SUMMARY
Tissue transglutaminase (tTG) is a GTP-binding protein/acyl transferase whose expression is up-regulated in glioblastoma and associated with decreased patient survival. Here we delineate a unique mechanism by which tTG contributes to the development of gliomas, by using two glioblastoma cell lines, U87 and LN229, whose growth and survival are dependent on tTG. We show that tTG significantly enhances the signaling activity and lifetimes of EGF-receptors (EGFRs) in these brain cancer cells. Moreover, over-expressing tTG in T98G glioblastoma cells that normally express low levels of tTG, caused a marked up-regulation of EGFR expression and transforming activity. We further show that tTG accentuates EGFR-signaling by blocking c-Cbl-catalyzed EGFR ubiquitylation, through the ability of tTG to bind GTP and adopt a specific conformation that enables it to interact with c-Cbl. These findings demonstrate that tTG contributes to gliomagenesis by interfering with EGFR down-regulation and thereby promoting transformation.
INTRODUCTION
Glioblastoma multiforme, also known as glioblastoma or grade IV astrocytoma, represents one of the most prevalent and aggressive forms of primary brain tumor that occurs in humans. Glioblastomas are therapeutically challenging due to the collective effects of a number of traits frequently exhibited by these types of tumors including rapid growth rates, resistance to radiation and chemotherapy, a high recurrence rate following surgical resection, and an ability to infiltrate surrounding normal tissue (Furnari et al., 2007). As a result, patients with glioblastomas tend to survive only 12-17 months following their initial diagnosis, even despite having received a multimodal therapy regiment (Stupp et al., 2005). Thus, there continues to be an over-riding need to develop additional strategies to manage this devastating form of cancer.
In an effort to identify new potential targets for therapeutic intervention, we searched for proteins whose expression is up-regulated in glioblastoma and correlated with a poor patient prognosis. One intriguing candidate that emerged is tTG, a GTP-binding protein/acyl transferase previously reported to be one of eleven metastasis-associated proteins selectively amplified in human lung and breast cancers (Jiang et al., 2003a; Jiang et al., 2003b). tTG promotes the growth and survival of several different cancer cell types (Kim et al., 2011; Li et al., 2010), outcomes that are largely thought to be dependent on its acyl transferase (protein crosslinking) activity. In order to determine how tTG contributes to the development of malignant brain cancer, we used glioblastoma cell lines whose aberrant growth and survival are highly dependent upon tTG. Here we show that tTG plays an important role in the transformed properties of these cancer cells by having a major influence on EGFR protein levels and signaling activities. The ability of tTG to affect EGFR expression and function has significant implications for brain cancer given that this receptor tyrosine kinase has been shown to trigger mitogenic and survival responses in both normal astrocytes and brain tumor-derived cell lines (Lund-Johansen et al., 1990; Rousselet et al., 2012). Moreover, ectopic expression of the EGFR in normal cell types induces their transformation in a ligand-dependent manner, suggesting that increased signaling by the EGFR plays a critical role in promoting human malignancies (Moscatello et al., 1996).
At the protein level, the EGFR is over-expressed in approximately 60-90% of all glioblastomas, with the extent of EGFR expression being correlated with poor patient outcomes (Shinojima et al., 2003; Umesh et al., 2009). Although amplification of the gene encoding the EGFR can account for the aberrant EGFR expression detected in 30-40% of primary brain tumors or brain tumor-derived cell lines (Guillaudeau et al., 2009; Libermann et al., 1985), additional mechanisms must be involved to account for the increased EGFR protein levels observed in those glioblastoma cases where gene amplification does not occur, as well as for the excessive and sustained EGFR-signaling that is characteristic of these brain cancers. Thus, it seemed likely that the disruption of the normal (negative) regulation of EGFRs contributes to the aberrant EGFR-signaling capabilities exhibited in at least some glioblastomas. Indeed, it is through the regulation of EGFR degradation where tTG appears to exert a major influence, as we show that it affects the ability of c-Cbl, an E3 ubiquitin ligase, to target the EGFR for lysosomal degradation. This involves the ability of tTG, when bound to GTP and having adopted a specific GTP-induced conformational state, to associate with c-Cbl and block the c-Cbl-catalyzed ubiquitylation and degradation of EGFRs, thereby significantly enhancing and extending EGFR-signaling activities.
RESULTS
tTG is over-expressed in human brain tumors
tTG functions both as a GTPase and acyl transferase whose expression and activation have been shown to be up-regulated in several different types of human cancer including breast, ovarian, and pancreatic cancer (Miyoshi et al., 2010; Singer et al., 2006; Verma et al., 2006). In addition, tTG has been demonstrated to play an important role in the growth, survival, migration and invasive activity of aggressive cancer cells (Li et al., 2011). These findings, coupled with the fact that tTG was identified as a downstream signaling partner of the EGFR, promoting the transformed characteristics of human breast cancer SKBR3 cells (Li et al., 2010), as well as having an essential function in the EGF-stimulated migration and invasion of different cancer cells (Antonyak et al., 2009; Boroughs et al., 2011), made it attractive to consider that tTG plays an important role in the development of brain cancer.
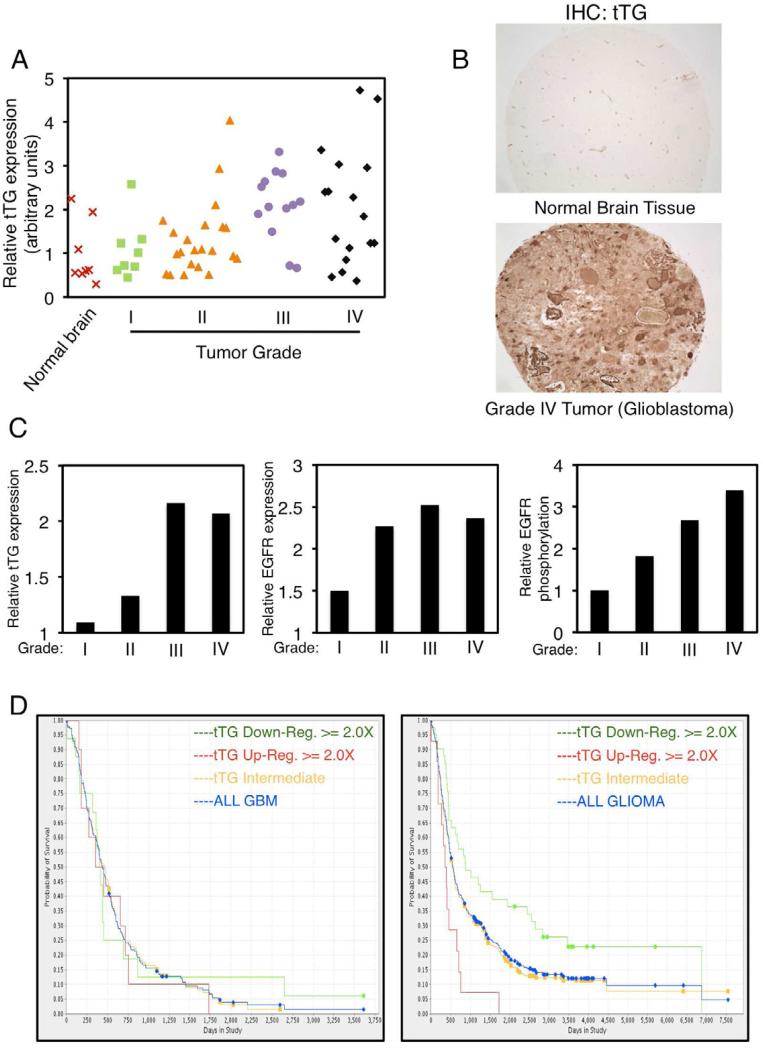
We began by examining tTG expression levels in human primary brain tumors. A tissue array consisting of normal brain tissues and astrocytomas ranging from grade I to grade IV (grade IV astrocytomas are also referred to as glioblastomas) was immuno-stained for tTG. We found that, like the case for the normal human astrocyte (NHA) controls, tTG expression in normal brain is generally low (Figures 1A, 1B, and S1A, top panel). Similarly, tTG is often expressed at relatively low levels in the lower grade (grades I and II) astrocytomas (Figure 1C, left panel). However, in the more advanced/aggressive brain tumors (i.e. grades III and IV astrocytomas), tTG expression was frequently up-regulated. For example, greater than 80% of the grade III astrocytomas and nearly 60% of the grade IV astrocytomas showed increased tTG expression (Figure 1A). Figure 1B shows a representative comparison of tTG expression in a normal brain sample versus a grade IV astrocytoma. We then took our analysis one step further by comparing tTG transcript levels in the four sub-types of glioblastomas that have been identified according to their unique molecular signatures (Verhaak et al., 2010) using The Cancer Genome Atlas (TCGA). Figure S1B shows that tTG transcript levels are increased in the mesenchymal (~45%) and classical (~16%) tumor sub-types.
Figure 1. tTG expression is up-regulated in high grade human brain tumors and correlates with poor patient outcomes.
(A-C) Tissue arrays of human primary brain tumors of increasing grades and normal brain samples were subjected to immunohistochemical analysis using tTG, EGFR and phospho-EGFR antibodies.
(A) Expression levels of tTG detected in the tissue array. Each symbol on the chart represents an individual normal brain or tumor sample as indicated.
(B) Representative images of a normal brain sample and a grade IV tumor (glioblastoma) stained for tTG. Magnification is ×10.
(C) The expression levels of tTG (left) and the EGFR (middle), and the levels of EGFR phosphorylation (right) shown represent their enhanced expression in brain tumor tissue relative to their expression in normal brain tissue (which was set to 1).
(D) Kaplan-Meier Survival Plots for glioblastoma (GBM) patients (left panel) and glioma patients (right panel) with differential tTG expression levels. Data cited from REMBRANDT, National Cancer Institute, accessed 2013, March 18.
See also Figure S1.
Overall, the trend we observed for tTG expression matched that for EGFR expression and phosphorylation (Figure 1C, middle and right panels). While we were not able to draw a strict correlation between tTG expression and the survival of glioblastoma patients (Figure 1D, left panel), we did find a correlation based on tumor grade, similar to what has been observed for the EGFR (Quaranta et al., 2007; Umesh et al., 2009). Individuals with gliomas of various grades whose tumors showed a 2-fold or greater increase in tTG expression had an ~25% reduction in their expected lifespan, whereas those individuals with a 2-fold or greater decrease in tTG expression benefited by an ~20% increase in their survival (Figure 1D, right panel).
tTG is essential for the transformed properties of glioblastoma cell lines
In order to learn more about the potential role played by tTG in brain cancer, we set out to identify human glioblastoma cell lines that exhibited tTG-dependent transformed phenotypes. Three commonly used human glioblastoma cell lines, U87, LN229, and T98G cells, were examined for tTG expression and acyl transferase activity. As shown in Figure S1A, top panel, U87 and LN229 cells exhibited significantly higher levels of tTG expression compared to NHAs, while T98G cells exhibited only a modest increase in tTG levels. The same was true when comparing the enzymatic transamidation activity catalyzed by tTG in lysates prepared from these different cell lines, as read-out by assaying the incorporation of biotinylated pentylamine (BPA) into their lysate proteins (Figure S1A, bottom panel) (Antonyak et al., 2004).
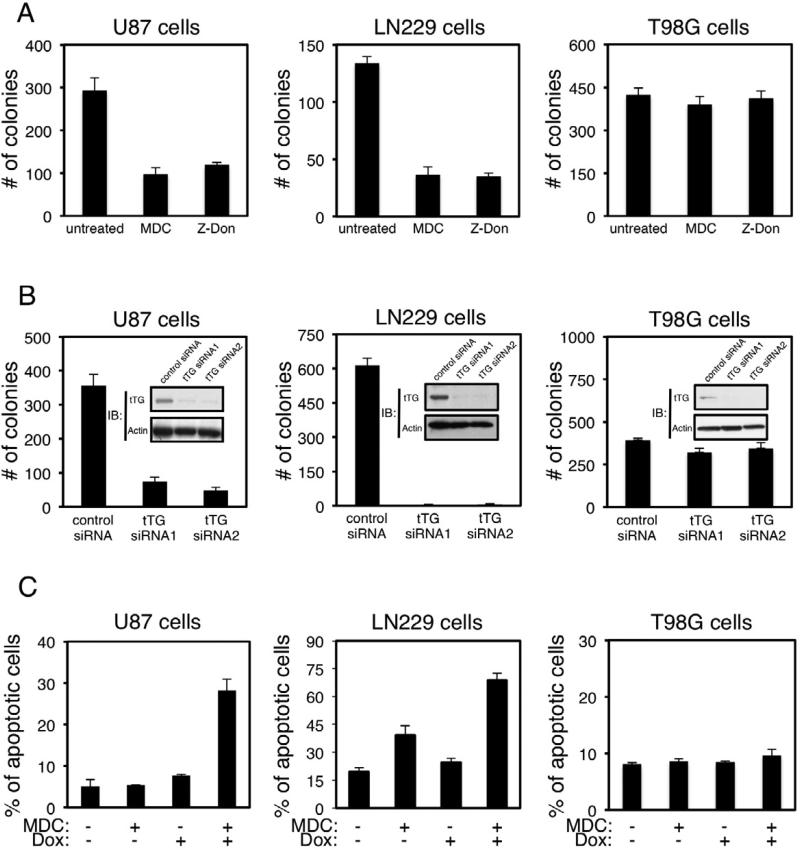
We next asked whether the expression of tTG in these glioblastoma cells contributed to their ability to exhibit transformed phenotypes. The anchorage-independent growth of U87, LN229, and T98G cells was assayed under conditions where tTG activity was blocked using two distinct tTG inhibitors, mono-dansyl cadaverine (MDC) and Z-Don, or after tTG expression was knocked-down by siRNA (Figures S2A and S2B). Figure 2A shows that the ability of both U87 and LN229 cells to form colonies in soft agar was sensitive to MDC and Z-Don, with the number of colonies formed by each of these cell lines being reduced by at least 60% upon treatment with the inhibitors. Likewise, the knock-down of tTG expression in U87 and LN229 cells using two different siRNAs (Figure 2B, insets) inhibited colony formation (Figure 2B). In contrast, the anchorage-independent growth of T98G glioblastoma cells was not dependent on tTG, as it was insensitive to tTG inhibitors MDC and Z-Don, as well as to siRNAs targeting tTG (Figures 2A and 2B).
Figure 2. tTG is essential for the transformed characteristics of U87 and LN229 glioblastoma cells.
(A) Soft agar colony formation assays were performed on U87, LN229 and T98G cells treated without or with MDC or Z-Don. After 10 days of growth, the colonies that formed were counted.
(B) U87, LN229, and T98G cells transfected with control or tTG siRNAs were either lysed and immunoblotted (IB) with tTG and actin antibodies (insets), or subjected to soft agar colony formation assays. After 10 days of growth, the colonies that formed were counted.
(C) Apoptotic assays were performed on U87, LN229 and T98G cells cultured in serum-free medium supplemented without or with MDC, and without or with doxorubicin (Dox), for 1 day. Apoptotic cells were identified by the presence of condensed or blebbed nuclei.
(A-C) Data are represented as mean ± SEM.
See also Figure S2.
It was previously shown that these brain cancer cells are resistant to chemotherapy (Qian et al., 2012; Ren et al., 2010; Weaver et al., 2003), and indeed challenging the different glioblastoma cell lines with doses of doxorubicin that potently killed other types of human cancer cells (i.e. SKBR3 breast cancer cells) (Antonyak et al., 2004), induced only modest increases in cell death beyond that normally observed in the absence of any treatment (Figure 2C). When assaying the survival of these glioblastoma cell lines following their exposure to various combinations of MDC and doxorubicin, we found that MDC treatment alone caused little or no increase in their rates of apoptosis (Figure 2C). However, when the cells were treated with both MDC and doxorubicin, an ~2-3 fold increase in apoptosis occurred in U87 and LN229 cells compared to what was observed with doxorubicin alone (Figure 2C, left and middle panels), whereas, treatment of T98G cells with the combination of MDC and doxorubicin showed essentially no effect (Figure 2C, right panel). Together, these findings demonstrate that the over-expression of tTG that occurs in highly aggressive human brain tumors and glioblastoma cell lines (U87 and LN229) strongly contributes to their aberrant growth and chemoresistance.
tTG regulates EGFR levels and the extent of EGFR-signaling activities in brain tumor cells
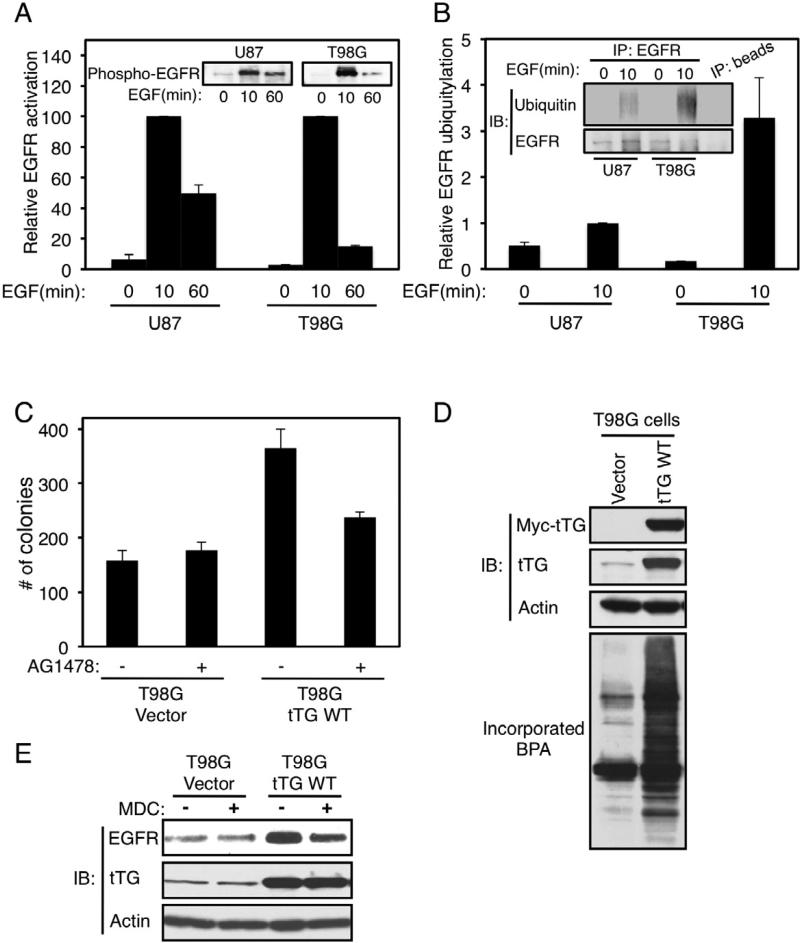
Both tTG and EGFR expression levels are frequently up-regulated in high grade brain tumors. Thus, we examined whether EGFR-signaling might be enhanced and/or extended in a glioblastoma cell line that showed relatively high tTG expression and whose transformed properties were dependent upon tTG (U87 cells), compared to a cell line that expressed relatively low levels of tTG and was not dependent upon it for transformation (T98G cells). Indeed, we found that the signaling lifetimes of activated EGFRs in U87 cells were extended, compared to the case for T98G cells (Figure 3A). This appeared to be preceded by a significantly greater extent of EGFR ubiquitylation in T98G cells compared to U87 cells (Figure 3B).
Figure 3. EGFR signaling is potentiated in U87 glioblastoma cells.
(A) Extracts from U87 and T98G cells that had been cultured in serum-free medium and then further stimulated without or with EGF for increasing lengths of time, were immunoblotted (IB) using a phospho-EGFR antibody (insets). The relative activity of the EGFR in each cell line was plotted (the EGFR activity detected at 10 min of EGF stimulation was set as the maximal activity for each cell line).
(B) Immunoprecipitations with an EGFR antibody (IP: EGFR) were performed on the extracts from U87 and T98G cells that had been cultured in serum-free medium and then further stimulated without or with EGF for 10 min. The resulting immuno-complexes were immunoblotted (IB) with EGFR and ubiquitin antibodies, and the relative ubiquitylation levels of the EGFR for each sample were plotted (the level of EGFR ubiquitylation detected in U87 cells stimulated with EGF for 10 min was set as 1).
(C) Soft agar colony formation assays were performed on the T98G stable cell lines treated without or with AG1478.
(A-C) Data are represented as mean ± SEM.
(D) Extracts from T98G cells stably expressing the vector-alone or a Myc-tagged wild-type tTG (tTG WT) were immunoblotted (IB) with Myc, tTG, and actin antibodies (top three panels), as well as assayed for their enzymatic transamidation activity, as read-out by the incorporation of BPA into lysate proteins (bottom panel).
(E) Extracts from the T98G stable cell lines cultured in serum-free medium supplemented without or with MDC were immunoblotted (IB) with EGFR, tTG and actin antibodies.
We then set out to establish that tTG is responsible for enhancing EGFR-signaling in glioblastoma. First, we took advantage of the fact that T98G glioblastoma cells have relatively low levels of tTG expression and are not dependent upon tTG for their survival or transformed growth properties. Interestingly, these brain cancer cells also showed virtually no dependence on EGFR-signaling for their transformed characteristics, as indicated by their insensitivity to the EGFR tyrosine kinase inhibitor AG1487 (Figure 3C, compare the first and second histograms). We then generated T98G cells that stably expressed either vector-alone or a Myc-tagged form of tTG (Figure 3D, top two panels). As expected, lysates from the cells over-expressing Myc-tagged tTG exhibited significantly more transamidation activity than lysates from cells expressing vector-alone (Figure 3D, bottom panel). While T98G cells that expressed just the vector-alone were capable of forming colonies in soft agar, cells that ectopically expressed tTG showed a significant increase (i.e. ~2-fold) in their anchorage-independent growth (Figure 3C, compare the first and third bars). Moreover, upon ectopically expressing tTG in T98G cells, the EGFR protein levels increased by 3- to 4-fold (Figure 3E, top panel, compare the first and third lanes). This increase in EGFR levels was off-set upon treatment of the cells with the tTG inhibitor MDC (Figure 3E, compare the third and fourth lanes), thereby directly demonstrating that tTG influences EGFR expression. We then asked whether the ability of tTG to potentiate EGFR levels in T98G transfectants was important for the enhanced transforming capabilities exhibited by the T98G cells stably over-expressing tTG. As shown in Figure 3C, the enhanced colony formation exhibited by T98G cells upon the ectopic expression of tTG was nearly completely eliminated by AG1478 (compare the third and fourth histograms), indicating that the enhancement in transformation accompanying over-expression of tTG was dependent upon EGFR tyrosine kinase activity.
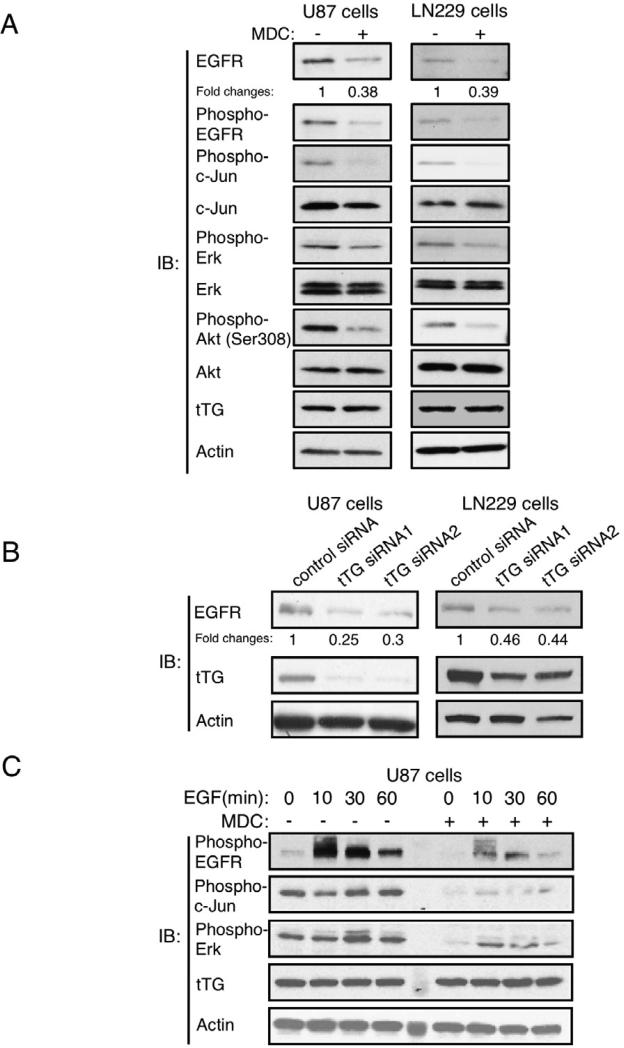
We then made use of the U87 and LN229 glioblastoma cell lines to further establish that tTG mediates the up-regulation of EGFR expression and signaling. Figure 4A (top two panels) shows that treatment of both U87 and LN229 cells with the tTG inhibitor MDC consistently reduced the basal levels of EGFR expression and activation, as read-out using an anti-pan-EGFR antibody and an anti-phospho-EGFR antibody that detects EGFR auto-phosphorylation, respectively. Likewise, MDC treatment markedly reduced the amount of EGFR-stimulated phosphorylation of c-Jun, ERK and Akt in both cell lines (Figure 4A, third, fifth and seventh panels), signaling outcomes occurring downstream from the EGFR that are frequently observed in primary brain tumors and brain tumor-derived cell lines, including U87 cells (Figure S3A) (Antonyak et al., 2002; Hu et al., 2011; Mizoguchi et al., 2006). To further confirm a role for tTG in regulating EGFR expression levels, we examined whether a decrease in the cellular steady-state levels of the EGFR accompanied siRNA-mediated knock-downs of tTG. Indeed, the siRNA-induced reduction in tTG levels by ~80% in U87 cells and ~50% in LN229 cells (Figure 4B, second panels from the top), resulted in corresponding decreases in EGFR expression (Figure 4B, top panels).
Figure 4. tTG regulates EGFR levels and signaling activity in glioblastoma cells.
(A) Extracts from U87 and LN229 cells cultured in serum-free medium supplemented without or with MDC for 1 day were immunoblotted (IB) using the indicated antibodies. The fold changes in EGFR levels, as determined using ImageJ, are highlighted.
(B) Extracts from U87 cells and LN229 cells expressing control or tTG siRNAs were immunoblotted (IB) using EGFR, tTG, and actin antibodies. The fold changes in EGFR levels, as determined using ImageJ, are highlighted.
(C) Extracts from U87 cells that had been cultured in serum-free medium and then treated without or with MDC for 30 min before being further stimulated with EGF for increasing lengths of time, were immunoblotted (IB) using the indicated antibodies.
See also Figure S3.
We next examined whether tTG influenced the extent of EGFR-signaling activities in these glioblastoma cells. Serum-starved cultures of U87 cells were treated with EGF for increasing lengths of time, in the presence or absence of MDC. Figure 4C shows that a maximal activation of EGFR tyrosine kinase activity occurred within 10 minutes of growth factor treatment (top panel). EGF stimulation of U87 cells pretreated with MDC showed a marked reduction in the magnitude of EGFR activation. Cell surface biotin-labeling experiments performed on the cells showed that this was due to reductions in the EGFR levels at the plasma membrane (Figure S3B). Moreover, the phosphorylation of c-Jun (Figure 4C, second panel from the top) and ERK (Figure 4C, third panel from the top) was significantly reduced in U87 cells pretreated with MDC. While each of these signaling events showed little dependence on EGF stimulation, the basal activities of both were dependent on EGFR activity as indicated by the inhibition caused by the specific EGFR tyrosine kinase inhibitor AG1478 (Figure S3A). EGFR activation was also compromised in LN229 glioblastoma cells treated with the tTG inhibitor MDC (Figure S3C).
We also examined whether tTG could influence the levels of the EGFR variant type III (EGFRvIII), a commonly occurring and highly oncogenic mutant form of the EGFR that is defective in its down-regulation (Huang et al., 2009). Figure S3D shows that ectopic EGFRvIII expression in U87 cells was not affected by MDC treatment (compare the first and second lanes to the third and fourth lanes). This suggests that tTG is not important for extending the lifetime of this mutant EGFR, most likely because unlike the wild-type EGFR, this truncated EGFR variant has an inherent insensitivity to the degradative actions of c-Cbl (Han et al., 2006; also, see below).
tTG blocks ubiquitylation of the EGFR
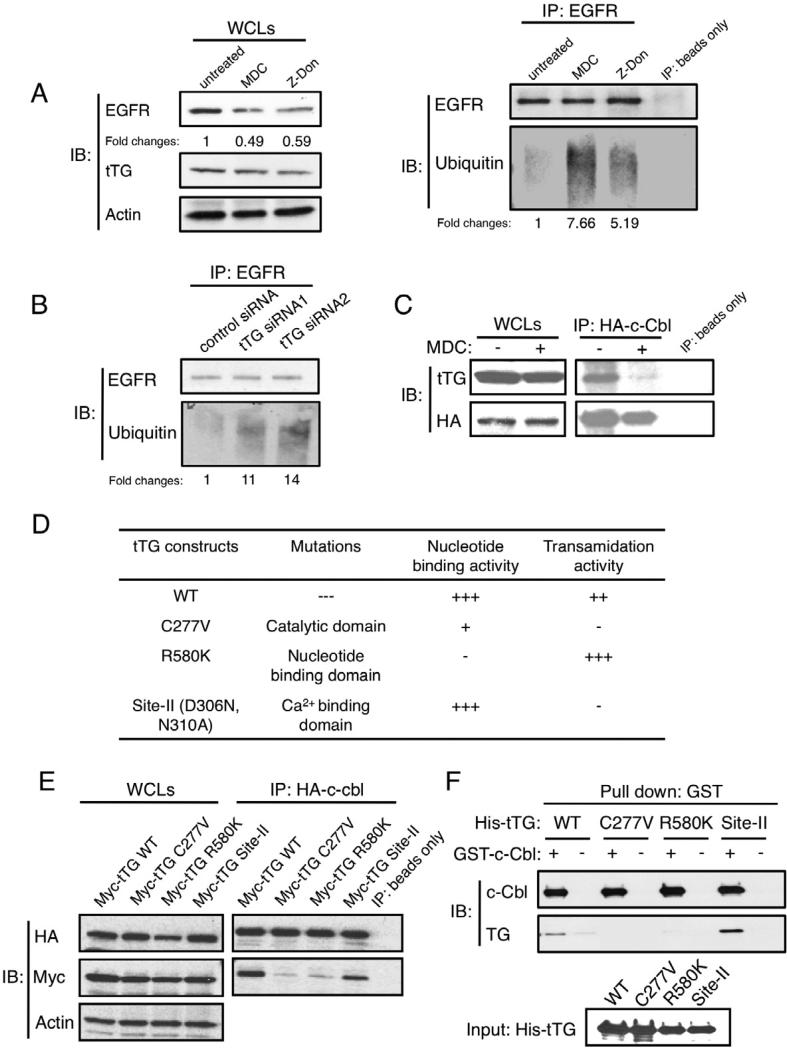
Taken together, the results presented in Figures 3 and 4, as well as those shown in Figure 5A, left panel, suggest that the actions of tTG are directed at enhancing the stability of wild-type EGFRs. The ubiquitylation-mediated down-regulation of the EGFR, as catalyzed by the E3 ubiquitin ligase c-Cbl, plays an essential role in terminating EGFR-signaling activities by targeting the receptor for degradation in the lysosomes (Levkowitz et al., 2005; Waterman et al., 1999). Figure 5A, right panel, shows that following treatment of U87 cells with MDC or Z-Don, the amount of detectable ubiquitylation of the immunoprecipitated EGFR was enhanced, compared to an equivalent amount of EGFR immunoprecipitated from untreated cells. It is worth noting that the overall levels of ubiquitylated proteins in U87 cells were largely unaffected by MDC or Z-Don treatment (Figure S4A), suggesting that the changes in the ubiquitylation status of the EGFR caused by tTG represented a specific regulatory event. Similar increases in EGFR ubiquitylation were observed when tTG expression was knocked-down by siRNAs in U87 cells (Figure 5B), or when LN229 cells were exposed to MDC (Figure S4B).
Figure 5. tTG influences the ubiquitylation of the EGFR by associating with E3 ubiquitin ligase c-Cbl.
(A) Immunoprecipitations with an EGFR antibody (IP:EGFR) were performed on the extracts from U87 cells that were cultured in serum-free medium supplemented without or with MDC or Z-Don for 1 day. The resulting immuno-complexes as well as samples of the whole cell lysates (WCLs) were immunoblotted (IB) with EGFR, ubiquitin, tTG and actin antibodies. The fold changes in ubiquitinylated EGFR, as determined using ImageJ, are highlighted.
(B) Immunoprecipitations with an EGFR antibody (IP: EGFR) were performed on the extracts from U87 cells expressing control or tTG siRNAs. The resulting immuno-complexes were then immunoblotted (IB) with EGFR and ubiquitin antibodies.
(C) Immunoprecipitations with an HA antibody (IP: HA-c-Cbl) were performed on extracts from U87 cells transfected with HA-tagged c-Cbl (HA-c-Cbl) and cultured in serum-free medium supplemented without or with MDC for 1 day. The resulting immuno-complexes, as well as the whole cell extracts collected (WCLs), were immunoblotted (IB) with tTG and HA antibodies.
(D) List of the various tTG constructs used in this study including: the name of each construct, the sites of the mutations, and their functional consequences on nucleotide binding and enzymatic transamidation activity are indicated.
(E) Immunoprecipitations with an HA antibody (IP: HA-c-Cbl) were performed on extracts from U87 cells transfected with HA-tagged c-Cbl and either Myc-tagged wild-type tTG (Myc-tTG WT) or one of the indicated Myc-tagged mutant forms of tTG listed in D. The resulting immuno-complexes, as well as the whole cell extracts collected (WCLs), were immunoblotted (IB) with HA, Myc, and actin antibodies.
(F) Purified, recombinant His-tagged, wild-type (WT) tTG, or one of the mutant forms of tTG, were incubated alone or together with an equal amount of recombinant GST-tagged c-Cbl. The resulting protein complexes were precipitated with GST beads and then immunoblotted with tTG and c-Cbl antibodies (Pull down: GST). Each form of His-tagged tTG used in the pull down assay was also immunoblotted with a tTG antibody to confirm that equal amounts of recombinant tTG were used in the experiment (Input).
See also Figure S4.
In an effort to understand the molecular mechanism by which tTG inhibits EGFR ubiquitylation, we examined whether tTG might be capable of associating with c-Cbl in cells. Figure 5C shows that endogenous tTG can be co-immunoprecipitated with HA-tagged c-Cbl from U87 cells. This interaction was greatly reduced when the cells were treated with MDC. Based on these findings, our initial assumption was that the acyl transferase or transamidation activity of tTG was in some way involved in its ablity to associate with and/or negatively regulate the function of c-Cbl. Such an idea was consistent with several findings that suggested the enzymatic transamidation activity of tTG was necessary for mediating many of its effects in cells (Antonyak et al., 2004; Datta et al., 2006; Verma et al., 2006).
We therefore set out to further examine this possibility by using various tTG mutants that we have developed in the laboratory (Figure 5D). The results in Figure 5E (panels on the right) show that a Myc-tagged wild-type tTG construct was capable of associating with HA-tagged c-Cbl, similar to what we had observed with endogenous tTG, whereas, a transamidation-defective mutant, tTG C277V, in which the active site cysteine residue had been changed to a valine (Li et al., 2010), was incapable of being co-immunoprecipitated with c-Cbl. This seemed to support the suggestion that the transamidation activity of tTG was involved in the interaction. However, surprisingly, we found that another tTG mutant, tTG D306N, N310A (referred to as the “Site-II mutant”), that was incapable of catalyzing transamidation because of two substitutions at one of the major Ca2+-binding sites essential for catalysis (Datta et al., 2006), was able to associate with c-Cbl (Figure 5E, panels on the right). Moreover, a GTP-binding-defective tTG mutant (tTG R580K), that exhibits greatly enhanced transamidation activity because its enzymatic activity is not subject to the same negative regulation that accompanies the binding of GTP to wild-type tTG (Datta et al., 2007), was incapable of associating with c-Cbl. Collectively, these results ruled out the possibility that the transamidation activity of tTG was required for its ability to associate with and inhibit the E3 ubiquitin ligase activity of c-Cbl.
We then asked whether the ability of tTG to associate with c-Cbl in cells was the outcome of a direct binding interaction. Purified recombinant, His-tagged wild-type tTG, as well as different tTG mutants, were incubated with purified recombinant GST-c-Cbl, and then the proteins were examined for complex formation by precipitating the GST-c-Cbl with glutathione-coated agarose beads. Figure 5F (first lane) shows that His-tagged wild-type tTG was co-precipitated with GST-c-Cbl, indicating that these proteins undergo a direct binding interaction. Likewise, the recombinant His-tagged tTG Site-II mutant was able to bind GST-c-Cbl, whereas His-tagged tTG R580K and His-tagged tTG C277V were ineffective (Figure 5F), consistent with our co-immunoprecipitation data in cells.
The GTP-bound ‘closed’ form of tTG is responsible for inhibiting c-Cbl function
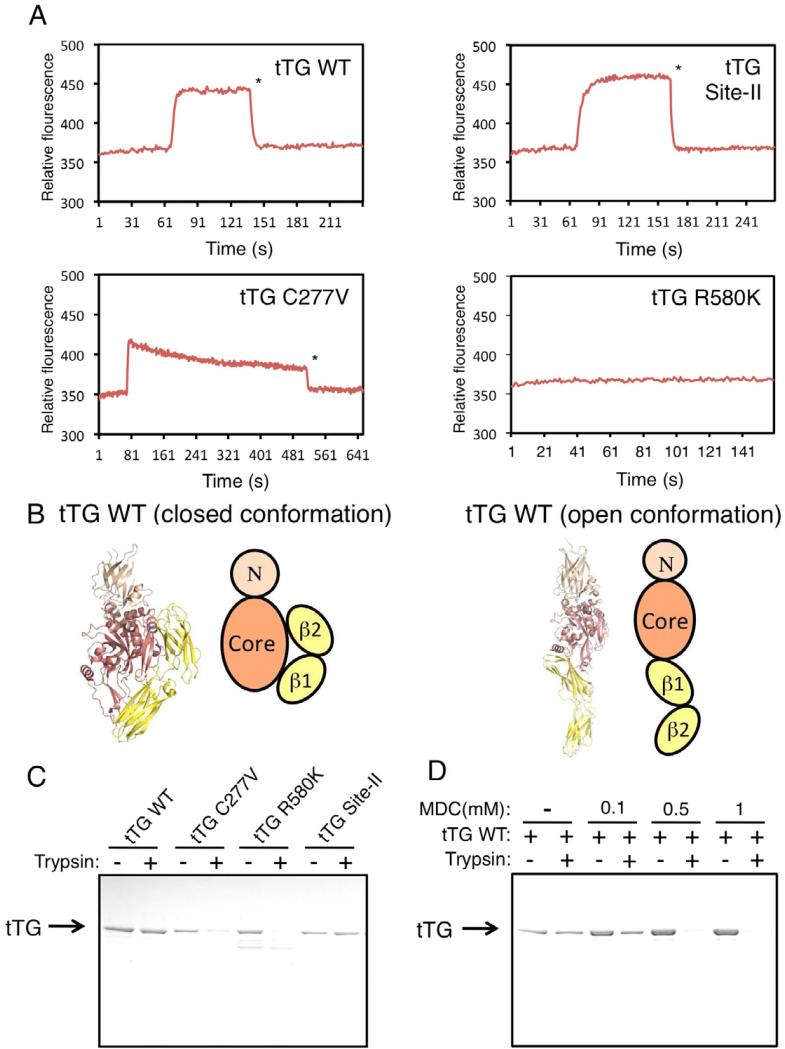
We next examined whether the GTP-binding activity of tTG is involved in its ability to associate with c-Cbl. The GTP-binding capabilities of the different tTG mutants, relative to the wild-type protein, were assayed by taking advantage of the ability of the fluorescent GTP-analog, BODIPY-GTP, to rapidly bind tTG and undergo a significant change in its fluorescence emission (Datta et al., 2007). Figure 6A shows that, like the case for wild-type tTG (top left panel), BODIPY-GTP was capable of rapidly associating with the tTG Site-II mutant (top right panel), with the binding being sustained until the addition of unlabeled GTPγS, which caused a rapid reversal of the interaction with the labeled GTP-analog. BODIPY-GTP also showed a rapid association with the tTG C277V mutant (Figure 6A, bottom left panel); however, this was followed by a steady dissociation of the labeled GTP-analog, indicative of a much weaker interaction compared to either wild-type tTG or the tTG Site-II mutant. The tTG R580K mutant showed essentially no ability to bind BODIPY-GTP (Figure 6A, bottom right panel), consistent with our earlier studies that showed this mutant to be GTP-binding-defective (Datta et al., 2007).
Figure 6. tTG adopts distinct conformations depending on whether it is bound to GTP.
(A) Purified recombinant forms of wild-type tTG (tTG WT), tTG C227V, tTG R580K, and the tTG Site-II mutant were incubated with BODIPY-GTP and the resulting changes in fluorescence caused by the binding of BODIPY-GTP to the proteins were determined. * indicates the point in the assay when an excess of unlabeled GTPγS was added to the tTG proteins to compete off BODIPY-GTP.
(B) X-ray crystal structures and schematic representations of the closed and open conformations of tTG. tTG adopts a closed conformation when it is bound to GDP (PDB: 1KV3) (left side), or an open conformation when it is in a nucleotide free state (PDB: 2Q3Z). The domains highlighted in the tTG diagrams include the N-terminal β sandwich (N), the catalytic core (core), and the two C-terminal β-barrels (β1 and β2).
(C) Purified recombinant forms of wild-type tTG (tTG WT), tTG C277V, tTG R580K, tTG Site-II (3.0 μg of each protein) were incubated without or with trypsin for 2 h before being resolved by SDS-PAGE and then stained with Coomassie blue to visualize the proteins.
(D) Purified recombinant wild-type tTG (3.0 μg) pre-treated without or with increasing amounts of MDC was incubated with trypsin for 2 h before being resolved by SDS-PAGE and then stained with Coomassie blue to visualize the proteins.
X-ray crystallographic studies have shown that tTG can exist in two distinct conformational states. When bound to guanine nucleotide (i.e. GTP-bound in cells), tTG adopts what is referred to as a ‘closed’ conformational state where the β1 and β2 barrel domains fold over the central core of the protein that contains the transamidation catalytic site (Liu et al., 2002) (Figure 6B, left side). However, in the absence of bound GTP, the protein assumes a more extended or ‘open’ conformation in which the catalytic site is fully accessible (Figure 6B, right side). This was first shown when the x-ray structure was solved for tTG bound to a peptide that mimics the inflammatory gluten peptide substrate that binds to the transamidation active site (Pinkas et al., 2007). Thus, when the structural data is taken together with the results of the coimmunoprecipitation and GST pull-down experiments presented in Figures 5E and 5F, as well as the GTP-binding data in Figure 6A, it would appear that it is the GTP-bound, closed state of tTG that is best suited for associating with and functionally inactivating c-Cbl.
Further support for this idea came from studies where we examined the protease-sensitivity of wild-type tTG and the different tTG mutants. Figure 6C shows that wild-type tTG, and the tTG Site-II mutant, exhibited similar capabilities for resisting trypsin proteolysis, whereas, the tTG C277V mutant behaved in a similar manner to the GTP-binding-defective tTG R580K mutant and was highly sensitive to protease treatment. This indicates that the tTG Site-II mutant adopts an overall conformation similar to that of the GTP-bound, wild-type protein, as indicated by its ability to resist trypsin proteolysis, whereas the tTG C277V mutant assumes a conformation more like that of the GTP-binding-defective tTG R580K mutant. Interestingly, we found that MDC, in a dose-dependent manner, enhanced the protease sensitivity of wild-type tTG, thus causing it to behave more like the tTG R580K and tTG C277V mutants (Figure 6D). These findings might then explain why MDC treatment inhibits the ability of tTG to interact with c-Cbl, as read-out in co-immunoprecipitation experiments (Figure 5C). Specifically, rather than weakening the interaction between tTG and c-Cbl by blocking access to the transamidation active site of tTG, the binding of the inhibitor helps tTG to assume a more open conformation that has very weak affinity for c-Cbl.
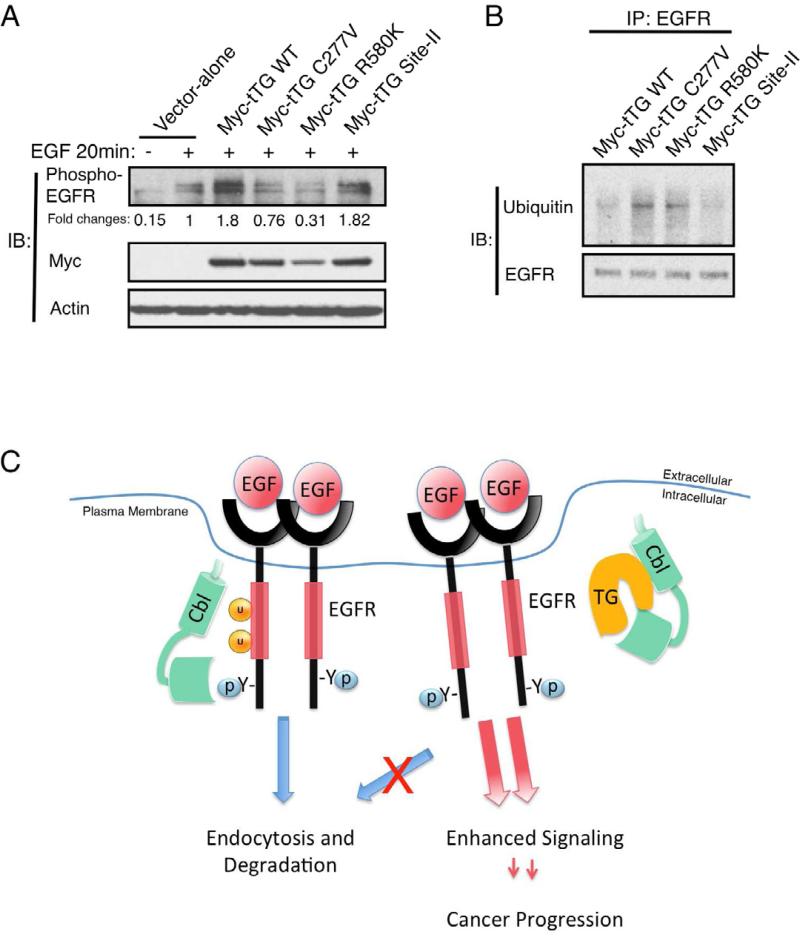
The relative effectiveness of these different mutants to associate with c-Cbl could be correlated with their effects on EGFR activation (auto-phosphorylation) and receptor ubiquitylation. Figure 7A shows that when the different mutants were transiently expressed in U87 cells as Myc-tagged proteins, both wild-type tTG and the Myc-tTG Site-II mutant were able to increase the levels of activated EGFR upon EGF treatment, whereas the Myc-tTG C277V mutant and the GTP-binding-defective Myc-tTG R580K mutant were completely ineffective. Although we were not able to make reliable comparisons of the effects of the different mutants on EGFR ubiquitylation in U87 cells, we were able to compare their actions in the human breast cancer cell line, SKBR3, which lacks endogenous tTG expression. The results of these experiments suggested that only wild-type tTG and the tTG Site-II mutant were able to reduce EGFR ubiquitylation (Figure 7B), which was fully consistent with their abilities to associate with c-Cbl.
Figure 7. Ectopic expression of tTG forms that are in the “closed” conformation enhances EGFR signaling.
(A) Cell extracts from U87 cells ectopically expressing the vector-alone, wild-type tTG (Myc-tTG WT), or one of the different mutant forms of tTG stimulated without or with EGF (100 ng/ml) for 20 min, were immunoblotted (IB) using phospho-EGFR, Myc, and actin antibodies.
(B) Immunoprecipitations with an EGFR antibody (IP: EGFR) were performed on the cell extracts from SKBR3 cells transfected with wild-type (Myc-tTG WT) or one of the different mutant forms of tTG indicated. The resulting immuno-complexes were then immunoblotted (IB) with ubiquitin and EGFR antibodies.
(C) Schematic representation depicting the effects of tTG on EGFR signaling. In normal conditions, c-Cbl is recruited to the activated EGFR and mediates its ubiquitylation. The ubiquitylated EGFR is then endocytosed and targeted for degradation in the lysosome. In brain tumor cells where tTG is abundantly expressed, tTG forms a complex with c-Cbl and prevents it from ubiquitylating the EGFR. This disrupts the normal down-regulation of the EGFR, extending its signaling lifetime and enhancing cellular transformation.
See also Figure S5.
DISCUSSION
In this study, we set out to gain new insights into the molecular mechanisms contributing to the development of malignant gliomas. These efforts led us to tTG, a GTP-binding protein and Ca2+-dependent acyl transferase, whose expression is up-regulated in several types of cancer (Li et al., 2010; Miyoshi et al., 2010; Verma et al., 2006). We show that this is also true for malignant gliomas. While the levels of tTG were consistently low in normal brain, as well as in most grade I and II tumors, tTG expression was noticeably up-regulated in the more aggressive, high grade (i.e. grades III and IV) tumors. In fact, we found that it was over-expressed in ~70% of these cases, making the up-regulation of tTG expression one of the most frequently occurring events in human brain tumors. Moreover, there appears to be a correlation between the increased expression of tTG in brain cancer and a poor survival prognosis for patients.
A number of studies have implicated tTG in cancer cell growth, chemoresistance, invasiveness, and metastasis (Li et al., 2011; Verma et al., 2006), and to be a downstream signaling partner of the EGFR (Antonyak et al., 2009; Boroughs et al., 2011; Li et al., 2010). Its involvement in EGFR-signaling was particularly intriguing to us, since EGFR over-expression and activating EGFR mutations are recurring themes in human cancer and are especially important for the aggressive phenotypes exhibited by high grade primary brain tumors (Frederick et al., 2000; Huang et al., 2009; Layfield et al., 2006). In fact, over-expression of the EGFR has been reported in a large majority of the high grade (grade IV) tumors or glioblastomas, while it is rarely seen in the lower grade I and II brain tumors. Consequently, EGFR status is considered to be a prognostic indicator for patients with brain tumors (Jung et al., 2012; Quaranta et al., 2007; Shinojima et al., 2003).
It has been well established that the amplification of the gene encoding the EGFR represents one common mechanism through which EGFR expression levels are increased in glioblastomas (Layfield et al., 2006). However, 10-30% of brain cancers that show high EGFR expression contain a normal receptor gene copy number. Therefore, additional mechanisms must account for the relatively high EGFR protein levels and enhanced EGFR-signaling observed in some brain cancers (Huang et al., 2009). In light of these findings, we began to consider the idea that a previously unappreciated functional interplay might exist between tTG, the EGFR, and brain tumor progression. This in fact turned out to be the case. We now describe how tTG helps maintain EGFR protein levels in glioblastomas, enhancing the extent and lifetime of EGFR activation and down-stream signaling activities, through a mechanism that does not involve gene amplification, but rather is due to the ability of tTG to interfere with receptor ubiquitylation and degradation that is normally catalyzed by the EGFR-adaptor protein and negative regulator, c-Cbl.
Work by Vivanco et al. showed that PTEN loss can result in a diminished c-Cbl-dependent degradation of EGFRs (Vivanco et al., 2010). Because previous studies from our laboratory showed that the PI3K/Akt pathway is necessary for the up-regulation of tTG expression in breast cancer cells upon growth factor treatment (Li et al., 2010), we asked whether the inactivation of PTEN could regulate tTG levels and that perhaps changes in tTG expression could explain the effects of PTEN on EGFR degradation. Consistent with previous reports, U87 cells contain a truncated version of PTEN, whereas, LN229 cells express wild-type PTEN, while T98G cells contain PTEN harboring a point mutation (Furnari et al., 1997). However, although an inverse correlation appears to exist between the expression levels of tTG and PTEN in the different glioblastoma cells that we examined (Figure S5A), we did not observe any significant change in the levels of tTG when we ectopically expressed increasing amounts of PTEN in U87 cells (Figure S5B) or mimicked PTEN function by inhibiting downstream signaling events with a PI3K inhibitor LY294002 (Figure S5C), suggesting that up-regulation of tTG in these brain cancer cells is not due to inactivation of PTEN.
tTG is best known for its enzymatic, Ca2+-dependent acyl transferase activity that catalyzes the crosslinking of proteins through the formation of covalent linkages between glutamine residues on ‘acceptor’ proteins and lysine residues on ‘donor’ proteins (Li et al., 2011). However, interestingly, tTG is also capable of binding and hydrolyzing GTP similar to other classical G-proteins (Li et al., 2011). These two activities are reciprocally regulated, such that GTP-bound tTG exhibits little detectable transamidation activity, whereas millimolar levels of Ca2+ weaken GTP binding to tTG and thereby stimulate its enzymatic activity. This would imply that intracellular tTG exists in its GTP-bound state, given the high cellular concentrations of GTP and typically low concentrations of Ca2+, whereas upon its secretion, tTG may then be capable of catalyzing protein crosslinking events. In fact, as schematized in Figure 7C and discussed further below, our data suggest it is the GTP-bound, closed state of tTG that is responsible for preventing c-Cbl from exerting its negative regulatory effects on the EGFR.
Two key findings have provided us with a mechanistic picture of how tTG blocks the actions of c-Cbl and thereby helps maintain EGFR protein levels in glioblastoma cells. U87 and LN229 human glioblastoma cell lines are highly dependent on EGFR-signaling for their transformed phenotypes. Interestingly, these cell lines also express inordinately high levels of tTG. Moreover, when tTG expression was knocked-down in these cells by siRNA, corresponding reductions were observed in the expression and activation of the EGFR, as well as in the transforming capabilities of these cancer cell lines.
Perhaps even more striking were the results from experiments that took advantage of the unique qualities of the T98G glioblastoma cell line. Unlike U87 and LN229 cells, T98G cells are not dependent on the EGFR for their transforming potential. Moreover, they do not express, nor are they dependent upon, tTG for their transformed properties. However, when a Myc-tagged form of tTG was stably introduced into these cells, EGFR expression was increased between 3- and 4-fold compared to the vector-alone expressing control cells. Importantly, T98G cells over-expressing tTG were capable of forming nearly twice as many colonies in soft agar as their control counterparts, an outcome that was dependent on the enhanced EGFR activity associated with these cells. Thus, these findings demonstrate that the over-expression of tTG in at least certain brain tumor cell lines is sufficient to maintain relatively high EGFR levels and exacerbate their transformed phenotypes. Since tTG is over-expressed in several additional types of human cancer (i.e. breast, ovarian, and pancreatic cancer), it will be interesting to see whether this interplay between tTG and the EGFR holds up in these cancer types as well.
How does tTG have such profound effects on the actions of c-Cbl, and consequently, EGFR expression in glioblastomas? Given that knock-downs of tTG expression in U87 and LN229 cells, as well as the treatment of these cells with the tTG crosslinking inhibitor MDC or Z-Don, markedly increased the amounts of ubiquitylated EGFRs, we had initially assumed that the transamidation activity of tTG was responsible for its ability to inactivate the E3 ubiquitin ligase activity of c-Cbl. However, a careful analysis of the interaction of tTG with c-Cbl revealed that it did not rely upon, and indeed occurred independently of, tTG's transamidation activity, but instead appeared to be dependent on its GTP-binding capability. This is especially interesting in light of structural studies that show the binding of GTP to tTG causes the protein to adopt a ‘closed conformation’ that maintains it in an enzymatically-inactive state by blocking substrate access to the catalytic domain. Under conditions of high Ca2+ (as might occur when tTG is extracellular), GTP dissociates and tTG adopts an ‘open conformation’, in which the transamidation active site is accessible and able to catalyze the crosslinking of proteins. Thus, the only mutant form of tTG that constitutively adopts the closed state (i.e. Site-II mutant) is capable of binding to c-Cbl and blocking its ability to catalyze EGFR ubiquitylation. The ability of MDC treatment to block tTG from protecting the EGFR from c-Cbl-mediated ubiquitylation is most likely an outcome of this competitive tTG inhibitor causing the protein to adopt an open conformation which has weak affinity for c-Cbl. Similarly, the x-ray structure for Z-Don covalently linked to the active site cysteine of tTG (PDB accession # 3S3J) shows that the inhibitor-tTG complex adopts an open conformation, consistent with the ability of Z-Don to block the protective effects of tTG. Therefore, we believe that it is the GTP-bound, closed state of tTG, perhaps acting in a manner analogous to classical signaling G-proteins, that enables it to associate with c-Cbl and thereby provides a unique mode of regulation of EGFR expression (Figure 7C).
In closing, we have identified a novel mechanism where through the actions of tTG, relatively high EGFR protein levels can be maintained and EGFR-signaling activities enhanced and extended in human brain tumors, thus contributing to their oncogenic phenotypes. Given that a number of therapeutic strategies directed at blocking EGFR activation and function, primarily through the use of tyrosine kinase inhibitors and monoclonal antibodies, have only had limited success in the clinics, there remains an over-riding need to develop novel approaches that target the EGFR. Our findings that tTG is frequently over-expressed in high grade brain tumors, coupled with the fact that it interferes with the proper down-regulation of the EGFR, raises the interesting possibility that developing approaches that block the ability of tTG to interact with c-Cbl may offer potentially new strategies for therapeutic intervention.
EXPERIMENTAL PROCEDURES
Materials
Cell-culture reagents, EGF, Lipofectamine, Lipofectamine 2000, protein-G agarose beads, and the control and tTG siRNAs were obtained from Invitrogen. G418, MDC, AG1478, and doxorubicin were from Calbiochem. Z-Don was from Zedira and BPA was from Pierce. Antibodies for tTG and actin were purchased from Thermo Fisher Scientific. Antibodies for EGFR, phospho-EGFR, phospho-Akt, Akt, phospho-c-Jun, c-Jun, phospho-ERK, ERK, PTEN and phospho-PTEN were from Cell Signaling. Ubiquitin and c-Cbl antibodies were from Santa Cruz, and HA and Myc antibodies were from Covance. Avidin/Biotin blocking solutions, Elite ABC reagent, and chromogen solution for immunohistochemical analysis were obtained from Vector Labs.
Cell culture, transfections, inhibitor treatments
U87 and SKBR3 cells were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), the LN229 and T98G cells were maintained in DMEM supplemented with 10% FBS, while NHAs were maintained in AGM (Lonza) supplemented with the BulletKit (Lonza). The expression constructs were introduced into cells using Lipofectamine, while the control and tTG siRNAs were introduced into cells using Lipofectamine 2000. T98G cells stably expressing the vector-alone or Myc-tagged tTG were selected by supplementing the growth medium with 1 mg/ml G418. Where indicated, cells were treated with 50 μM MDC, 50 μM Z-Don, 10 μM AG1478, 1.0 μM doxorubicin, and 100 ng/ml EGF.
Tumor analysis
Tissue array slides composed of paraffin embedded sections of normal brain samples and human primary brain tumors of various grades (#GL208; U.S. Biomax) were subjected to immunohistochemical analysis. Briefly, the slides were deparaffinized by baking them at 60°C for 30 min and then rehydrating them in water containing 3% hydrogen peroxide for 30 min. After washing with phosphate buffered saline (PBS), the slides were boiled for 10 min in a 10 mM sodium citrate buffer (pH 6.0) and allowed to cool. The slides were then blocked with 2.5% horse serum and with Avidin and Biotin blocking solutions. The tissue arrays were incubated with a tTG, EGFR, or phospho-EGFR antibody, followed by a biotinylated secondary antibody and treatment with the Elite ABC reagent for 30 min. After washing with PBS, the slides were processed with chromogen solution. Lastly, the slides were dehydrated and mounted. The resulting staining obtained with each antibody was quantified using ImageJ software.
REMBRANDT (Repository of Molecular Brain Neoplasia Data) analysis
The REMBRANDT database (http://rembrandt.nci.nih.gov) was used to correlate tTG expression levels in brain tumors with patient survival rates. The parameters were set to include those brain tumor samples (343 gliomas or 181 glioblastomas) with a 2-fold or greater increase or a 2-fold or greater decrease in tTG expression. The results were presented as Kaplan-Meier Survival Plots.
TCGA analysis
tTG transcript levels in glioblastoma patient samples deposited in TCGA (https://tcga-data.nci.nih.gov/tcga/) were analyzed using the cBio Cancer Genomics Portal (z-score of 0.5). The percentage of patients with increased tTG levels were plotted for each glioblastoma sub-type.
Immunoblot analysis and immunoprecipitation
Cells were lysed with cell lysis buffer (25 mM Tris, 100 mM NaCl, 1% Triton X-100, 1 mM EDTA, 1 mM DTT, 1 mM Na3VO4, 1 mM β-glycerolphosphate, 1 μg/mL leupeptin and 1 μg/mL aprotinin). The extracts were resolved by SDS-PAGE and transferred onto PVDF membranes. The membranes were incubated with primary antibodies diluted in TBST (20 mM Tris, 135 mM NaCl, and 0.02% Tween 20). Horseradish-peroxidase-conjugated secondary antibodies were used to detect the primary antibodies followed by exposure to ECL reagent. For immunoprecipitations, cell extracts (600 μg) were incubated with a particular antibody for 2 h, followed by the addition of protein-G beads for 1 h. The beads were washed with cell lysis buffer before being subjected to immunoblot analysis.
Soft agar colony formation assays
Cells (3×103) were suspended in growth medium supplemented without or with the indicated inhibitors and 0.3% agarose and plated on top of a layer of growth medium containing 0.6% agarose in a six-well plate. The growth medium and inhibitors were replenished on the cultures every fourth day, and after 10 days of growth the colonies that formed were counted and graphed.
Apoptotic assays
Cultures of cells were placed in serum-free medium containing various combinations of doxorubicin and MDC. After 1 day, the cells were collected and stained with DAPI for visualization by fluorescence microscopy. Cells undergoing apoptosis were identified by nuclei condensation/blebbing.
Transamidation activity assays
Cell extracts (15 μg of each) were incubated in a buffer containing 10 mM DTT, 10 mM CaCl2, and 50 μM BPA for 10 min. The reactions were stopped with the addition of Laemmli sample buffer, followed by boiling, and then the proteins were resolved on a SDS-PAGE gel, transferred to PDVF membranes, and blocked overnight in BBST (100 mM boric acid, 20 mM sodium borate, 0.01% SDS, 0.01% Tween 20, and 80 mM NaCl) containing 10% bovine serum albumin. The membranes were incubated with horseradish-peroxidase-conjugated streptavidin, diluted at 1:2000 in BBST containing 5% BSA for 1 h, followed by extensive washing with BBST. The proteins that incorporated BPA were visualized on x-ray film after exposing the membranes to ECL reagent.
Cell surface protein isolation assays
Cell surface EGFR was isolated using the cell surface protein isolation kit according to the manufacturer's protocol (Pierce). Briefly, U87 cells exposed to different culturing conditions were incubated with a cell-impermeable biotinylation reagent that labels the exposed primary amines of proteins expressed on the cell surface before being lysed. The EGFR was then immunoprecipitated from the extracts and the cell surface EGFR was detected using HRP-streptavidin.
BODIPY-GTP binding assays
Recombinant tTG (600 nM final concentration) was added into buffer containing 1 mM BODIPY-GTP, 50 mM Tris-HCl, 2 mM DTT and 1 mM EDTA. Fluorescence changes were measured using a Varian eclipse spectrofluorimeter. The excitation and emission wavelengths for BODIPY fluorescence were set at 504 nm and 520 nm, respectively.
Trypsin digestion assays
The recombinant tTG proteins (3 μg of each) were combined with 80 ng of trypsin in a buffer containing 20 mM Tris, 300 mM NaCl and 10% glycerol. The reaction was carried-out on ice for 2 h and stopped with the addition of Laemmli sample buffer, followed by boiling. The proteins were then resolved by SDS-PAGE and the gels were stained with Coomassie blue to visualize the proteins.
In vitro binding assays
Purified, recombinant His-tagged, wild-type tTG, or one of the mutant forms of tTG, was combined without or with purified, GST-tagged c-Cbl (1.0 μg of each protein) in a tube and rotated at 4°C for 2 h. Glutathione-coated agarose beads were then added to each tube for an additional 60 min, at which time the precipitated complexes were subjected to immunoblot analysis using tTG and c-Cbl antibodies.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants from the National Institutes of Health (GM061762 and GM040654). We would also like to thank Cindy Westmiller for her expert administrative assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Antonyak MA, Kenyon LC, Godwin AK, James DC, Emlet DR, Okamoto I, Tnani M, Holgado-Madruga M, Moscatello DK, Wong AJ. Elevated JNK activation contributes to the pathogenesis of human brain tumors. Oncogene. 2002;21:5038–5046. doi: 10.1038/sj.onc.1205593. [DOI] [PubMed] [Google Scholar]
- Antonyak MA, Li B, Regan AD, Feng Q, Dusaban SS, Cerione RA. Tissue transglutaminase is an essential participant in the epidermal growth factor-stimulated signaling pathway leading to cancer cell migration and invasion. J. Biol. Chem. 2009;284:17914–17925. doi: 10.1074/jbc.M109.013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonyak MA, Miller AM, Jansen JM, Boehm JE, Balkman CE, Wakshlag JJ, Page RL, Cerione RA. Augmentation of tissue transglutaminase expression and activation by epidermal growth factor inhibit doxorubicin-induced apoptosis in human breast cancer cells. J. Biol. Chem. 2004;279:41461–41467. doi: 10.1074/jbc.M404976200. [DOI] [PubMed] [Google Scholar]
- Boroughs LK, Antonyak MA, Johnson JL, Cerione RA. A unique role for heat shock protein 70 and its binding partner tissue transglutaminase in cancer cell migration. J. Biol. Chem. 2011;286:37094–37107. doi: 10.1074/jbc.M111.242438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Antonyak MA, Cerione RA. Importance of Ca2+-dependent transamidation activity in the protection afforded by tissue transglutaminase against doxorubicin-induced apoptosis. Biochemistry. 2006;45:13163–13174. doi: 10.1021/bi0606795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta S, Antonyak MA, Cerione RA. GTP-binding-defective forms of tissue transglutaminase trigger cell death. Biochemistry. 2007;46:14819–14829. doi: 10.1021/bi701422h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederick L, Wang XY, Eley G, James CD. Diversity and frequency of epidermal growth factor receptor mutations in human glioblastomas. Cancer Res. 2000;60:1383–1387. [PubMed] [Google Scholar]
- Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- Furnari FB, Lin H, Huang HS, Cavenee WK. Growth suppression of glioma cells by PTEN requires a functional phosphatase catalytic domain. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:12479–12484. doi: 10.1073/pnas.94.23.12479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaudeau A, Durand K, Pommepuy I, Robert S, Chaunavel A, Lacorre S, DeArmas R, Bourtoumieux S, El Demery M, Moreau JJ, Labrousse F. Determination of EGFR status in gliomas: usefulness of immunohistochemistry and fluorescent in situ hybridization. Appl. Immunohistochem. Mol. Morphol. 2009;17:220–226. doi: 10.1097/pai.0b013e31818db320. [DOI] [PubMed] [Google Scholar]
- Han W, Zhang T, Yu H, Foulke JG, Tang CK. Hypophosphorylation of residue Y1045 leads to defective downregulation of EGFRvIII. Cancer biology & therapy. 2006;5:1361–1368. doi: 10.4161/cbt.5.10.3226. [DOI] [PubMed] [Google Scholar]
- Hu J, Jo M, Cavenee WK, Furnari F, VandenBerg SR, Gonias SL. Crosstalk between the urokinase-type plasminogen activator receptor and EGF receptor variant III supports survival and growth of glioblastoma cells. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:15984–15989. doi: 10.1073/pnas.1113416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PH, Xu AM, White FM. Oncogenic EGFR signaling networks in glioma. Sci. Signal. 2009;2:re6. doi: 10.1126/scisignal.287re6. [DOI] [PubMed] [Google Scholar]
- Jiang D, Ying W, Lu Y, Wan J, Zhai Y, Liu W, Zhu Y, Qiu Z, Qian X, He F. Identification of metastasis-associated proteins by proteomic analysis and functional exploration of interleukin-18 in metastasis. Proteomics. 2003a;3:724–737. doi: 10.1002/pmic.200300411. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Ablin R, Douglas-Jones A, Mansel RE. Expression of transglutaminases in human breast cancer and their possible clinical significance. Oncol. Rep. 2003b;10:2039–2044. [PubMed] [Google Scholar]
- Jung Y, Joo KM, Seong DH, Choi YL, Kong DS, Kim Y, Kim MH, Jin J, Suh YL, Seol HJ, Shin CS, Lee JI, Kim JH, Song SY, Nam DH. Identification of prognostic biomarkers for glioblastomas using protein expression profiling. Int. J. Oncol. 2012;40:1122–1132. doi: 10.3892/ijo.2011.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DS, Choi YB, Han BG, Park SY, Jeon Y, Kim DH, Ahn ER, Shin JE, Lee BI, Lee H, Hong KM, Kim SY. Cancer cells promote survival through depletion of the von Hippel-Lindau tumor suppressor by protein crosslinking. Oncogene. 2011;30:4780–4790. doi: 10.1038/onc.2011.183. [DOI] [PubMed] [Google Scholar]
- Layfield LJ, Willmore C, Tripp S, Jones C, Jensen RL. Epidermal growth factor receptor gene amplification and protein expression in glioblastoma multiforme: prognostic significance and relationship to other prognostic factors. Appl. Immunohistochem. Mole. Morphol. 2006;14:91–96. doi: 10.1097/01.pai.0000159772.73775.2e. [DOI] [PubMed] [Google Scholar]
- Levkowitz G, Waterman H, Zamir E, Kam Z, Oved S, Langdon WY, Beguinot L, Geiger B, Yarden Y. c-Cbl/Sli-1 regulates endocytic sorting and ubiquitination of the epidermal growth factor receptor. Genes Dev. 1998;12:3663–3674. doi: 10.1101/gad.12.23.3663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Antonyak MA, Druso JE, Cheng L, Nikitin AY, Cerione RA. EGF potentiated oncogenesis requires a tissue transglutaminase-dependent signaling pathway leading to Src activation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:1408–1413. doi: 10.1073/pnas.0907907107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Cerione RA, Antonyak M. Tissue transglutaminase and its role in human cancer progression. Adv. Enzymol. Relat. Areas Mol. Biol. 2011;78:247–293. doi: 10.1002/9781118105771.ch6. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Nusbaum HR, Razon N, Kris R, Lax I, Soreq H, Whittle N, Waterfield MD, Ullrich A, Schlessinger J. Amplification, enhanced expression and possible rearrangement of EGF receptor gene in primary human brain tumours of glial origin. Nature. 1985;313:144–147. doi: 10.1038/313144a0. [DOI] [PubMed] [Google Scholar]
- Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc. Natl. Acad. Sci. U.S.A. 2002;99:2743–2747. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund-Johansen M, Bjerkvig R, Humphrey PA, Bigner SH, Bigner DD, Laerum OD. Effect of epidermal growth factor on glioma cell growth, migration, and invasion in vitro. Cancer Res. 1990;50:6039–6044. [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Mimori K, Tanaka F, Hitora T, Tei M, Sekimoto M, Doki Y, Mori M. TGM2 is a novel marker for prognosis and therapeutic target in colorectal cancer. Ann. Surg. Oncol. 2010;17:967–972. doi: 10.1245/s10434-009-0865-y. [DOI] [PubMed] [Google Scholar]
- Mizoguchi M, Betensky RA, Batchelor TT, Bernay DC, Louis DN, Nutt CL. Activation of STAT3, MAPK, and AKT in malignant astrocytic gliomas: correlation with EGFR status, tumor grade, and survival. Journal of neuropathology and experimental neurology. 2006;65:1181–1188. doi: 10.1097/01.jnen.0000248549.14962.b2. [DOI] [PubMed] [Google Scholar]
- Moscatello DK, Montgomery RB, Sundareshan P, McDanel H, Wong MY, Wong AJ. Transformational and altered signal transduction by a naturally occurring mutant EGF receptor. Oncogene. 1996;13:85–96. [PubMed] [Google Scholar]
- Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X, Ren Y, Shi Z, Long L, Pu P, Sheng J, Yuan X, Kang C. Sequence-dependent synergistic inhibition of human glioma cell lines by combined temozolomide and miR-21 inhibitor gene therapy. Mol. Pharm. 2012;9:2636–2645. doi: 10.1021/mp3002039. [DOI] [PubMed] [Google Scholar]
- Quaranta M, Divella R, Daniele A, Di Tardo S, Venneri MT, Lolli I, Troccoli G. Epidermal growth factor receptor serum levels and prognostic value in malignant gliomas. Tumori. 2007;93:275–280. doi: 10.1177/030089160709300308. [DOI] [PubMed] [Google Scholar]
- Ren Y, Zhou X, Mei M, Yuan XB, Han L, Wang GX, Jia ZF, Xu P, Pu PY, Kang CS. MicroRNA-21 inhibitor sensitizes human glioblastoma cells U251 (PTEN-mutant) and LN229 (PTEN-wild type) to taxol. BMC Cancer. 2010;10:27. doi: 10.1186/1471-2407-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousselet E, Traver S, Monnet Y, Perrin A, Mandjee N, Hild A, Hirsch EC, Zheng TS, Hunot S. Tumor necrosis factor-like weak inducer of apoptosis induces astrocyte proliferation through the activation of transforming-growth factor-α/epidermal growth factor receptor signaling pathway. Mol. Pharmacol. 2012;82:948–957. doi: 10.1124/mol.112.079608. [DOI] [PubMed] [Google Scholar]
- Shinojima N, Tada K, Shiraishi S, Kamiryo T, Kochi M, Nakamura H, Makino K, Saya H, Hirano H, Kuratsu J, Oka K, Ishimaru Y, Ushio Y. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63:6962–6970. [PubMed] [Google Scholar]
- Singer CF, Hudelist G, Walter I, Rueckliniger E, Czerwenka K, Kubista E, Huber AV. Tissue array-based expression of transglutaminase-2 in human breast and ovarian cancer. Clin. Exp. Metastasis. 2006;23:33–39. doi: 10.1007/s10585-006-9015-0. [DOI] [PubMed] [Google Scholar]
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- Umesh S, Tandon A, Santosh V, Anandh B, Sampath S, Chandramouli BA, Sastry Kolluri VR. Clinical and immunohistochemical prognostic factors in adult glioblastoma patients. Clin. Neuropathol. 2009;28:362–372. [PubMed] [Google Scholar]
- Verhaak RG, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer cell. 2010;17:98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A, Wang H, Manavathi B, Fok JY, Mann AP, Kumar R, Mehta K. Increased expression of tissue transglutaminase in pancreatic ductal adenocarcinoma and its implications in drug resistance and metastasis. Cancer Res. 2006;66:10525–10533. doi: 10.1158/0008-5472.CAN-06-2387. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Rohle D, Versele M, Iwanami A, Kuga D, Oldrini B, Tanaka K, Dang J, Kubek S, Palaskas N, et al. The phosphatase and tensin homolog regulates epidermal growth factor receptor (EGFR) inhibitor response by targeting EGFR for degradation. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6459–6464. doi: 10.1073/pnas.0911188107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman H, Levkowitz G, Alroy I, Yarden Y. The RING finger of c-Cbl mediates desensitization of the epidermal growth factor receptor. J. Biol. Chem. 1999;274:22151–22154. doi: 10.1074/jbc.274.32.22151. [DOI] [PubMed] [Google Scholar]
- Weaver KD, Yeyeodu S, Cusack JC, Jr., Baldwin AS, Jr., Ewend MG. Potentiation of chemotherapeutic agents following antagonism of nuclear factor kappa B in human gliomas. J. Neurooncol. 2003;61:187–196. doi: 10.1023/a:1022554824129. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.