Abstract
The objective of the study is to investigate pulmonary responses to Pseudomonas aeruginosa (P. aeruginosa) and methicillin-resistant Staphylococcus aereus (MRSA) using ovine and mice models of sepsis with emphasis on lung cytokine expression, asymmetric dimethylarginine concentration, and the arginase pathway.
Methods
Sheep were instilled with either MRSA, P. aeruginosa or saline under deep anesthesia, mechanically ventilated, resuscitated with fluid, and sacrificed after 24 hrs. Mice were instilled with either MRSA, P. aeruginosa or saline under deep anesthesia and sacrificed after 8 hours. Lungs were assessed for ADMA concentration, arginase activity, oxidative stress, cytokine expression, and plasma was assessed for nitrate/nitrite concentrations.
Results
The severity of lung injury was more pronounced in P. aeruginosa sepsis compared to MRSA. The significant changes in sheep lung function after P. aeruginosa sepsis were associated with significantly increased ADMA concentrations and arginase activity compared to MRSA. However, the plasma concentration of nitrites and nitrates were significantly increased in MRSA sepsis compared to P. aeruginosa sepsis. In the mice model, P. aeruginosa significantly increased lung cytokine expression (IL-1 and 13), protein oxidation, and arginase activity compared to MRSA. Our data suggest that the greater expression of cytokines and ADMA concentrations may be responsible for severity of acute lung injury in P. aeruginosa sepsis. The lack of arginase activity may explain the greater nitric oxide production in MRSA sepsis.
Keywords: oxidative stress, arginine metabolism, sepsis, nitric oxide, cytokine
INTRODUCTION
In the United States there are approximately 750,000 patients that suffer from sepsis, resulting in 225,000 deaths annually (24). It is the tenth leading cause of death in the United States (2, 33). Septic infections from gram-negative Pseudomonas aeruginosa (P. aeruginosa) and gram-positive methicillin-resistant Staphylococcus aereus (MRSA) are significant contributors to morbidity and mortality. Despite the significant advances in critical care, the mortality of septic patients remains the same over the past two decades (23). This may be related to increased presence of bacterial agents resistant to antibiotics. Also, the specific host response to infection is related to the nature of the causative bacteria, and there is a lack of different treatments based on the different types of bacteria.
We have recently reported that the severity of acute lung injury (ALI) during P. aeruginosa-sepsis is more pronounced than in MRSA sepsis (9, 17). However, the microvascular hyperpermeability was significantly higher in MRSA sepsis. The vascular leakage from MRSA sepsis was associated with 2–3-fold increase in the plasma levels of nitrites and nitrates (NOx). However, the mechanisms underlying these differences remain not completely understood.
To date, there are no studies that compare host responses in large animals between MRSA and P. aeruginosa, and consequently an effective treatment against both bacteria has not been developed. We hypothesize that pro-inflammatory cytokines, protein oxidation and asymmetric dimethylarginine (ADMA) are responsible for the severity of ALI in P. aeruginosa sepsis, and that the significantly higher levels of plasma NOx in MRSA sepsis may be associated with the moderately activated arginase pathway. We tested our hypothesis using the well-established ovine and mice models of MRSA and P. aeruginosa sepsis.
MATERIALS AND METHODS
Animal Care and Use
This study was approved by the Institutional Animal Care and Use Committee of the University of Texas Medical Branch and conducted in compliance with the guidelines of the National Institutes of Health and of the American Physiology Society for the care, handling and use of laboratory animals. The studies were completed at UTMB’s Investigative Intensive Care Unit, which is a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC).
Ovine Model
The model of the P. aeruginosa- and MRSA-induced sepsis has been previously developed by our team and has been described in detail (9). Briefly, 18 adult female Merino sheep (body weight, 30–40 kg) were surgically prepared under isoflurane anesthesia with a right femoral artery catheter (Intracath, 16GA, 24IN, Becton Dickinson Vascular Access, Sandy, UT), a thermodilution catheter (Model 131F7, Edwards Lifesciences LLC, Irvine, CA), and a left atrial catheter (0.062 in. ID, 0.125 in. OD; Dow Corning, Midland, MI). After a 7-day recovery period, sheep were randomly divided into three groups: sham (non-injured, non-treated; n = 6), P. aeruginosa (n = 6), and MRSA (n = 6). A stock solution of either live P. aeruginosa or MRSA (2–5 × 1011 CFU, P. aeruginosa Human Strain IRS 12-4-4, MRSA Human Strain USA300) was suspended in 30 mL of 0.9% NaCl solution. P. aeruginosa 12-4-4 was used because it is the most common form of P. aeruginosa isolated from burn patients, and it was isolated from a burn patient at Brook Army Medical Center in San Antonio. MRSA USA300 is the most common MRSA strain in the United States. Ten milliliters of solution either P. aeruginosa or MRSA was instilled in the right middle and lower lobes and the left lobe of the lung by bronchoscope after isoflurane anesthesia. Based on the dose response curves of each bacteria (13, 29), the quantity of bacteria given was based on achieving similar cardiovascular responses and hemodynamic variables such as cardiac output, mean arterial pressure (MAP) and systemic vascular resistance index (SVRI). Sham animals were anesthetized with isoflurane anesthesia and treated in the same manner as the injured sheep but were instilled with physiological saline. After the injury or the sham procedure, all sheep were awakened and placed on a ventilator with positive end expiratory pressure set to 5 cm H2O and tidal volume maintained at 15 mL/kg. A large tidal volume is required for sheep because their ratio of lung dead space to tidal volume (Vd/Vt) is 0.6 compared to only 0.3 for humans (39). The sheep were ventilated with 100% oxygen for the first 3 hrs after injury and the inspired oxygen was further adjusted according to arterial oxygen and saturation. Respiratory rate was initially set at 20 breaths/minute and thereafter adjusted to keep PaCO2 between 25–35 mm Hg. All sheep were resuscitated with Ringer’s solution with an initial rate of 2 mL•kg−1•hr−1. The fluid rate was further adjusted to maintain hematocrit levels close to baseline values (±3). The experiment continued for 24 hours.
Murine Model
The model of the P. aeruginosa-and MRSA-induced sepsis in mice has been previously described in detail (20). Briefly, 18 C57Bl/6J mice (Jackson Laboratories, Bar Harbor, ME, Catalog No. 000664; body weight, 15–20 g) were randomly divided into three groups: sham (non-injured, non-treated, n = 6), P. aeruginosa (n = 6), and MRSA (n = 6). A stock solution of either live P. aeruginosa or MRSA (3.2 × 107 CFU, P. aeruginosa Human Strain 12-1-1, MRSA Human Strain USA300) was suspended in 20 µL of 0.9% NaCl solution. Under deep anesthesia with isoflurane, mice were placed in a supine position and 20 µL of either P. aeruginosa or MRSA was inserted into each nostril. Sham animals were anesthetized with isoflurane anesthesia and treated in the same manner as the injured mice but were instilled with physiological saline. The experiment continued for 8 hours.
Measured Variables
Lung Histology and Scoring
The protocol for lung histology and scoring has previously been described in detail (6). Briefly, a 1 cm transverse slice was taken through the middle of the lower lobe of the right ovine lung and injected with 10% buffered formalin at necropsy. The tissue was then immersed in fixative for three to five days and sampled into blocks. Following standardized paraffin embedding protocols, 4 µm sections were obtained and stained with hematoxylin and eosin. An experienced pathologist scored the slides without knowledge of the experimental group. Interstitial edema, perivascular edema, vascular congestion, intra-alveolar hemorrhage, and the presence of clusters of bacteria within alveoli were scored subjectively for the degree of the abnormality, using 0 for absent, 1 for mild, 2 for mild to moderate, 3 for moderate, 4 for moderate to severe and 5 for severe. Intra-alveolar edema, atelectasis and the extent of neutrophils in alveoli, on the other hand, were scored according to the percentage of the section occupied by the each, using 0 for none, 1 for 1–20%, 2 for 20–40%, 3 for 40–60%, 4 for 60–80% and 5 for 80–100%.
Lung Homogenate Arginase Activity
The protocol to determine arginase activity in lung tissue homogenate in mice after 8 hrs and in sheep after 24 hrs has previously been described in detail (42). It is a colorimetric assay that uses α-isonitrosopropiophenone to assess urea formation. Protein levels for each sample were determined using the Protein Quantification Kit (Dojindo Molecular Technologies, Rockville, MD) the according to manufacturer’s instructions. All results are expressed as µM urea/µg protein.
Plasma Nitrite/Nitrate (NOx)
The plasma NO levels were evaluated in sheep by measuring the intermediate and end products, which are nitrates and nitrites (NOx). Concentrations were determined at 0, 6, 12, 18, 24 hrs using a commercially available assay (Nitrate/Nitrite Colorimetric Assay Kit, Cayman Chemicals, Ann Arbor, MI, Catalog No. 780001).
Plasma ADMA
Plasma ADMA levels were evaluated in sheep by using a commercially available assay (ADMA Direct ELISA Kit, ALPCO Diagnostics, Salem, NH, Catalog No. 30-7814).
Lung OxyBlot Detection of Protein Carbonylation
Lung homogenate detection of protein oxidation in mice was evaluated by using a commercially available assay after 8 hrs (OxyBlot Protein Oxidation Kit, Milipore, Billerica, MA, Catalog No. S7150).
Lung Homogenate IL-13 and IL-1 Protein Expression Western Blot
IL-13 and IL-1 protein expression in mice from lung homogenate was determined using anti-IL-13 antibody (Santa Cruz, Santa Cruz, CA, Catalog No. sc-1776) with anti-rat IgG HRP secondary antibody (R&D Systems, Minneapolis, MN, Catalog No. HAF005) and anti-IL-1 antibody (Abcam, Cambridge, MA, Catalog No. ab8155) with anti-goat IgG HRP secondary antibody (R&D Systems, Minneapolis, MN, Catalog No. HAF109) as described previously (15). Blots were completed using 20 µg of protein and were quantified by National Institutes of Health IMAGE J (Image and Processing and Analysis in Java) scanning densitometry, and normalized to total actin (I-19) (sc-1616) expression.
Statistical Analysis
All values are expressed as mean ± SEM using GraphPad Prism Software (GraphPad Software, La Jolla, CA, USA). Results were compared between groups using repeated measures (analysis of variance) and the Newman-Keuls multiple comparison tests. A value of p < 0.05 was accepted as statistically significant.
RESULTS
All sheep and mice in each of the three study groups survived throughout the experimental time period.
P. aeruginosa Induced a Reduced PaO2:FiO2 Ratio compared to MRSA in Sheep
Pulmonary gas exchange, measured by the ratio of the partial pressure of arterial oxygen (PaO2) to the fractional concentration of oxygen in inspired air (FiO2), is significantly decreased in P. aeruginosa and MRSA-treated sheep compared to uninjured sheep after 24 hours (p < 0.05; 205 ± 72 in P. aeruginosa-treated animals, 319 ± 82 in MRSA-treated animals, and 600 ± 10 in sham animals, Figure 1). The PaO2/FiO2 ratio was significantly lower with P. aeruginosa compared to MRSA. Significant increases in alveolar edema were found between P. aeruginosa-treated and sham animals (0.67 ± 0.14 P.aeruginosa vs 1.83 ± 0.43 sham, p<0.05, 1.56 ± 0.55 MRSA, data not shown). Significant increases were also seen in atelectasis between P. aeruginosa-treated and sham animals (0.20 ± 0.20 sham vs 1.67 ± 0.47 P.aeruginosa, p<0.05, 0.70 ± 0.18 MRSA, data not shown), as well as in polymorphonuclear cells in the alveoli (0.15 ± 0.10 sham vs 1.58 ± 0.46 P.aeruginosa, p<0.05, 0.95 ± 0.42 MRSA, data not shown).
Figure 1.
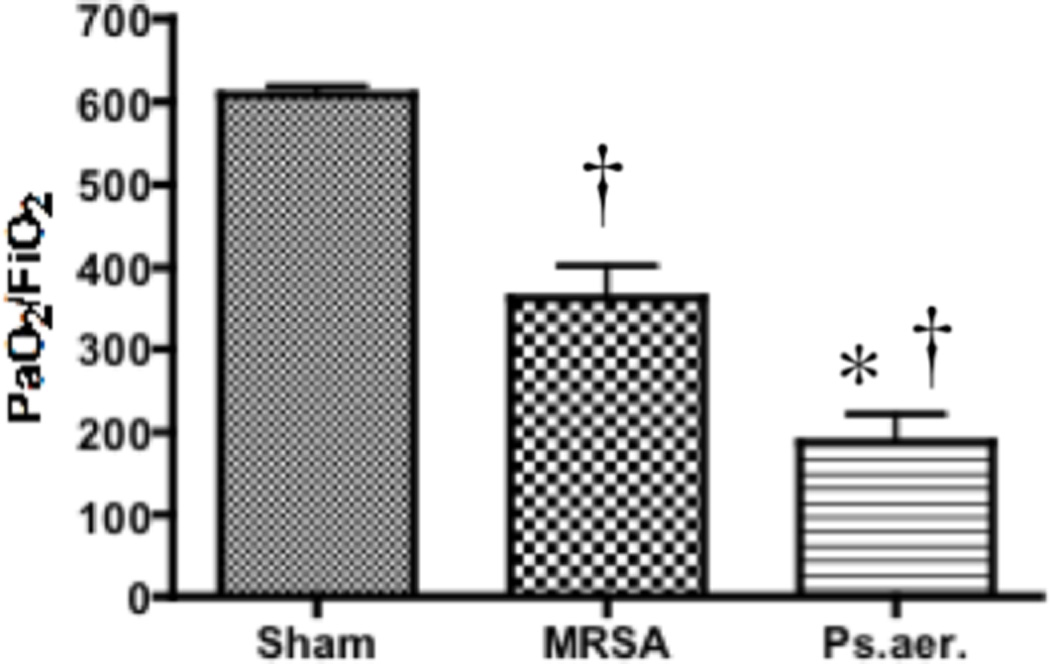
Effect of MRSA and P. aeruginosa on PaO2:FiO2 in sheep. Each group includes 6 animals. Data are shown as means ± SEM. †P < 0.05 versus uninjured sham animals, *P < 0.05 versus MRSA.
Significantly Increased Lung Arginase Activity with P. aeruginosa-sepsis was Associated with Moderately Increased NO Production and Significantly Increased Plasma ADMA Concentrations in Sheep
Lung tissue arginase activity was significantly increased with P. aeruginosa treatment (0.16 ± 0.16 µM urea/µg protein) compared to uninjured sheep (0.10 ± 0.01 µM urea/µg protein; p < 0.05; Figure 2A). Arginase activity of MRSA-treated animals (0.13 ± 0.11 µM urea/µg protein) moderately increased compared to sham animals, but there was no statistical significance between the two groups.
Figure 2.
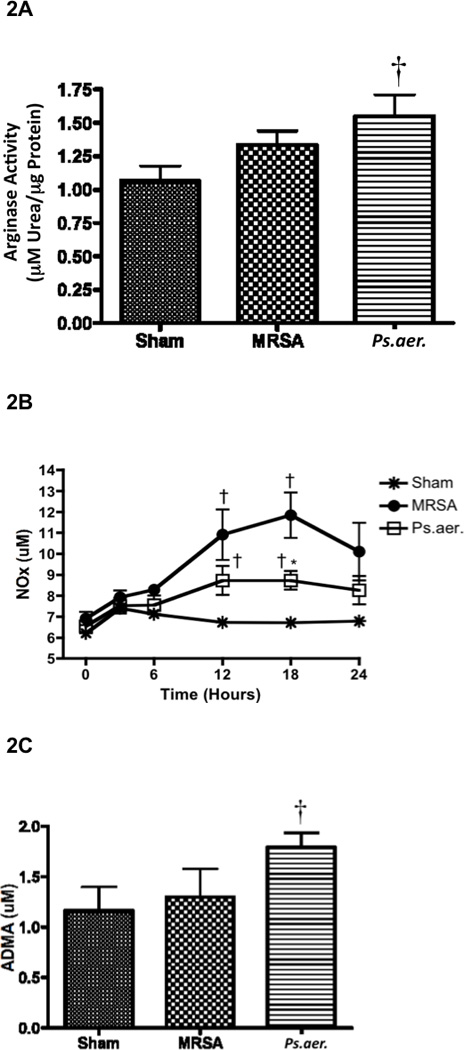
Effect of MRSA and P. aeruginosa on arginase activity, NO synthesis, and ADMA concentrations in sheep. Each group includes 6 animals. (A) Lung homogenate was used to measure arginase activity. Plasma was collected over time to measure (B) the products of nitric oxide, nitrates and nitrites (NOx), and (C) ADMA concentrations. Data are shown as means ± SEM. †P < 0.05 versus uninjured sham animals, *P < 0.05 versus MRSA.
The stable end products of NO, which include nitrates and nitrites (NOx), significantly increased after treatment with MRSA and P. aeruginosa after 18 hours in plasma compared to uninjured animals. However, the plasma NOx levels were significantly higher in MRSA sepsis compared to P. aeruginosa (11.84 ± 1.09 µM in MRSA-treated animals, 8.73 ± 0.44 in P. aeruginosa-treated animals, and 6.72 ± 0.18 in sham animals after 18 hours, p < 0.05; Figure 2B).
Plasma concentrations of ADMA significantly increased in animals treated with P. aeruginosa compared to uninjured animals (1.79 µM ± 0.14 in P. aeruginosa-treated animals versus 1.163 ± 0.18 in uninjured animals; p < 0.05; Figure 2C) and MRSA-treated animals. There is essentially no increase in ADMA after treatment with MRSA (1.30 ± 0.28 µM; Figure 2C) compared to uninjured animals after 24 hours.
P. aeruginosa was Associated with Significantly Increased Lung Arginase Activity, Protein Oxidation, and IL-13 and IL-1 Cytokine Expression in Mice
P. aeruginosa induced a significantly higher arginase activity (0.17 ± 0.01 µM urea/µg protein; p < 0.05; Figure 3A) than uninjured animals (0.11 ± 0.007 µM urea/µg protein) and a higher activity than MRSA-treated animals (0.13 ± 0.007 µM urea/µg protein) when the mice were sacrificed 8 hours after the injury.
Figure 3.
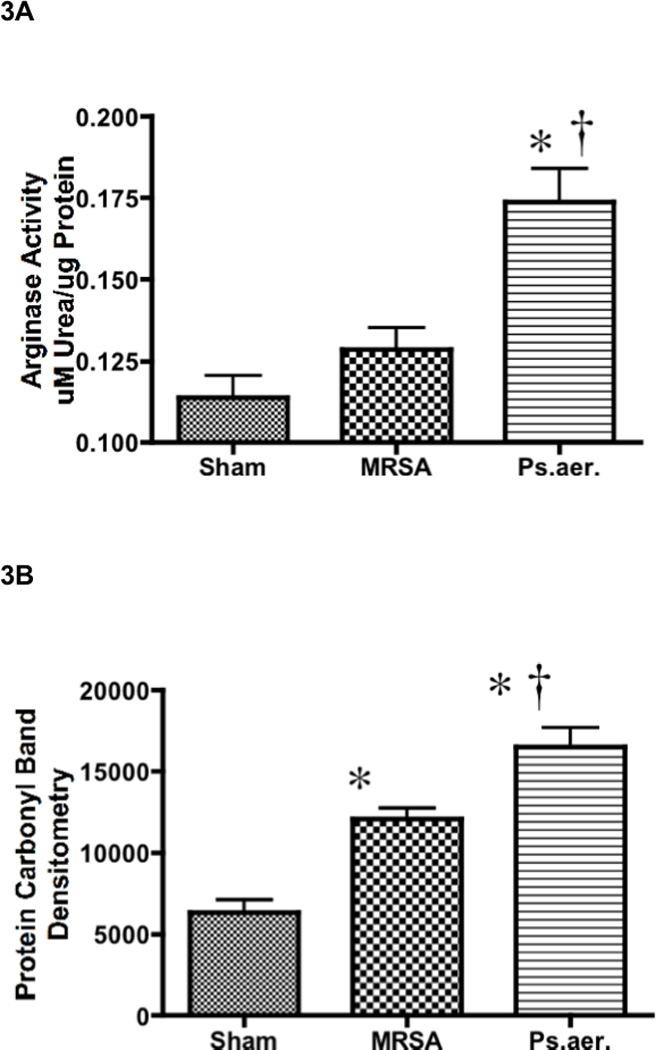
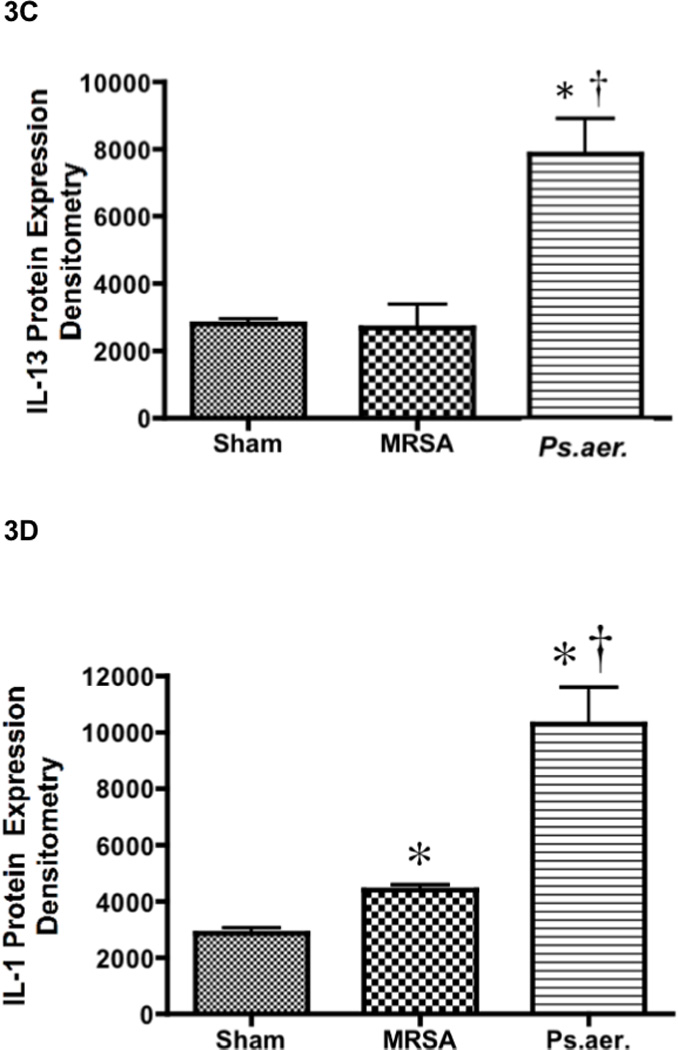
Effect of MRSA and P. aeruginosa on arginase activity, protein oxidation, and expression of IL-13 and IL-1 in mice. Each group includes 6 animals. Lung homogenate was used to measure (A) arginase activity, (B) protein oxidation, (C) IL-13 protein expression, and (D) IL-1 protein expression. Equality of protein loading was confirmed by the expression of B-actin, and each band was quantified by densitometric analysis. Data are shown as means ± SEM. †P < 0.05 versus uninjured sham animals, *P < 0.05 versus MRSA.
P. aeruginosa-treated mice had significantly higher protein oxidation compared to MRSA-treated and sham mice (16510 ± 1208 in P. aeruginosa-treated mice versus 12090 ± 648 in MRSA-treated mice and 6339 ± 792 in sham mice; p < 0.05; Figure 3B). MRSA-treated mice had a moderate but significantly higher oxidation compared to sham mice.
Western blot analysis was used in the mice model due to the lack of specificity of sheep antibodies. P. aeruginosa induced a significantly higher IL-13 expression (7859 ± 1057; p < 0.05; Figure 3A) than uninjured animals (2802 ± 155) and MRSA-treated mice (2693 ± 693). A similar trend existed with IL-1 expression. P. aeruginosa -treated mice had significantly higher IL-1 protein compared to MRSA-treated and sham mice (10310 ± 1301 in P. aeruginosa-treated mice versus 4416 ± 174 in MRSA-treated mice and 2877 ± 195 in sham mice; p < 0.05; Figure 3B). MRSA-treated mice had moderate but significantly higher IL-1 expression compared to sham mice.
DISCUSSION WITH CONCLUSIONS
Acute respiratory distress syndrome (ARDS) is a life-threatening condition that results from a manifestation of many serious diseases, including sepsis and smoke inhalation injury (14, 25). It represents a clinical syndrome defined by acute pulmonary dysfunction with deteriorated gas exchange (PaO2/FiO2 less than 200 mmHg) and bilateral pulmonary edema not related to myocardial contractility failure (3, 41). Despite intensive research, the exact mechanisms that lead to ARDS are not completely understood. The present study compares the severity of ALI in the ovine and murine sepsis models induced by two different pathogenic agents: P. aeruginosa and MRSA. P. aeruginosa 12-4-4 was used because it is the most common form of P. aeruginosa isolated from burn patients, and it was isolated from a burn patient at Brook Army Medical Center in San Antonio. MRSA USA300 is the most common MRSA strain in the United States. We aimed to explore underlying mechanisms of these different pulmonary responses and focused on cytokine expression, on oxidative stress, and on the ADMA, NOS and arginase pathway using our well-established ovine and murine models of sepsis (9, 20, 29). Results from this study demonstrate that P. aeruginosa causes a more severe lung injury compared to MRSA. In our study, the PaO2/FiO2 ratio decreases to a greater extent after P. aeruginosa sepsis compared MRSA sepsis. The severity of P. aeruginosa lung injury was associated with increased cytokine expression, oxidant stress, ADMA, and arginase activity, while MRSA sepsis is associated with moderately increased plasma nitrates and nitrites.
It is well established that pro-inflammatory cytokines mediate lung injury in sepsis (20, 21). In the present study, pro-inflammatory cytokines such as IL-13 and IL-1 were significantly more expressed in P. aeruginosa sepsis compared to MRSA sepsis in mice, which may explain why the degree of lung injury was more severe with P. aeruginosa. IL-1 represents an acute-phase inflammatory cytokine, while IL-13 represents a late-phase inflammatory cytokine (28, 46). Mouse lungs were used to study inflammatory cytokines because of the difficulty and lack of specific antibodies for sheep lungs. IL-13 was significantly increased after only 8 hours in P. aeruginosa, indicating the severity of the injury. The severe lung injury may also be explained by more pronounced oxidative stress observed with P. aeruginosa sepsis. Lung injury during sepsis is associated with reactive oxygen species (ROS), and the administration of antioxidants reduce organ injury (1). Liaw, et al., demonstrated that an inhibitor of ROS formation attenuated intraperitoneal injury from sepsis in the rat model (22). There is also a direct relationship between ROS and pro-inflammatory cytokines in the sepsis model (35, 40, 45).
The release of ROS is mediated through various pathways. ADMA is an analog of arginine and an endogenous inhibitor of all three isoforms of NOS (7, 36, 37). It is synthesized by hydrolysis of proteins with methylated arginine residues (5). In the presence of increased ADMA, NOS can become uncoupled to produce superoxide (O2−) (18, 31, 32, 38, 43, 44). Thus, in the presence of ADMA, the increase in the NOS uncoupling increases ROS. In the present study, the plasma levels of ADMA are significantly higher with P. aeruginosa compared to MRSA. Thus it is possible that severity of lung injury in P. aeruginosa sepsis is mediated by increased ADMA, resulting in excessive ROS formation. The recent report that the lung is a major source of the ADMA and that elevated circulating levels of ADMA can contribute to abnormal airway physiology may support our findings (4, 42). Future studies will measure ADMA using gas chromatography-mass spectrometry instead of ELISA in order to determine arginine concentrations as well. This will be a more accurate determinant of whether ADMA inhibits NOS in our sepsis model.
An interesting finding was that MRSA sepsis was associated with a more excessive production of NO by measuring its stable metabolites, nitrates and nitrites, in plasma despite it only causing a moderate lung injury. In previous studies, we have reported the beneficial effects of NOS inhibitors in P. aeruginosa sepsis. The non-specific NOS inhibitor L-NAME, as well as neuronal NOS and inducible NOS inhibitors have significantly improved pulmonary gas exchange following smoke inhalation injury and pneumonia with P. aeruginosa (8, 10–12). Despite the excessively expressed NO in MRSA-sepsis, the animals showed a milder lung dysfunction compared to P. aeruginosa. The reason and mechanism is unknown. The future studies exploring these mechanisms are necessary. In previous studies, we have demonstrated that reactive nitrogen species (RNS) such as peroxynitrite play a critical role in ARDS induced by P. aeruginosa (26) and by cutaneous burn and smoke inhalation injury (19). Peroxynitrite, which is formed from NO and superoxide, is more toxic in high concentrations than NO. It causes lipid peroxidation, protein oxidation and nitration, enzyme inactivation and eventually cell necrosis (34). Although we did not measure RNS in this study, it is possible that the increased oxidative stress in P. aeruginosa sepsis caused excessive RNS formation. The latter may partly explain why P. aeruginosa-induced lung injury was more severe despite its association with moderately increased NO.
As mentioned, P. aeruginosa sepsis was associated with significantly increased plasma concentrations of ADMA compared to MRSA sepsis. It is well known that ADMA is a potent endogenous inhibitor of NOS. Thus, the increased ADMA in P. aeruginosa sepsis could explain the only moderately increased NO production compared to MRSA. Also, it is noteworthy that MRSA sepsis did not affect the plasma levels of ADMA. Another explanation for the differences in NO production between the two pathogens could be related to the different responses in arginase activity. In the present study, we report that lung arginase activity was significantly higher in P. aeruginosa sepsis. It is well known that NOS and arginase compete for arginine. NOS converts arginine into citrulline and NO, and arginase converts arginine to urea and ornithine (27, 30). Enhanced arginase activity results in less arginine substrate for leading to moderate production of NO in Ps. aeruginosa sepsis. We also report that expressions of pro-inflammatory cytokines, including Th2 cytokines such as IL-13, were more pronounced in P. aeruginosa sepsis. Because the induction of arginase is Th2 cytokine dependent (16), the higher arginase activity in P. aeruginosa sepsis may be a result of more excessively expressed IL-13. Future studies will examine additional acute and late-phase Th1 and Th2 cytokines such as TNF-α and IL-6 using both western blots and ELISA and their effects on both arginase expression and activity.
Based on our previous studies and our current findings, P. aeruginosa-sepsis increases lung cytokine expressions, which may cause increased reactive species and more severe pulmonary dysfunction. The severe lung injury associated with P. aeruginosa sepsis may occur because of significantly increased cytokine expression, oxidative stress, ADMA concentrations and arginase activity compared to MRSA in ovine and murine models. Increased ADMA concentration and arginase activity in P. aeruginosa may explain the moderate production of NO in P. aeruginosa sepsis compared to MRSA. Our findings are preliminary in the ovine model, and future studies will accurately determine the causal relationship between ROS, NO, ADMA, and cytokines after MRSA and P. aeruginosa sepsis using endothelial and epithelial cell culture and various knock-out mice. This is the first report of the mechanistic differences between P. aeruginosa and MRSA sepsis, which suggests that treatment strategies for P. aeruginosa and MRSA should consider their different host responses.
ACKNOWLEDGEMENTS
The authors would like to thank the staff of the Investigational Intensive Care Unit at the University of Texas Medical Branch for their valuable assistance, especially J.R. Salsbury, E. Kraft, R. Cox, C. Moncebaiz, J. Jinkins, T. Walker, and C. Hallum. The authors also thank R. Connelly for consultation; K. Bansal, A. Esechie for assistance with molecular techniques; and S. Assmussen and Y. Zhu for laboratory support.
GRANTS
This work was supported by Grant GM66312 from the National Institute of General Medical Science, Grant 0565028Y from the American Heart Association (AHA), and Grant 8630 from the Shrine Hospitals for Children (SHC). The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the NIH, AHA or SHC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: None
REFERENCES
- 1.Andrades M, Ritter C, de Oliveira MR, Streck EL, Fonseca Moreira JC, Dal-Pizzol F. Antioxidant Treatment Reverses Organ Failure in Rat Model of Sepsis: Role of Antioxidant Enzymes Imbalance, Neutrophil Infiltration, and Oxidative Stress. J Surg Res. 2009;167:307–313. doi: 10.1016/j.jss.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 2.Balk RA. Severe sepsis and septic shock. Definitions, epidemiology, and clinical manifestations. Crit Care Clin. 2000;16:179–192. doi: 10.1016/s0749-0704(05)70106-8. [DOI] [PubMed] [Google Scholar]
- 3.Bernard GE, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, LeGall JR, Morris A, Spragg R. The american-european consensus conference on ARDS. Am Rev Respir Dis. 1993;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 4.Bulau P, Zakrzewicz D, Kitowska K, Leiper J, Gunther A, Grimminger F, Eickelberg O. Analysis of methylarginine metabolism in the cardiovascular system identifies the lung as a major source of ADMA. Am J Physiol Lung Cell Mol Physiol. 2007;292:L18–L24. doi: 10.1152/ajplung.00076.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cantoni GL. Biological methylation: selected aspects. Annu Rev Biochem. 1975;44:435–451. doi: 10.1146/annurev.bi.44.070175.002251. [DOI] [PubMed] [Google Scholar]
- 6.Cox RA, Burke AS, Oliveras G, Enkhbaatar P, Traber LD, Zwischenberger JB, Jeschke MG, Schmalstieg FC, Herndon DN, Traber DL, Hawkins HK. Acute bronchial obstruction in sheep: histopathology and gland cytokine expression. Exp Lung Res. 2005;31:819–837. doi: 10.1080/01902140600574967. [DOI] [PubMed] [Google Scholar]
- 7.Dowling RB, Newton R, Robichaud A, Cole PJ, Barnes PJ, Wilson R. Effect of inhibition of nitric oxide synthase on Pseudomonas aeruginosa infection of respiratory mucosa in vitro. Am J Respir Cell Mol Biol. 1998;19:950–958. doi: 10.1165/ajrcmb.19.6.2904. [DOI] [PubMed] [Google Scholar]
- 8.Enkhbaatar P, Connelly R, Wang J, Nakano Y, Lange M, Hamahata A, Horvath E, Szabo C, Jaroch S, Holscher P, Hillmann M, Traber LD, Schmalstieg FC, Herndon DN, Traber DL. Inhibition of neuronal nitric oxide synthase in ovine model of acute lung injury. Crit Care Med. 2009;37:208–214. doi: 10.1097/CCM.0b013e318193226a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Enkhbaatar P, Joncam C, Traber L, Nakano Y, Wang J, Lange M, Connelly R, Kulp G, Saunders F, Huda R, Cox R, Schmalstieg F, Herndon D, Traber D. Novel Ovine Model of Methicillin-Resistant Staphylococcus Aureus-Induced Pneumonia and Sepsis. Shock. 2007;29:642–649. doi: 10.1097/shk.0b013e318158125b. [DOI] [PubMed] [Google Scholar]
- 10.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, McGuire R, Schmalstieg F, Cox R, Hawkins H, Jodoin J, Lee S, Traber L, Herndon D, Traber D. Inhibition of neuronal nitric oxide synthase by 7-nitroindazole attenuates acute lung injury in an ovine model. Am J Physiol Regul Integr Comp Physiol. 2003;285:R366–R372. doi: 10.1152/ajpregu.00148.2003. [DOI] [PubMed] [Google Scholar]
- 11.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips G, Parkinson J, Salsbury JR, Biondo N, Schmalstieg F, Burke A, Cox R, Hawkins H, Herndon D, Traber D. Inducible nitric oxide synthase dimerization inhibitor prevents cardiovascular and renal morbidity in sheep with combined burn and smoke inhalation injury. Am J Physiol Heart Circ Physiol. 2003;285:H2430–H2436. doi: 10.1152/ajpheart.00055.2003. [DOI] [PubMed] [Google Scholar]
- 12.Enkhbaatar P, Murakami K, Shimoda K, Mizutani A, Traber L, Phillips GB, Parkinson JF, Cox R, Hawkins H, Herndon D, Traber D. The inducible nitric oxide synthase inhibitor BBS-2 prevents acute lung injury in sheep after burn and smoke inhalation injury. Am J Respir Crit Care Med. 2003;167:1021–1026. doi: 10.1164/rccm.200209-1031PP. [DOI] [PubMed] [Google Scholar]
- 13.Enkhbaatar P, Murakami K, Traber LD, Cox R, Parkinson JF, Westphal M, Esechie A, Morita N, Maybauer MO, Maybauer DM, Burke AS, Schmalstieg FC, Hawkins HK, Herndon DN, Traber DL. The Inhibition of Inducible Nitric Oxide Synthase in Ovine Sepsis Model. Shock. 2006;25:522–527. doi: 10.1097/01.shk.0000209525.50990.28. [DOI] [PubMed] [Google Scholar]
- 14.Enkhbaatar P, Traber DL. Pathophysiology of acute lung injury in combined burn and smoke inhalation injury. Clin Sci (Lond) 2004;107:137–143. doi: 10.1042/CS20040135. [DOI] [PubMed] [Google Scholar]
- 15.Gillett AM, Wallace MJ, Gillespie MT, Hooper SB. Increased expansion of the lung stimulates calmodulin 2 expression in fetal sheep. Am J Physiol Lung Cell Mol Physiol. 2002;282:L440–L447. doi: 10.1152/ajplung.00202.2001. [DOI] [PubMed] [Google Scholar]
- 16.Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM, Cheever AW, Pearce EJ, Wynn TA. Differential regulation of nitric oxide synthase-2 and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous pathology is shaped by the pattern of L-arginine metabolism. J Immunol. 2001;167:6533–6544. doi: 10.4049/jimmunol.167.11.6533. [DOI] [PubMed] [Google Scholar]
- 17.Jonkam CC, Bansal K, Traber DL, Hamahata A, Maybauer MO, Maybauer DM, Cox RA, Lange M, Connelly RL, Traber LD, Djukom CD, Salsbury JR, Herndon DN, Enkhbaatar P. Pulmonary vascular permeability changes in an ovine model of methicillin-resistant Staphylococcus aureus sepsis. Crit Care. 2009;13:R19. doi: 10.1186/cc7720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klatt P, Schmidt K, Uray G, Mayer B. Multiple catalytic functions of brain nitric oxide synthase. Biochemical characterization, cofactor-requirement, and the role of N omega-hydroxy-L-arginine as an intermediate. J Biol Chem. 1993;268:14781–14787. [PubMed] [Google Scholar]
- 19.Lange M, Enkhbaatar P, Nakano Y, Traber DL. Role of nitric oxide in shock: the large animal perspective. Front Biosci. 2010;14:1979–1989. doi: 10.2741/3357. [DOI] [PubMed] [Google Scholar]
- 20.Lange M, Hamahata A, Traber DL, Esechie A, Jonkam C, Bansal K, Nakano Y, Traber LD, Enkhbaatar P. A murine model of sepsis following smoke inhalation injury. Biochem Biophys Res Commun. 2010;39:1565–1560. doi: 10.1016/j.bbrc.2009.12.124. [DOI] [PubMed] [Google Scholar]
- 21.Lange M, Nakano Y, Traber DL, Hamahata A, Esechie A, Jonkam C, Bansal K, Traber LD, Enkhbaatar P. Role of different nitric oxide synthase isoforms in a murine model of acute lung injury and sepsis. Biochem Biophys Res Commun. 2010;399:286–291. doi: 10.1016/j.bbrc.2010.07.071. [DOI] [PubMed] [Google Scholar]
- 22.Liaw WJ, Chen TH, Lai ZZ, Chen SJ, Chen A, Tzao C, Wu JY, Wu CC. Effects of a membrane-permeable radical scavenger, Tempol, on intraperitoneal sepsis-induced organ injury in rats. Shock. 2005;23:88–96. doi: 10.1097/01.shk.0000145937.70085.89. [DOI] [PubMed] [Google Scholar]
- 23.Martin G, Brunkhorst FM, Janes JM, Reinhart K, Sundin DP, Garnett K, Beale R. The international PROGRESS registry of patients with severe sepsis: drotrecogin alfa (activated) use and patient outcomes. Crit Care. 2009;13:R103. doi: 10.1186/cc7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 25.Mathru M, Rooney MW, Dries DJ, Hirsch LJ, Barnes L, Tobin MJ. Urine hydrogen peroxide during adult respiratory distress syndrome in patients with and without sepsis. Chest. 1994;105:232–236. doi: 10.1378/chest.105.1.232. [DOI] [PubMed] [Google Scholar]
- 26.Maybauer DM, Maybauer MO, Szabo C, Westphal M, Traber LD, Enkhbaatar P, Murthy KG, Nakano Y, Salzman AL, Herndon DN, Traber DL. Lung-protective effects of the metalloporphyrinic peroxynitrite decomposition catalyst WW-85 in interleukin-2 induced toxicity. Biochem Biophys Res Commun. 2008;19:786–791. doi: 10.1016/j.bbrc.2008.10.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moncada S, Palmer RM, Higgs EA. The discovery of nitric oxide as the endogenous nitrovasodilator. Hypertension. 1988;12:365–372. doi: 10.1161/01.hyp.12.4.365. [DOI] [PubMed] [Google Scholar]
- 28.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 29.Murakami K, Bjertnaes LJ, Schmalstieg FC, McGuire R, Cox RA, Hawkins HK, Herndon DN, Traber LD, Traber DL. A novel animal model of sepsis after acute lung injury in sheep. Crit Care Med. 2002;30:2083–2090. doi: 10.1097/00003246-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Murakami K, Enkhbaatar P, Yu YM, Traber LD, Cox RA, Hawkins HK, Tompkins RG, Herndon D, Traber DL. L-Arginine Attenuates Acute Lung Injury after Smoke Inhalation and Burn Injury in Sheep. Shock. 2007;28:477–483. doi: 10.1097/shk.0b013e31804a59bd. [DOI] [PubMed] [Google Scholar]
- 31.Pou S, Pou WS, Bredt DS, Snyder SH, Rosen GM. Generation of superoxide by purified brain nitric oxide synthase. J Biol Chem. 1992;267:24173–24176. [PubMed] [Google Scholar]
- 32.Pritchard KA, Jr, Groszek L, Smalley DM, Sessa WC, Wu M, Villalon P, Wolin MS, Stemerman MB. Native low-density lipoprotein increases endothelial cell nitric oxide synthase generation of superoxide anion. Circ Res. 1995;77:510–518. doi: 10.1161/01.res.77.3.510. [DOI] [PubMed] [Google Scholar]
- 33.Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 34.Rudolph V, Freeman BA. Cardiovascular consequences when nitric oxide and lipid signaling converge. Circ Res. 2009;105:511–522. doi: 10.1161/CIRCRESAHA.109.202077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simon F, Fernandez R. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. J Hypertens. 2009;27:1202–1216. doi: 10.1097/HJH.0b013e328329e31c. [DOI] [PubMed] [Google Scholar]
- 36.Ueda S, Kato S, Matsuoka H, Kimoto M, Okuda S, Morimatsu M, Imaizumi T. Regulation of cytokine-induced nitric oxide synthesis by asymmetric dimethylarginine: role of dimethylarginine dimethylaminohydrolase. Circ Res. 2003;92:226–233. doi: 10.1161/01.res.0000052990.68216.ef. [DOI] [PubMed] [Google Scholar]
- 37.Vallance P, Leone A, Calver A, Collier J, Moncada S. Endogenous dimethylarginine as an inhibitor of nitric oxide synthesis. Journal of Cardiovascular Pharmacology. 1992;20(Suppl 12):S60–S62. doi: 10.1097/00005344-199204002-00018. [DOI] [PubMed] [Google Scholar]
- 38.Vasquez-Vivar J, Kalyanaraman B, Martasek P, Hogg N, Masters BS, Karoui H, Tordo P, Pritchard KA., Jr Superoxide generation by endothelial nitric oxide synthase: the influence of cofactors. Proc Natl Acad Sci U S A. 1998;95:9220–9225. doi: 10.1073/pnas.95.16.9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vidal Melo MF, Harris RS, Layfield D, Musch G, Venegas JG. Changes in regional ventilation after autologous blood clot pulmonary embolism. Anesthesiology. 2002;97:671–681. doi: 10.1097/00000542-200209000-00022. [DOI] [PubMed] [Google Scholar]
- 40.Wang YH, Shen YC, Liao JF, Lee CH, Chou CY, Liou KT, Chou YC. Anti-inflammatory effects of dimemorfan on inflammatory cells and LPS-induced endotoxin shock in mice. Br J Pharmacol. 2008;154:1327–1338. doi: 10.1038/bjp.2008.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 42.Wells SM, Buford MC, Migliaccio CT, Holian A. Elevated Asymmetric Dimethylarginine Alters Lung Function and Induces Collagen Deposition in Mice. Am J Respir Cell Mol Biol. 2008;40:179–188. doi: 10.1165/rcmb.2008-0148OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wells SM, Holian A. Asymmetric Dimethylarginine Induces Oxidative and Nitrosative Stress in Murine Lung Epithelial Cells. Am J Respir Cell Mol Biol. 2007;36:520–528. doi: 10.1165/rcmb.2006-0302SM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xia Y, Tsai AL, Berka V, Zweier JL. Superoxide generation from endothelial nitric-oxide synthase. A Ca2+/calmodulin-dependent and tetrahydrobiopterin regulatory process. J Biol Chem. 1998;273:25804–25808. doi: 10.1074/jbc.273.40.25804. [DOI] [PubMed] [Google Scholar]
- 45.Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong L, Hou L, Wang G. Hydrogen Gas Improves Survival Rate and Organ Damage in Zymosan-Induced Generalized Inflammation Model. Shock. 2010;34:495–501. doi: 10.1097/SHK.0b013e3181def9aa. [DOI] [PubMed] [Google Scholar]
- 46.Ying S, Meng Q, Barata LT, Robinson DS, Durham SR, Kay AB. Associations between IL-13 and IL-4 (mRNA and protein), vascular cell adhesion molecule-1 expression, and the infiltration of eosinophils, macrophages, and T cells in allergen-induced late-phase cutaneous reactions in atopic subjects. J Immunol. 1997;158:5050–5057. [PubMed] [Google Scholar]


