, and that such processes may be modulated by higher order factors such as acculturation and cultural expectations. These factors might have different influences on the affective and behavioral dimensions of pain and therefore the results of the two dimensions diverged. Such possibilities are speculative however, and require additional research.
Second, our findings suggest the importance of studying children and using multiple pain modalities. Previous studies in adult samples demonstrated elevated pain intensity among African-Americans relative to Caucasians (Campbell, et al., 2005; Sheffield, et al., 2000). On the contrary, the current study revealed lower heat pain intensity among African-Americans compared with Caucasians. Our sample consisted of children ranging in age from 8 to 18 years, whereas the previous studies mainly had samples of adults (Campbell, et al., 2005; Sheffield, et al., 2000). Even after anticipatory anxiety was controlled for, the differences between African Americans and Caucasians remained and became even more pronounced and consistent across pain domains and tasks, such that African Americans had lower pain intensity and unpleasantness for both heat and pressure pain tasks compared with Caucasians. Thus, the differences between African Americans and Caucasians were consistent in both affective and sensory domains across both pain modalities.
One explanation for the different findings between our child sample and adult samples is that psychosocial factors that lead to ethnic differences in pain may weigh or even function differently for children and adults. For example, it has been shown that ignoring pain and diverting attention were associated with less pain among African American adults (R. R. Edwards, Moric, Husfeldt, Buvanendran, & Ivankovich, 2005; Jordan, Lumley, & Leisen, 1998); however, diverting attention was associated with more pain and attentive coping was associated with less pain among African American children compared with Caucasian children (Evans, Lu, Tsao, & Zeltzer, 2008). It is also possible that African American adults may experience prolonged exposure to high levels of stress associated with racism and discrimination, and thus experience more adverse health effects compared with children. Other minority groups also face stresses linked with racism and discrimination but prior comparative work with adults and children has not been done in these other groups, and thus it is unknown whether such statements are also applicable to these groups. Future studies that directly compare children and adults within a developmental perspective will help to give a fuller picture of ethnic disparities in pain.
It should also be noted that another difference between the current study and the majority of existing studies among adults is the type of heat pain stimulus used. The current study used radiant heat stimulus that emitted infra-red light to skin. In contrast, the majority of adult studies used contact heat stimulus that directly contacted skin (Campbell, et al., 2005; Rahim-Williams, et al., 2007; Sheffield, et al., 2000), with the exception of one earlier study (Chapman & Jones, 1944). A recent animal study showed that skin with more pigmentation had less pain reaction to infra-red heat (Wen, Ansonoff, & Pintar, 2009). Darker skin with higher pigmentation density may allow less heat to penetrate skin and thus reduce the pain sensation for radiant heat. Future research that use both radiant and contact heat stimuli in one study will help to solve the puzzle.
Third, as mentioned above, our findings suggest important ethnic differences in the affective and sensory dimensions of pain reactions among multiple ethnic groups. The greatest group difference emerged in the affective dimension of pain. Psychophysical studies have revealed that pain sensation and pain unpleasantness are two distinct dimensions of pain that demonstrate reliably different relationships to nociceptive stimulus intensity, and are separately influenced by various psychological factors (Price, 2002). For example, Rainville and colleagues reported that hypnosis selectively altered the unpleasantness of noxious stimuli without changing the perceived intensity (Rainville, et al., 1997). Brain imaging also suggests a segregation of function and underlining neuronal pathways between pain affect and sensation, with the anterior cingulate cortex (ACC) activity reflecting the emotional experience that provokes our reactions to pain, and primary and secondary somatosensory cortices (SI and SII) for the processing of the sensory-discriminative aspects of pain (Rainville, et al., 1997). The separation of pain dimensions is also implied by the substantial variation in group differences across different pain measures in laboratory pain studies among adults (Rahim-Williams, et al., 2012). The current study revealed the greatest ethnic differences in the affective dimension of pain without controlling for anticipatory anxiety; patterns of ethnic differences in both affective and sensory dimensions of pain became more consistent after controlling for anticipatory anxiety. There results provide further evidence on the segregation of the two dimensions of pain, as well as the differential influence of anxiety on ethnic differences in pain unpleasantness and intensity.
This study has important clinical implications. Ethnic differences in pain intensity and unpleasantness revealed from this study represent more than minimal clinically significant differences in children, defined as 10mm on a 0-100 mm VAS pain scale (i.e. equivalent to 1 unit difference in the VAS pain score in the current study), which is considered a noticeable difference by most children and adults (Powell, Kelly, & Williams, 2001). Pain intensity ratings in the current study were higher than pediatric patients with chronic pain (Gragg, et al., 1996). The present findings might be particularly relevant for clinicians who manage acute pain in children from diverse ethnic backgrounds. For example, our findings suggest that anxiety in anticipation of pain may differ depending on children’s racial/ethnic background. Since anticipatory anxiety appears to play a more prominent role for Asian children compared with the other groups, efforts to calm such fears may be particularly important for these children prior to undergoing pain stimulus, such as that experienced during injections and other painful medical procedures.
The current findings may also have additional clinical implications as anxiety is often associated with clinical pain symptoms (McWilliams, Cox, & Enns, 2003). According to the fear-avoidance model of pain (Asmundson, Norton, & Norton, 1999), fear of activities or situations that have been associated with an exacerbation of pain in the past (e.g., physical exertion) may lead to the avoidance of such activities which leads to further de-conditioning, increased pain, and so forth. It may be that children of certain ethnic backgrounds experience higher levels of anxiety in anticipation of feared situations/activities, which leads to the exacerbation and maintenance of clinical pain. Given the artificial nature of the experimental procedures, caution should be exercised when generalizing the findings to clinical pain. However, several studies have demonstrated that laboratory pain responses are predictive of clinical and chronic pain (Clauw et al., 1999; Fillingim, Maixner, Kincaid, Sigurdsson, & Harris, 1996). As such, the current findings may inform healthcare providers to be aware of possible sources of underestimating pain among certain groups, a contributing factor to ethnic disparities in pain treatment (Green, et al., 2003). For example, Asians may not exhibit pain behaviors even if they experience a large amount of pain. Under circumstances that subjective pain experience cannot be reported due to an emergency situation or language barrier, clinical judgment based on behavioral aspects of pain should take into consideration patients’ cultural background. A better understanding of patients’ cultural background may facilitate a more accurate evaluation of patients’ subjective pain experience based on their behavior. Whenever possible, clinicians who work with pain patients should evaluate behavioral, sensory, and affective components of pain experience.
Several caveats are worth mentioning. Our findings are not consistent with a previous study (Widmalm SE, et al., 1995) which found that African-American children experienced more oral/facial pain symptoms than Caucasian children; thus the current study may have limited generalizability to chronic pain. However, the age range of the prior sample (4-6 years) was younger than that of the current sample. The larger study from which the current participants were drawn encompassed a wide age range in order to include participants at all stages of pubertal development. On one hand, this broad age range increases the generalization of the present findings to both children and adolescents. On the other hand, it is possible that racial/ethnic differences in pain responses may vary by age and/or pubertal development. Our sample was not large enough to detect possible interaction effects of participants’ ethnicity with their age or sex. There is little research on ethnic differences in pain responses from a developmental perspective and further work should be conducted on this topic. Second, although our sample was limited to English-speaking individuals, it is reasonable to infer that ethnic differences revealed in the study might be even more evident in non-English speaking samples in the US. In addition, cell sizes were not large for some ethnic groups; however, the cell sizes were similar to earlier studies among adults and the patterns of ethnic differences were consistent across pain modalities, even after controlling for age, sex, SES, and the interaction of experimenter’s ethnicity and participants’ ethnicity. Furthermore, although no evidence emerged for experimenter effects, we did not have enough Caucasian and African-American experimenters to test their influences. Future studies should try to reduce experimenter effect by matching experimenters and participants’ ethnicities. Finally, the study was designed to describe ethnic differences in a laboratory pain setting rather than examining potential mechanisms explaining such differences. Although anxiety accounted for some ethnic differences between Asians and other minorities, mediation test was conducted in a post hoc. Future studies are warranted to elucidate how cultural, psychosocial, physiological, and genetic factors influence ethnic differences in response to laboratory-induced pain among children. Attempting to decipher precise mechanisms and quantifying the extent to which such mechanisms mediate ethnic differences in pain will be a difficult but important task.
Despite these limitations, the current study has revealed consistent ethnic differences in pain responses across multiple laboratory pain modalities in a multi-ethnic sample of children. Overall, Asians had greater pain sensitivity compared with Caucasians, who had greater pain sensitivity compared with African Americans and Hispanics. The patterns of ethnic differences in pain were largely consistent in all three pain response indices across pressure and heat pain tasks, particularly after anticipatory anxiety was controlled for. A variety of factors embraced by the biopsychosocial model may contribute to ethnic differences in pain, such as cultural beliefs, the meaning of pain, pain expression, coping with pain, genetic factors, physiological reactions, and the interaction of these systems. Future studies are needed to replicate the findings and to better understand under what conditions and why ethnic differences emerge by utilizing multiple pain modalities, multiple-ethnic samples, and a life-span perspective within the biopsychosocial model of pain.
Footnotes
Critical cut-off values of α were calculated using this formula, α = 0.05/k, and k = n, n-1, n-2, n-3…, 1 (n = the total number of post hoc multiple comparisons). The p values were ordered for all comparisons from lowest to highest, and then compared these p values with corresponding critical values of α according to the Bonferroni-Holm step down test. In the case of comparisons among three minority groups, critical alphas were set to be 0.017, 0.025, and 0.05 (as k = 3, 2, 1, and 3 is the total number of post hoc comparisons). If the p value was less than the critical value, then null-hypothesis would be rejected and the next p value would be compared to the next critical value. If the p value was larger than the critical value, the null-hypothesis would not be rejected, and the comparisons would be stopped.
Contributor Information
Qian Lu, University of Houston.
Lonnie Zeltzer, University of California, Los Angeles.
Jennie Tsao, University of California, Los Angeles.
References
- Allen LB, Lu Q, Tsao JCI, Worthman CM, Zeltzer LK. Sex differences in the association between cortisol concentrations and laboratory pain responses in healthy children. Gender Medicine. 2009;6(Part 2):193. doi: 10.1016/j.genm.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asmundson GJG, Norton PJ, Norton GR. Beyond pain: The role of fear and avoidance in chronicity. Clinical Psychology Review. 1999;19(1):97–119. doi: 10.1016/s0272-7358(98)00034-8. doi: 10.1016/s0272-7358(98)00034-8. [DOI] [PubMed] [Google Scholar]
- Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113(1-2):20. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Campbell CM, France CR, Robinson ME, Logan HL, Geffken GR, Fillingim RB. Ethnic differences in the nociceptive flexion reflex (NFR) Pain. 2008;134(1-2):91–96. doi: 10.1016/j.pain.2007.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman WP, Jones CM. Variations In Cutaneous And Visceral Pain Sensitivity In Normal Subjects. J Clin Invest. 1944;23(1):81–91. doi: 10.1172/JCI101475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauw DJ, Williams D, Lauerman W, Dahlman M, Aslami A, Nachemson AL, Wiesel SW. Pain Sensitivity as a Correlate of Clinical Status in Individuals With Chronic Low Back Pain. Spine. 1999;24(19):2035. doi: 10.1097/00007632-199910010-00013. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. Erlbaum; Hillsdale, NJ: 1988. [Google Scholar]
- Davis DA, Luecken LJ, Zautra AJ. Are Reports of Childhood Abuse Related to the Experience of Chronic Pain in Adulthood?: A Meta-analytic Review of the Literature. The Clinical Journal of Pain. 2005;21(5):398–405. doi: 10.1097/01.ajp.0000149795.08746.31. [DOI] [PubMed] [Google Scholar]
- Edwards CL, Fillingim RB, Keefe F. Race, ethnicity and pain. Pain. 2001;94(2):133. doi: 10.1016/S0304-3959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic Differences in Pain Tolerance: Clinical Implications in a Chronic Pain Population. Psychosom Med. 2001;63(2):316–323. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Fillingim RB. Ethnic Differences in Thermal Pain Responses. Psychosom Med. 1999;61(3):346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- Edwards RR, Moric M, Husfeldt B, Buvanendran A, Ivankovich O. Ethnic Similarities and Differences in the Chronic Pain Experience: A Comparison of African American, Hispanic, and White Patients. Pain Medicine. 2005;6(1):88–98. doi: 10.1111/j.1526-4637.2005.05007.x. doi: doi:10.1111/j.1526-4637.2005.05007.x. [DOI] [PubMed] [Google Scholar]
- Evans S, Lu Q, Tsao JCI, Zeltzer LK. The role of coping and race in healthy children’s experimental pain responses. Journal of Pain Management. 2008;1(2):151–162. [PMC free article] [PubMed] [Google Scholar]
- Fillingim RB, Maixner W, Kincaid S, Sigurdsson A, Harris MBMS. Pain Sensitivity in Patients with Temporomandibular Disorders: Relationship to Clinical and Psychosocial Factors. Clinical Journal of Pain. 1996;12(4):260–269. doi: 10.1097/00002508-199612000-00005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald M. The development of nociceptive circuits. Nat Rev Neurosci. 2005;6(7):507–520. doi: 10.1038/nrn1701. 10.1038/nrn1701. [DOI] [PubMed] [Google Scholar]
- Fortier MA, Anderson CT, Kain ZN. Ethnicity Matters in the Assessment and Treatment of Children’s Pain. Pediatrics. 2009;124(1):378–380. doi: 10.1542/peds.2008-3332. doi: 10.1542/peds.2008-3332. [DOI] [PubMed] [Google Scholar]
- Gelfand S. The Relationship Of Experimental Pain Tolerance To Pain Threshold. Can J Psychol. 1964;18:36–42. doi: 10.1037/h0083283. [DOI] [PubMed] [Google Scholar]
- Gil KM, Edens JL, Wilson JJ, Raezer LB, Kinney TR, Schultz WH, Daeschner C. Coping strategies and laboratory pain in children with sickle cell disease. Ann Behav Med. 1997;19(1):22–29. doi: 10.1007/BF02883423. [DOI] [PubMed] [Google Scholar]
- Gragg RA, Rapoff MA, Danovsky MB, Lindsley CB, Varni JW, Waldron SA, Bernstein BH. Assessing Chronic Musculoskeletal Pain Associated with Rheumatic Disease: Further Validation of the Pediatric Pain Questionnaire. J. Pediatr. Psychol. 1996;21(2):237–250. doi: 10.1093/jpepsy/21.2.237. doi: 10.1093/jpepsy/21.2.237. [DOI] [PubMed] [Google Scholar]
- Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, Vallerand AH. The Unequal Burden of Pain: Confronting Racial and Ethnic Disparities in Pain. Pain Medicine. 2003;4(3):277–294. doi: 10.1046/j.1526-4637.2003.03034.x. doi: doi:10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. d. B. Four factor index of social status. Yale University, Dept. of Sociology; New Haven, Conn.: 1975. [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand. J. Statist. 1979;6:65–70. [Google Scholar]
- Hsu L, Woody SR, Lee H-J, Peng Y, Zhou X, Ryder AG. Social anxiety among East Asians in North America: East Asian socialization or the challenge of acculturation? Cultural Diversity and Ethnic Minority Psychology. 2012;18(2):181–191. doi: 10.1037/a0027690. doi: 10.1037/a0027690. [DOI] [PubMed] [Google Scholar]
- Jordan MS, Lumley MA, Leisen JCC. The relationships of cognitive coping and pain control beliefs to pain and adjustment among African–American and Caucasian women with rheumatoid arthritis. Arthritis Care & Research. 1998;11(2):80–88. doi: 10.1002/art.1790110203. doi: 10.1002/art.1790110203. [DOI] [PubMed] [Google Scholar]
- Lau AS, Fung J, Wang S.-w., Kang S-M. Explaining elevated social anxiety among Asian Americans: Emotional attunement and a cultural double bind. Cultural Diversity and Ethnic Minority Psychology. 2009;15(1):77–85. doi: 10.1037/a0012819. doi: 10.1037/a0012819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis M, Ramsay D, Kawakami K. Differences between Japanese infants and Caucasian American infants in behavioral and cortisol response to inoculation. Child Development. 1993;64(6):1722–1731. [PubMed] [Google Scholar]
- Lu Q, Stanton AL. How benefits of expressive writing vary as a function of writing instructions, ethnicity and ambivalence over emotional expression. Psychology & Health. 2010;25(6):669–684. doi: 10.1080/08870440902883196. [DOI] [PubMed] [Google Scholar]
- Lu Q, Zeltzer LK, Tsao JCI, Kim SC, Turk N, Naliboff BD. Heart rate mediation of sex differences in pain tolerance in children. Pain. 2005;118(1–2):185–193. doi: 10.1016/j.pain.2005.08.008. [DOI] [PubMed] [Google Scholar]
- McGrath PA, Gillespie J. Pain assessment in children and adolescents. In: Turk DC, Melzack R, editors. Handbook of pain assessment. 2nd Edition Guilford; New York: 2001. pp. 97–118. [Google Scholar]
- McWilliams LA, Cox BJ, Enns MW. Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain. 2003;106(1-2):127–133. doi: 10.1016/s0304-3959(03)00301-4. doi: 10.1016/s0304-3959(03)00301-4. [DOI] [PubMed] [Google Scholar]
- Mechlin B, Morrow AL, Maixner W, Girdler SS. The relationship of allopregnanolone immunoreactivity and HPA-axis measures to experimental pain sensitivity: Evidence for ethnic differences. Pain. 2007;131(1-2):142. doi: 10.1016/j.pain.2006.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier PM, Berde CB, DiCanzio J, Zurakowski D, Sethna NF. Quantitative assessment of cutaneous thermal and vibration sensation and thermal pain detection thresholds in healthy children and adolescents. Muscle Nerve. 2001;24(10):1339–1345. doi: 10.1002/mus.1153. [DOI] [PubMed] [Google Scholar]
- Miller A, Barr RG, Young SN. The cold pressor test in children: Methodological aspects and the analgesic effect of intraoral sucrose. Pain. 1994;56(2):175. doi: 10.1016/0304-3959(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum B, Adams J, Aceves J. The Influence of Culture on Pain in Anglo and Hispanic Children with Cancer. Journal of the American Academy of Child & Adolescent Psychiatry. 1990;29(4):642–647. doi: 10.1097/00004583-199007000-00020. doi: 10.1097/00004583-199007000-00020. [DOI] [PubMed] [Google Scholar]
- Powell CV, Kelly A-M, Williams A. Determining the minimum clinically significant difference in visual analog pain score for children. Annals of Emergency Medicine. 2001;37(1):28. doi: 10.1067/mem.2001.111517. [DOI] [PubMed] [Google Scholar]
- Rahim-Williams B, Riley Iii JL, Herrera D, Campbell CM, Hastie BA, Fillingim RB. Ethnic identity predicts experimental pain sensitivity in African Americans and Hispanics. Pain. 2007;129(1-2):177. doi: 10.1016/j.pain.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahim-Williams B, Riley JL, Williams AKK, Fillingim RB. A Quantitative Review of Ethnic Group Differences in Experimental Pain Response: Do Biology, Psychology, and Culture Matter? Pain Medicine. 2012;13(4):522–540. doi: 10.1111/j.1526-4637.2012.01336.x. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainville P, Duncan GH, Price DD, Carrier B. t., Bushnell MC. Pain Affect Encoded in Human Anterior Cingulate But Not Somatosensory Cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- Rowell LN, Mechlin B, Ji E, Addamo M, Girdler SS. Asians differ from non-Hispanic Whites in experimental pain sensitivity. European Journal of Pain. 2011;15(7):764–771. doi: 10.1016/j.ejpain.2010.11.016. doi: 10.1016/j.ejpain.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield D, Biles PL, Orom H, Maixner W, Sheps DS. Race and Sex Differences in Cutaneous Pain Perception. Psychosom Med. 2000;62(4):517–523. doi: 10.1097/00006842-200007000-00010. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. In: Leinhardt S, editor. Sociological Methodology. American Sociological Association; Washington, DC: 1982. pp. 290–312. 1982. [Google Scholar]
- Tsao JCI, Lu Q, Myers CD, Kim SC, Turk N, Zeltzer LK. Parent and Child Anxiety Sensitivity: Relationship to Children’s Experimental Pain Responsivity. The Journal of Pain. 2006;7(5):319. doi: 10.1016/j.jpain.2005.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao JCI, Myers CD, Craske MG, Bursch B, Kim SC, Zeltzer LK. Role of Anticipatory Anxiety and Anxiety Sensitivity in Children’s and Adolescents’ Laboratory Pain Responses. Journal of Pediatric Psychology. 2004;29(5):379–388. doi: 10.1093/jpepsy/jsh041. doi: 10.1093/jpepsy/jsh041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau . An Older and More Diverse Nation by Midcentury. U.S. Census Press Releases; 2008. [Google Scholar]
- Wade JB, Dougherty LM, Archer CR, Price DD. Assessing the stages of pain processing: a multivariate analytical approach. Pain. 1996;68(1):157. doi: 10.1016/S0304-3959(96)03162-4. [DOI] [PubMed] [Google Scholar]
- Watson Latif, Rowbotham Ethnic differences in thermal pain responses: A comparison of South Asian and White British healthy males. Pain. 2005;118(1-2):194. doi: 10.1016/j.pain.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Wen T, Ansonoff MA, Pintar JE. The tail pigmentation pattern of C57BL/6J mice affects nociception/pain quantification in the tail flick test. European Journal of Pain. 2009;13(6):564. doi: 10.1016/j.ejpain.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widmalm SE, Christiansen RL, Gunn S. Race and gender as TMD risk factors in children. Cranio. 1995;13(3):163–166. doi: 10.1080/08869634.1995.11678062. [DOI] [PubMed] [Google Scholar]
- Zatzick DF, Dimsdale JE. Cultural variations in response to painful stimuli. Psychosom Med. 1990;52(5):544–557. doi: 10.1097/00006842-199009000-00007. [DOI] [PubMed] [Google Scholar]