1. Introduction
Bronchiolitis is the most common cause of infant respiratory hospitalizations,1–3 and is suggested to be a major risk factor in the later development of asthma.4 During winter virus season, 85% of infant bronchiolitis is caused by respiratory syncytial virus (RSV).3 There is no vaccine for RSV, and the only available treatment for bronchiolitis is supportive care. RSV circulation is known to follow distinct annual patterns similar to influenza, peaking primarily between November and March in the northern hemisphere and during April and September in the southern hemisphere.5–7
The timing of RSV epidemics varies according to location. Epidemic peak timing has been studied in multiple places around the globe, with results suggesting that in North America the annual epidemic occurs earliest in the southeast coastal areas of the US in September and October.8 Local differences in RSV rates are known to correlate with climate and other location-specific factors, though the reasons for those correlations are not all understood.2;5;6;9–11 Maternal smoking, socioeconomic status, gender and race are known to influence individual susceptibility.12–15 Exposure to high levels of particulate air pollution has also been suggested to increase susceptibility in some studies.16;17 Factors influencing particulate matter and other lung irritants (secondhand smoke and air pollution) have very uneven geographic distributions. In Tennessee, there are high levels of smoking in rural areas, whereas air pollution from external sources including roadways are more heavily focused in urban areas.
Cluster analysis is an effective method for finding times and locations where incidence varies significantly from expected rates. However, there have been very few cluster analyses of RSV in human populations to date.18;19 The studies that have been done previously were focused within highly localized areas (a single city) or in tropical areas. Other studies have compared strains from different regions to determine if the same strains circulate in nearby areas, rather than being designed as a thorough scan of disease clusters over continuous space.20
The purpose of this study was to investigate patterns of local clustering and RSV epidemic timing in TN to evaluate if consistent location-specific patterns in epidemic timing can exist within a relatively small geographic region. This is an important question in RSV ecology and modeling. Defining distinct, repeating, local patterns would help in future modeling of epidemics and in preventive initiatives by defining times and locations where the implementation of targeted preventive measures may be most effective.
2. Methods
2.1 Population
The TN Asthma and Bronchiolitis Study (TABS) is a retrospective birth cohort of children enrolled in TN Medicaid (TennCare) and followed longitudinally for the outcome of asthma. There were 458,837 children born between 7/1/1995 and 6/31/2008 included in this cohort. Infants born during this time frame who had an outpatient, emergency department, or hospital visit for bronchiolitis within the first six months of life were included in the cluster analysis. Only the first bronchiolitis visit was used; no subsequent doctor’s visits were counted for the same infant. As most bronchiolitis during winter virus season in infants less than 6 months of age is due to RSV, infant bronchiolitis served as a specific proxy measure for RSV infection.
Street addresses of infants at the time of infection were Geocoded using MapMarker USA, and latitude and longitude values were randomized to within a 100 meter radius to protect confidentiality. All study methods, including the use and protection of individual addresses, were approved by the Vanderbilt University Medical Center Institutional Review Board. Individuals were not consented as individual identification is not possible from this database. Of the total 458,367 infant addresses from the TennCare database, 95.3% were successfully geocoded to the street level. The remaining 4.7% were assigned by zip code centroid. Adding a small randomization level to the patient locations was necessary to protect patient confidentiality. Previous simulation studies21 have shown that the scan statistic is highly robust to perturbations larger than that used here when investigating smaller (city-wide) geographic areas. Therefore in our very large state-wide database, 100 meters of randomization is not expected to substantively alter the statistical outcome.
2.2 Rate calculations
For four major cities in TN: Knoxville, Nashville, Chattanooga and Memphis, weekly rates of infant bronchiolitis were calculated for individuals living in census tracts at least partially contained within city boundaries. Rates were standardized per 10,000 people according to the number of infants under 6 months old in TennCare living in the same city during the same week. Rates were collapsed for all years by week of year for graphical comparison of overall trends between cities.
2.3 City characteristics
In order to provide general information regarding land usage and the demographics of the population in the area we are studying that could influence disease rates, a brief assessment of land cover classifications between each of the 4 major cities in Tennessee as well as the prevalence of race and maternal smoking was completed. Using city boundary files and data from the 2006 national land cover database (NLCD), the percentage of each of 11 different land cover types in each city was calculated. The NLCD releases freely-available national raster files of land cover at 30 meter resolution. The land cover types were further collapsed to 8, reducing 3 types of upland forest and 2 types of wetland to 1 new variable each. The land cover reclassification was done to simplify variables that are not pertinent to this particular study. For instance, there are multiple types of wetland and upland forest that are not expected to differentially influence RSV rates. For our purposes, we are investigating those land cover types that may have differential impact on respiratory health and virus transmission through influencing air pollution levels or human transportation and interaction. The final land cover types were open water, wetland, pasture/grassland, row crop, upland forest, urban/developed, non-vegetated, and undefined. The race and maternal smoking prevalence by cities were calculated directly from individual TennCare data.
2.4 Space-Time Analysis
Cluster analysis was done throughout the calendar year, though only those clusters appearing between October and April were considered true clusters. This is because it is during these months that infant bronchiolitis is strongly correlated with RSV infection (85%) and therefore bronchiolitis can be used as a proxy measurement. The circular version of Kulldorff’s scan statistic, as implemented in the SatScan software (v. 9.0), was used to investigate possible clustering of bronchiolitis cases. While both the elliptical version and circular version of the scan statistic were tried on select years of data, for this analysis the results from the more compact and conservative circular version were finally used. The elliptical version tests many different oblong cluster variations at many different angles. Due to the very high number of cases and the seasonal variation, the elliptical version therefore resulted in suggesting an implausible number of clusters (hundreds), even when using options to restrict non-compact clusters.
A retrospective space-time permutation model was determined to be the most appropriate for these data due to both the nature of the data and the desired analysis. Cases did not have matched controls since infants who did not develop bronchiolitis were likely to also have contracted the virus (> 70% of all children are infected with RSV during infancy).22 The space-time permutation method of the scan statistic assigns clusters only when case counts deviate from what is expected at a certain location at a certain time, and from counts elsewhere in the entire study region. This is the most appropriate statistic to use here, as other methods would detect “seasonal peaks” instead of local deviations from the seasonal norm. The space-time permutation model uses the case population to look for times and locations where there are more cases than expected compared to the rest of the region. The clusters located in this analysis are therefore not indicative of the annual epidemic, but deviations in epidemic timing and intensity relative to elsewhere in TN. For example, if there were twice the number of cases in Knoxville as expected, while everywhere else experienced normal levels, then Knoxville would be deemed a cluster. If Knoxville had twice the number of normal cases and other areas in the state also had twice the number of normal cases, then Knoxville would not be deemed a cluster.
Kulldorff’s space-time permutation model, including significance testing, is described in detail elsewhere.23 When using this method to investigate multiple years, it is important to account for changes in the underlying population. To avoid possible bias from population shifting, each year was investigated separately. This analysis was not adjusted for covariates, because at this stage the global pattern is of interest. The sources of the data shown here come from multiple sources and time spans, so more detailed hierarchical modeling is required to investigate the causes of the differential patterns seen here.
A year was defined as beginning on July 1st and ending June 30th of the subsequent calendar year. The maximum size of the scanning bandwidth allowed was 50% of the state, and could include up to 25% of the total population. The temporal window was set at 10% of the year (approximately 35 days). Significant clusters were investigated to look for patterns in timing and location across all years studied. All clustering analyses were run using the computing cluster in Vanderbilt University’s Advanced Computing Center for Research (ACCRE).
3. Results
3.1 Population Description
Among the 458,837 children in the cohort, 52,468 (11.4%) of children had an outpatient, emergency department, or hospital visit for bronchiolitis within their first 6 months of life. Within this population, 33,158 of the infants (63.2%) were Caucasian, 14,185 (27.0%) were African-American, and 1,744 (3.3%) Hispanic. This was similar to the TennCare population as a whole, in which 58.4% of children were Caucasian and 30.8% were African-American. However, there were more Hispanics in the TennCare population (7.0%) than in the bronchiolitis cohort. As these data use health administrative records from Tennessee Medicaid, financially affluent areas tend to be underrepresented. The distribution of infant bronchiolitis cases over the entire time period is shown in figure 1. Supplementary figure 1 shows the distribution of the average number of infants by census tract in TN covered by TennCare.
Figure 1.
Case locations. The locations of bronchiolitis in infants less than 6 months old between 7/1/1995 and 6/31/2008 in the TABS cohort (n = 52,468). (Locations were randomized slightly to protect confidentiality.)
3.2 Bronchiolitis epidemic curves
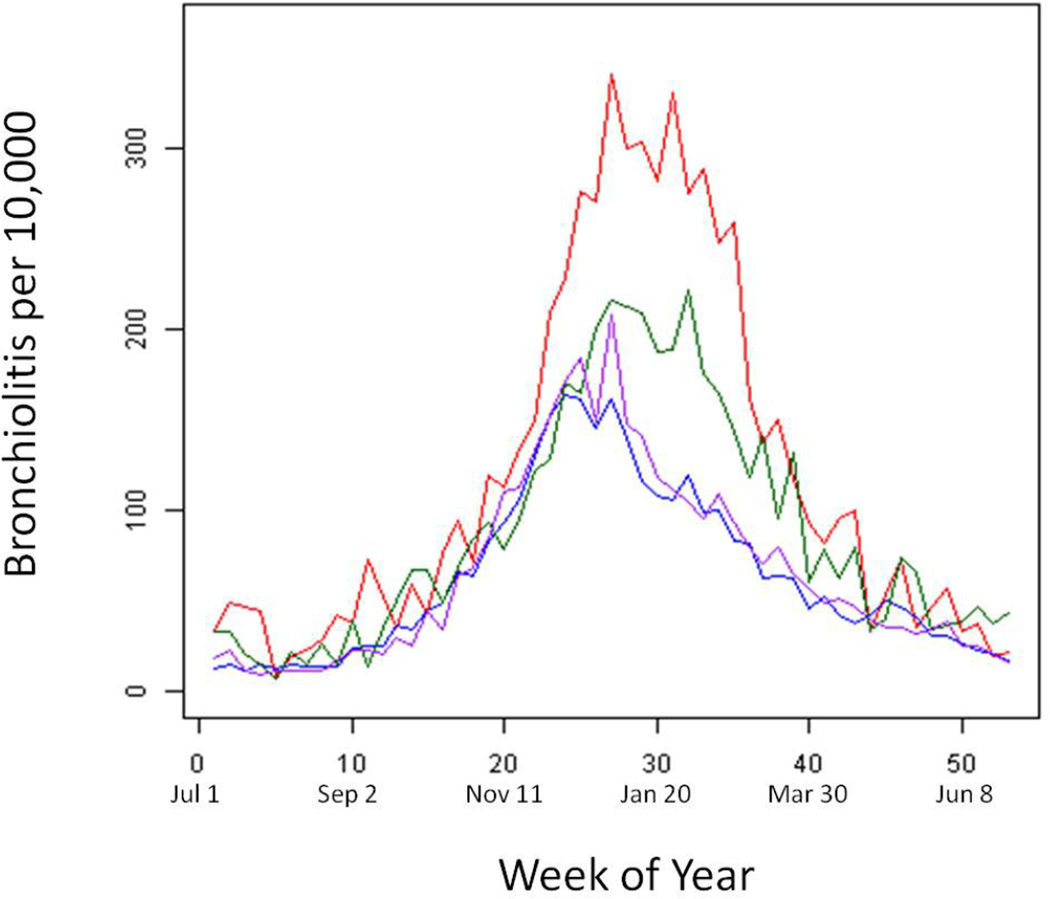
Bronchiolitis rates for each of the 4 major TN cities, collapsed by week are shown in figure 2. (Uncollapsed rates are shown for all years in supplementary figure 2). Epidemic peaks were usually near January 1st, on average, though this varied by location and year. Epidemic burden also varied, with Knoxville consistently experiencing the highest rates of infant bronchiolitis. Knoxville and Chattanooga also tended to have rates that persisted at higher levels into the spring season.
Figure 2.
Tennessee Infant bronchiolitis rates. Infant bronchiolitis rates for each of the largest 4 cities in TN for all July 1, 1995– June 30, 2007 collapsed to week of year.
3.3 City characteristics
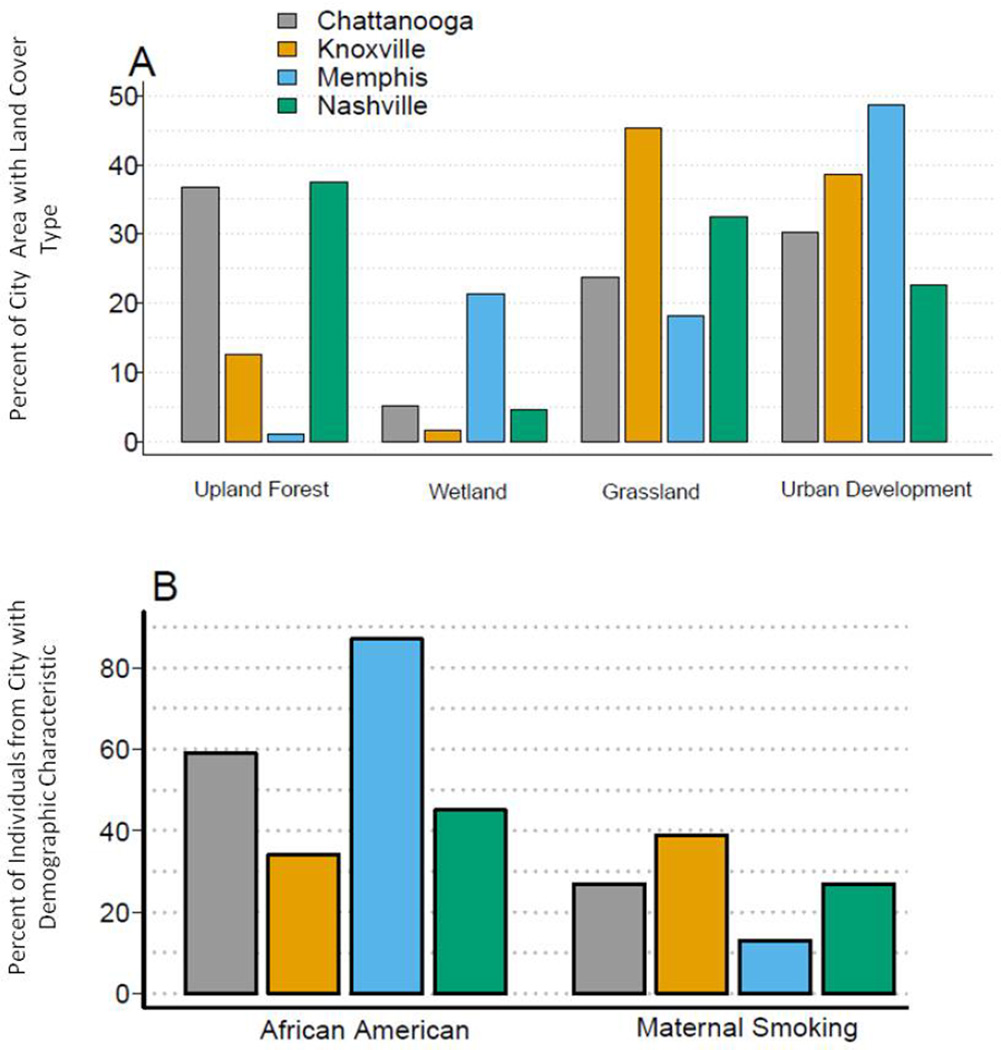
The land cover classifications that showed variation between the 4 major Tennessee cities as well as the percent of each city reporting African American race and maternal smoking are shown in figure 3. Other land cover variables, such as row crop and open water, did not show appreciable variation by city. Memphis had the lowest amount of upland forest and the highest amount of wetland and urban development. The city of Knoxville had the highest amount of grassland. Memphis had the highest percentage of African American infants in TennCare and the lowest percentage of maternal smoking, of which Knoxville had the highest.
Figure 3.
City characteristics. Land usage, percent African American and percent maternal smoking were compared between each of the 4 largest cities in Tennessee.
3.4 Clustering Results
There were a total of 26 significant disease clusters identified by the scan statistic, defined as having a p-value below 0.05. On average, there were 2 clusters detected per year during winter virus season. There were no significant clusters found in 1998, 2000 or 2004. A brief description of the clusters is given in Table 1 (with full results in Supplementary Table 1). The average cluster radius was 62 km, with a maximum of 191.68 km and minimum of 3.79 km. The smallest cluster existed in Nashville beginning November 13, 2008 and encompassed an area of 3.79 km. The largest cluster included the city of Memphis and encompassed 191.68 km radius (extending outside the state). This cluster began on January 24, 2007 and persisted for 29 days. The average duration of clusters was 28 days, with a maximum of 35 days and a minimum of 1 day. Several clusters lasted the maximum 35 days, and therefore likely persisted beyond the allowable time window. The most likely significant cluster was found in the Memphis area early in RSV season. The cluster began in November of 2007 and persisted into December (p <0.0001 (p=2 × 10−15)). There were 199 cases in this cluster area during this time window, compared to an expected 94. Most clusters had an observed/expected case ratio of about 2 (1.45, 10.72). The cluster with the 10.72 observed/expected ratio occurred in southeast TN beginning January 30, 1997. This cluster existed only 1 day and was likely the result of a sudden outbreak in a rural area.
Table 1.
Descriptions of the 26 statistically significant clusters found between July 1995 and June 2008 in state-wide cohort of infants less than 6 months old from the TennCare population. Full results are provided in supplementary table 1.
| Year | Region | Radius (km) | Start Date | Length (days) |
Observed Cases / Expected Cases (Ratio) |
P-Value |
|---|---|---|---|---|---|---|
| 1995 – 1996 | Nashville | 12.94 | 12/14/1995 | 5 | 23/5.54 (4.15) | 2.60E-02 |
| Memphis | 20.96 | 5/1/1996 | 24 | 29/7.85 (3.69) | 6.30E-03 | |
| 1996 – 1997 | Nashville | 9.45 | 11/18/1996 | 27 | 36/10.64 (3.38) | 1.60E-03 |
| Southeast | 32.01 | 1/30/1997 | 1 | 12/1.12 (10.72) | 4.60E-03 | |
| Johnson City | 170.49 | 2/5/1997 | 35 | 252/150.32 (1.68) | 1.10E-08 | |
| 1997 – 1998 | Memphis | 12.16 | 7/21/1997 | 25 | 34/7.21 (4.72) | 9.70E-07 |
| Nashville | 90.72 | 11/7/1997 | 31 | 199/122.94 (1.62) | 2.10E-04 | |
| Knoxville | 120.11 | 2/13/1998 | 34 | 183/97.35 (1.88) | 6.00E-09 | |
| 1999 – 2000 | Nashville | 6.06 | 12/10/1999 | 11 | 12/1.21 (9.94) | 1.60E-02 |
| 2001 – 2002 | Nashville | 44.9 | 11/8/2001 | 32 | 128/65.2 (1.96) | 7.20E-06 |
| Memphis | 57.97 | 11/30/2001 | 35 | 246/132.28 (1.86) | 1.90E-13 | |
| 2002 – 2003 | Knoxville | 104.78 | 2/8/2002 | 30 | 282/186.03 (1.52) | 3.70E-05 |
| Memphis | 14.88 | 11/9/2002 | 35 | 144/59.41 (2.42) | 1.00E-13 | |
| Nashville | 28.49 | 12/16/2002 | 34 | 106/58.15 (1.82) | 4.20E-02 | |
| 2003 – 2004 | Memphis | 78.3 | 10/4/2003 | 33 | 178/85.49 (2.08) | 6.40E-12 |
| Nashville | 3.79 | 11/13/2003 | 16 | 21/4.18 (5.02) | 1.80E-02 | |
| Johnson City | 50.53 | 1/27/2004 | 35 | 121/60.73 (1.99) | 3.00E-05 | |
| Chattanooga | 110.97 | 2/10/2004 | 35 | 168/105.11 (1.6) | 4.10E-02 | |
| 2005 – 2006 | Memphis | 22.16 | 11/27/2005 | 35 | 117/64.19 (1.82) | 3.90E-03 |
| Knoxville | 46.58 | 12/12/2005 | 35 | 148/84.14 (1.76) | 3.00E-04 | |
| SW Border | 131.26 | 2/15/2006 | 34 | 184/118.62 (1.55) | 2.50E-02 | |
| 2006 – 2007 | Nashville | 67.6 | 11/9/2006 | 35 | 324/207.29 (1.56) | 3.60E-08 |
| Memphis | 191.68 | 1/24/2007 | 29 | 320/221.39 (1.45) | 5.00E-04 | |
| 2007 – 2008 | Memphis | 21.68 | 11/12/2007 | 34 | 199/94 (2.12) | 2.00E-15 |
| Nashville | 41.08 | 12/16/2007 | 18 | 222/133.16 (1.67) | 2.00E-06 | |
| Knoxville | 119.46 | 1/19/2008 | 34 | 430/289.3 (1.49) | 2.30E-09 |
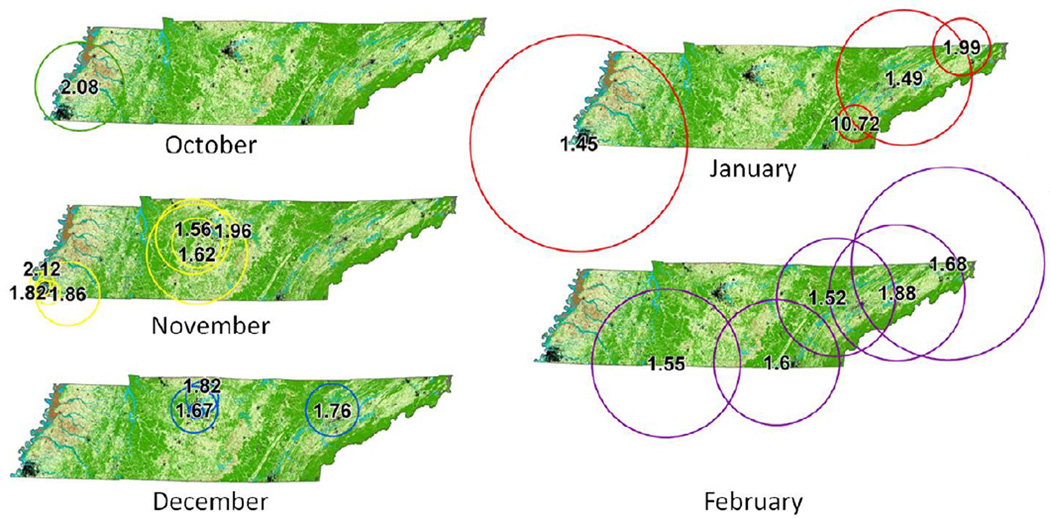
Clustering across years showed consistent patterns. Figure 4 shows the location and radius of each cluster area found during RSV season between October and February, displayed by the month of the start date. Most clusters appeared in urban areas, though 4 did not incorporate a major city. Several cluster areas incorporated a major city and its surrounding suburban and rural areas. Clusters including the city of Nashville occurred entirely in the months of November (1996, 1997, 2001, 2003, and 2006) or December (1995, 1999, 2002, 2007). East TN clusters including Knoxville and Johnson City always occurred in either December (2005), January (2004 and 2008) or February (1997, 1998 and 2002). Clusters appearing in Memphis occurred in October 2003, November (2001, 2002, 2005), and January (2007). Geographic sizes of clusters were variable year to year, though they did mostly appear to center over the metropolitan areas. The clusters that did not incorporate cities, including one found along the central southwest border, occurred in February.
Figure 4.
Cluster locations. Significant clusters (p<0.05) appearing in fall or winter for all years displayed by the month of their start date. Dots indicating locations of major TN cities are included for reference, with Memphis, Nashville and Knoxville labeled. Eastern TN clusters were found generally later in the winter virus season compared to Central and Southwestern Tennessee.
4. Discussion
This study demonstrates differences in the spatio-temporal epidemiology of infant bronchiolitis (RSV) in different regions of TN. This research advances our understanding of RSV ecology, which has not been thoroughly studied to date. It confirms that there are sociodemographic and environmental factors that influence RSV circulation in the human population, and that the geographic distribution of those factors can determine the timing of the epidemic peak.
Currently, immunoprophylaxis is the only effective prevention for RSV. Immunoprophylaxis is a series of very costly injections that need to be appropriately timed in conjunction with RSV circulation. Based on these results, it should be noted that even within the same state, there are frequent deviations in expected timing of the seasonal RSV epidemic. Immunoprophylaxis should potentially begin earlier in the Memphis area, and surveillance should begin earlier in the fall (September) than has been typical in the past. In east Tennessee (Knoxville area) infants can remain at high risk much later into the winter season. As hospitals become aware of these local seasonal peaks they can better prepare for the influx of patients each year based on local expected risk.
Having 13 years of available data is a major strength of this clustering analysis. This allowed for the observance of consistent patterns every year, and also demonstrated that East TN (Knoxville) and Central and West TN (Nashville and Memphis) have very different patterns of deviations from the state-wide mean of RSV epidemic timing. In East TN, the epidemics are shifted later in the season, occurring in January and February, compared to Central TN’s tendency to have epidemics shifted toward November and December. West TN appears to have epidemic timing influenced by some external factors, as timing tends to be earlier than in Eastern TN, but is not consistent year to year. Based on these results, a detailed study of the differences in climate, demography, and behavior that may be responsible for these trends in epidemics is underway.
Although the majority of clusters were detected during winter virus season, there were two clusters detected in Memphis in summer (May 1996 and July 1997). As described in the introduction, these should be interpreted with caution since infant bronchiolitis is predominantly caused by RSV in winter virus season, but other viruses are likely to be influencing these summertime peaks.
These analyses were conducted in a Medicaid Population, which has a different geographic distribution than the population as a whole. The result is that wealthier regions of the state were underrepresented, including high income areas outside of Nashville and Memphis. However, since Medicaid covers 50% of the births in TN, general coverage of the state is still very good; and it can be expected that any geographic bias in this particular dataset resulted in false negatives (Type II error), as there may be additional clusters that were missed due to the underrepresentation. This is among the reasons why the results were interpreted according to regional consistency of cluster timing over all years, instead of investigating the significance of each individual cluster.
In some underserved rural areas, it may be that individuals could seek treatment in bordering states. However, emergency care sought outside of Tennessee can still be billed to TennCare and reported as connected to their home address. As stated, there is a limitation to the dataset in that some high-income areas are underrepresented and therefore clusters in those areas might go undetected. However, the space-time permutation version of the statistic controls for underlying population variation by accounting for how many cases are expected in a particular area based on what the counts are in that area for the whole time period. Though more cases in the cities result in more power and more potential to detect a cluster (as with any statistical test), there is some correction for this built into the statistic.
The final assumption made in this analysis was that infant bronchiolitis rates were representative of RSV rates in the same location. Since bronchiolitis in the winter virus season is mostly due to RSV, and since RSV is so ubiquitously contracted, the investigators feel that this assumption is reasonable. However, it is possible that there are environmental influences in different locations that interact temporally with RSV infection and result in increased severity of disease requiring more medical attention.
The consistency with which clusters appeared around metropolitan areas is of interest. The high, nearly concentric clusters seen around Nashville are especially striking. Rural areas were well-represented in this cohort, so the low numbers of clusters found, for example between Nashville and Memphis was unexpected. In this study, either epidemic timing remains more consistent between rural areas and state-wide averages, or deviations are more difficult to detect in rural areas. Our assessment of demographic and land cover variables between cities shows that they are very different in their composition. This likely influences rates of RSV in each area, as factors such as maternal smoking or exposure to air pollution may increase infant susceptibility. Incorporating more direct measures of pollution, additional demographic characteristics and climate into models of geographically-explicit models of RSV spread is a topic of further study.
Respiratory syncytial virus epidemics show consistent temporal deviations that are closely related to geography, even across relatively small regions. Incorporating these deviations into models of RSV spread will result in improved predictive ability, and thus, improved accuracy in estimating the impact of implementing preventive measures in different locations.
Supplementary Material
Highlights.
RSV seasonal epidemics have temporal patterns that vary in ways that are annually consistent and tied to geography.
RSV seasonal epidemics typically occur later in the season in east Tennessee (February) than central or west Tennessee.
Environmental factors associated with local geography should be considered in models of RSV spread.
Acknowledgements
The authors wish to thank the Bureau of TennCare and the TN Department of Health for providing the data. This work was funded from the NIH/NIEHS K12 ES015855 (Vanderbilt VEHSS) program at Vanderbilt University Medical Center, and K24 AI77930.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Heymann PW, Carper HT, Murphy DD, et al. Viral infections in relation to age, atopy, and season of admission among children hospitalized for wheezing. Journal of allergy and clinical immunology. 2004;114:239–247. doi: 10.1016/j.jaci.2004.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nair H, Nokes DJ, Gessner BD, et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. The Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. New England Journal of Medicine. 2009;360:588–598. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu P, Dupont WD, Griffin MR, et al. Evidence of a causal role of winter virus infection during infancy in early childhood asthma. American journal of respiratory and critical care medicine. 2008;178:1123–1129. doi: 10.1164/rccm.200804-579OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Noyola DE, Mandeville PB. Effect of climatological factors on respiratory syncytial virus epidemics. Epidemiology and infection. 2008;136:1328–1332. doi: 10.1017/S0950268807000143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Welliver RC., Sr Temperature, humidity, and ultraviolet B radiation predict community respiratory syncytial virus activity. The Pediatric infectious disease journal. 2007;26:S29. doi: 10.1097/INF.0b013e318157da59. [DOI] [PubMed] [Google Scholar]
- 7.Walton NA, Poynton MR, Gesteland PH, Maloney C, Staes C, Facelli JC. Predicting the start week of respiratory syncytial virus outbreaks using real time weather variables. BMC Medical Informatics and Decision Making. 2010;10:68. doi: 10.1186/1472-6947-10-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stensballe LG, Devasundaram JK, Simoes EAF. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. The Pediatric infectious disease journal. 2003;22:S21. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 9.Yunus AS, Jackson TP, Crisafi K, et al. Elevated temperature triggers human respiratory syncytial virus F protein six-helix bundle formation. Virology. 2010;396:226–237. doi: 10.1016/j.virol.2009.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hambling MH. Survival of the respiratory syncytial virus during storage under various conditions. British journal of experimental pathology. 1964;45:647. [PMC free article] [PubMed] [Google Scholar]
- 11.Panozzo CA, Fowlkes AL, Anderson LJ. Variation in timing of respiratory syncytial virus outbreaks: lessons from national surveillance. The Pediatric infectious disease journal. 2007;26:S41. doi: 10.1097/INF.0b013e318157da82. [DOI] [PubMed] [Google Scholar]
- 12.Brownstein JS, Pena BG, Mandl KD. Characterizing socioeconomic disparities in the burden of influenza and RSV using surveillance data. Advances in Disease Surveillance. 2007;2:94. [Google Scholar]
- 13.Cohen S, Alper CM, Doyle WJ, Adler N, Treanor JJ, Turner RB. Objective and subjective socioeconomic status and susceptibility to the common cold. Health Psychology. 2008;27:268–274. doi: 10.1037/0278-6133.27.2.268. [DOI] [PubMed] [Google Scholar]
- 14.Wang M, Kleit RG, Cover J, Fowler C. Spatial Variations in US Poverty: Beyond Metropolitan and Non-Metropolitan; Community Vitality Project Working Papers; 2009. Ref Type: Report. [DOI] [PubMed] [Google Scholar]
- 15.Bradley JP, Bacharier LB, Bonfiglio JA, et al. Severity of respiratory syncytial virus bronchiolitis is affected by cigarette smoke exposure and atopy. Pediatrics. 2005;115:e7–e14. doi: 10.1542/peds.2004-0059. [DOI] [PubMed] [Google Scholar]
- 16.Segala C, Poizeau D, Mesbah M, Willems S, Maidenberg M. Winter air pollution and infant bronchiolitis in Paris. Environmental research. 2008;106:96–100. doi: 10.1016/j.envres.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 17.Ciencewicki J, Jaspers I. Air pollution and respiratory viral infection. Inhalation toxicology. 2007;19:1135–1146. doi: 10.1080/08958370701665434. [DOI] [PubMed] [Google Scholar]
- 18.Hendry RM, Talis AL, Godfrey E, Anderson LJ, Fernie BF, McIntosh K. Concurrent circulation of antigenically distinct strains of respiratory syncytial virus during community outbreaks. Journal of Infectious Diseases. 1986;153:291–297. doi: 10.1093/infdis/153.2.291. [DOI] [PubMed] [Google Scholar]
- 19.Omer SB, Sutanto A, Sarwo H, et al. Climatic, temporal, and geographic characteristics of respiratory syncytial virus disease in a tropical island population. Epidemiology and infection. 2008;136:1319–1327. doi: 10.1017/S0950268807000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cane PA. Molecular epidemiology of respiratory syncytial virus. Reviews in medical virology. 2001;11:103–116. doi: 10.1002/rmv.305. [DOI] [PubMed] [Google Scholar]
- 21.Malizia N. Inaccuracy, Uncertainty and the Space-Time Permutation Scan Statistic. PloS one. 2013;8:e52034. doi: 10.1371/journal.pone.0052034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Archives of Pediatrics & Adolescent Medicine. 1986;140:543. doi: 10.1001/archpedi.1986.02140200053026. [DOI] [PubMed] [Google Scholar]
- 23.Kulldorff M. A spatial scan statistic. Communications in statistics-theory and methods. 1997;26:1481–1496. doi: 10.1080/03610927708831932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.