Abstract
The present study demonstrated that defatted soybean flour (DSF) can sorb polyphenols from blueberry and cranberry juices while separating them from sugars. Depending on DSF concentration and juice dilution, the concentration of blueberry anthocyanins and total polyphenols sorbed to DSF ranged from 2 – 22 mg/g and 10 – 95 mg/g, respectively while the concentration of anthocyanins and proanthocyanidins in cranberry polyphenol-enriched DSF ranged from 2.5 – 17 mg/g and 21 – 101 mg/g, respectively. Blueberry polyphenols present in one serving of fresh blueberries (73g) were delivered in just 1.4 g of blueberry polyphenol-enriched DSF. Similarly, one gram of cranberry polyphenol-enriched DSF delivered the amount of proanthocyanidins available in three 240 ml servings of cranberry juice cocktail. The concentration of blueberry anthocyanins and total polyphenols eluted from DSF remained constant after 22 weeks of incubation at 37°C, demonstrating the high stability of the polyphenol-DSF matrix. LC-MS analysis of eluates confirmed DSF retained major cranberry and blueberry polyphenols remained intact. Blueberry polyphenol-enriched DSF exhibited significant hypoglycemic activities in C57bl/6J mice, and cranberry polyphenol-enriched DSF showed anti-microbial and anti-UTI activities in vitro, confirming its efficacy. The described sorption process provides a means to create protein-rich food ingredients containing concentrated plant bioactives without excess sugars, fats and water that can be incorporated in a variety of scientifically validated functional foods and dietary supplements.
Keywords: polyphenols, anthocyanins, proanthocyanidins, soybean flour, nutrition, diabetes, antibacterial
1. Introduction
Consumption of phytochemical-rich plant foods is associated with lowered risk of diabetes, cardiovascular disease (CVD), certain cancers and age-related degenerative diseases (Amin, Kucuk, Khuri & Shin, 2009; Bazzano, 2006; Joseph, Cole, Head & Ingram, 2009). Fruits, berries, vegetables, legumes, grains, nuts, seeds and teas contain a variety of antioxidant polyphenols/bioflavonoids, including anthocyanins, flavanols, proanthocyanidins, flavonols, and stilbenes, which neutralize or reduce free radical oxidative damage associated with chronic disease and aging. In addition to their role as antioxidants, polyphenols and their metabolites are able to modulate molecular signaling pathways (Williams, Spencer & Rice-Evans, 2004). In recognition of the health-protective properties of phytochemicals the five-a-day program was implemented in the United States. The United States Department of Agriculture recommends consumption of at least five servings of fruits and vegetables per day; however, nationwide surveillance data indicate that ~75% of the population have not achieved this objective (http://apps.nccd.cdc.gov/5ADaySurveillance/, 2011). New strategies are thus needed to increase consumption of the health-promoting polyphenols contained in fruits and vegetables.
North American blueberry (Vaccinium angustifolium Aiton; Vaccinium corymbosum L.) and cranberry (Vaccinium macrocarpon Ait) fruits are rich in anthocyanin pigments and flavan-3-ol polymers or proanthocyanidins (Neto, 2007). Blueberries have been used in traditional medicine for the complications of diabetes (Jellin, Gregory, Batz & Hitchens, 2005; Martineau et al., 2006) and a recent clinical study demonstrated significantly improved insulin sensitivity in men and women after dietary supplementation with blueberries formulated into a smoothie beverage (Stull, Cash, Johnson, Champagne & Cefalu, 2010). Blueberries contain up to 27 different anthocyanins (Wu & Prior, 2005), which contribute to their anti-diabetic effects (Grace et al., 2009; Takikawa, Inoue, Horio & Tsuda, 2010). Proanthocyanidins, contained in blueberries and cranberries, have also been reported to have anti-diabetic activity (Hanhineva et al., 2010). Cranberries and other berries have antimicrobial activity against several bacterial strains including Staphylococcus, Salmonella and Escherichia, which are responsible for food-borne illness and human disease (Puupponen-Pimia, Nohynek, Alakomi & Oksman-Caldentey, 2005). While the natural acidity of the berries is bactericidal, cranberry polyphenols elicit antimicrobial activity through mechanisms independent of pH (Lacombe, Wu, Tyler & Edwards, 2010). The weight of scientific evidence supports the use of cranberry juice, which contains Atype proanthocyanidins, as a prophylactic for urinary tract infections (UTIs) (Barbosa-Cesnik, Brown, Buxton, Zhang, DeBusscher & Foxman, 2011; Epp et al., 2010; Jepson & Craig, 2008), possibly by preventing adhesion of P-fimbriated E. coli to uroepithelial cells (Howell, Reed, Krueger, Winterbottom, Cunningham & Leahy, 2005; Sobota, 1984).
The anti-diabetic effects of blueberry anthocyanins are at least partially countered by considerable sugar contained in the fruits (mainly glucose and fructose) which contribute to their significant glycemic index of 53 (http://www.wildblueberries.com/health_benefits/glycemic.php, 2011). Cranberry fruit is mainly consumed as juice, but due to its tartness cranberry juice is unpalatable for most consumers without large amounts of added sugar. We have developed a simple and effective technology that captures and concentrates health-protective polyphenol compounds onto a protein-rich soy matrix while excluding water, sugars and highly nonpolar compounds/fats. Polyphenols are commonly concentrated by ion exchange resins such as Sephadex; however, polyphenols are also known to bind loosely structured proline-rich proteins with the interaction being strongest near the isoelectric pH (Hagerman & Butler, 1981). Protein-polyphenol binding is mediated by a combination of hydrogen and hydrophobic bonding depending on chemical (polarity) and structural (size/shape) properties of interacting molecules (Hagerman, Rice & Ritchard, 1998). Covalent interactions between purified glycinin, a soybean storage protein, and selected flavonoids and phenolic acids have also been reported (Rawel, Czajka, Rohn & Kroll, 2002).
2. Materials & methods
2.1. Sorption of polyphenols from blueberry juice and cranberry juice to different flours
Defatted soybean flour (DSF) (Hodgson Mill Inc., IL), white whole wheat flour (King Arthur Flour Company, Inc), white cornmeal (Goya Foods, Inc.), brown rice flour (Arrowhead Mills), and blueberry juice (R. W. Knudsen, 100% juice from blueberry juice concentrate) were purchased from the grocery store. Sorption capacity of different flours (5 g/l) was compared using 20 ml of this blueberry juice. Blueberry juice concentrate (65 Brix) was acquired from Oxford Frozen Foods, NS, Canada or Fruitsmart, WA and cranberry juice concentrate (50 Brix) was obtained from Urban Processing LLC Wisconsin Rapids, WI. Stoichiometric analysis of polyphenol sorption to DSF was determined by mixing increasing concentrations of DSF with 50 ml volumes of 2x, 5x or 15x dilutions of blueberry or cranberry concentrate on a magnetic stirring plate for 5 min at room temperature. Time dependence experiments were performed using DSF (100 g/l) mixed with 3x diluted blueberry concentrate or DSF (30 g/l) mixed with 5x diluted cranberry concentrate for 5, 15 or 30 min on a magnetic stir plate. Defatted soybean flour (DSF), soy protein concentrate (SPC) or soy protein isolate (SPI) were mixed with 50 ml volumes of 5x diluted blueberry juice to a final concentration of 5 g/l. In all cases triplicate samples were prepared for each condition tested. Juice-flour mixtures were centrifuged for 10 min at 12,000 rpm (Beckman, JA-17 rotor) and the supernatants were filtered prior to quantification of blueberry anthocyanins, cranberry proanthocyanidins or total polyphenols. Polyphenol-enriched DSF was freeze-dried and powdered for further analysis.
2.2. Quantification of total monomeric anthocyanins, total proanthocyanidins, and total polyphenols
The pH differential method (Lee, Durst & Wrolstad, 2005) was used to measure total monomeric anthocyanins in cranberry and blueberry juices and absorbance was measured at 520 nm and 700 nm with a UV/VIS spectrophotometer (Shimadzu UV-2450 or Synergy HT Multi-Detection Microplate Reader, BioTek). Concentration of monomeric anthocyanins (mg/l) was calculated as cyanidin 3-O-glucoside equivalents. A modified vanillin assay (Burns, 1971) was used to quantify the total proanthocyanidins. The vanillin-reagent consisted of 1.1% vanillin and 22% sulphuric acid in methanol. The blank reagent consisted of 11% sulphuric acid in methanol without vanillin. Sample absorbance was read at 500 nm after 30 min against a catechin (ChromaDex, Irvine CA) standard curve. Total polyphenols were quantified by the Folin-Ciocalteu method (Singleton & Rossi, 1965) and samples were read at 726 nm against a gallic acid (Sigma) standard curve. The concentration of anthocyanins, proanthocyanidins or total polyphenols bound to the DSF was calculated by subtracting their concentration in the DSF-treated juice supernatants from that measured in the untreated juice samples and dividing by grams of DSF added.
2.3. Compositional analysis of blueberry and cranberry components eluted from soybean flour
Blueberry juice concentrate was diluted 5x DSF (100 g/l) was added to 20 ml of juice to sorb polyphenols then juice and DSF were separated by centrifugation. C18 cartridge columns (J.T. Baker, NJ) were used to remove sugars from pre- and post-DSF-treated juices; polyphenols eluted from the C18 column were concentrated to a final volume of 1 ml by rotary evaporation (Büchi, Switzerland) prior to LC-MS. Polyphenols were eluted from the blueberry polyphenol-enriched DSF with acidic methanol (methanol: water: acetic acid, 75:20:5) were also concentrated prior to LC-MS. Samples were separated and analyzed with UPLC – MS (Dionex® Ultimate 3000 RSLC UPLC system) and a triple quadruple Varian 1200 (Varian Inc., Palo Alto, CA) mass spectrometer equipped with electrospray ionization (ESI) interface using a Phenomenex® RP C8 reverse phase column (size 150 mm × 2 mm, 3 μm). The mobile phase consisted of 0.5% acetic acid in water, pH 3–3.5 (Solvent A) and 100% acetonitrile (Solvent B) at a flow rate of 0.2 ml/min. The gradient used was: initially 90% A and 10% B using an isocratic flow for first 5 min; 85% A and 15% B over 15 min, followed by isocratic elution at 85% A and 15% B for 5 min; 65% A and 35% B over 15 min. There was an 8 min equilibration interval between injections. Quantification was performed against a standard curve of malvidin 3-O-glucoside (Polyphenols Laboratories, Norway).
Cranberry juice concentrate (5x dilution) was mixed with DSF (30 g/l) followed by centrifugation to separate juice and DSF. Cranberry juices pre- and post-DSF treatment were analyzed by LC-MS. Polyphenols were eluted from freeze-dried cranberry polyphenol-enriched DFS with methanol: acetone: 0.5% acetic acid in water (4:4:2), the organic solvents were evaporated, and the aqueous extract was freeze-dried. The sample (1 mg/ml) was prepared in 50% methanol and LC-MS analysis was performed using a Shimadzu LC-MS-IT-TOF instrument equipped with a Prominence HPLC system (SIL-20A HT autosampler, LC-20AD pump system, SDP-M20A diode array detector). The LC separation was performed using a C18 RP column (Ascentis Epress C18, 100 mm × 2.1 mm, 2.7 μm; Supelco Analytical) with a binary solvent system comprising 0.1% formic acid in water (mobile phase A) and 0.1% formic acid in methanol (mobile phase B). Compounds were eluted into the ion source at a flow rate of 0.2 ml/min with the following gradient: 5–10% mobile phase B over 5 min, 10–20% B over 10 min, 20–40% B over 15 min, 40–95% B over 1 min, isocratic at 95% B over 3 min, and a return to 5% B over 1 min. The column was re-equilibrated at initial conditions (5% mobile phase B) for 10 min prior to the next injection. Ionization was performed using a conventional ESI source in positive ionization mode. Compounds were characterized by their MS/MS spectra and LC retention time and comparison with available reference samples. Quantification of proanthocyanidin A2 was performed against a standard curve using a reference sample (ChromaDex).
2.4. Quantification of glucose and total carbohydrates
The concentration of glucose in untreated and flour-treated juice samples was quantified using the QuantiChrom™ Glucose assay kit (BioAssay Systems). Total carbohydrates were quantified by the phenol-sulphuric acid method (Masuko, Minami, Iwasaki, Majima, Nishimura & Lee, 2005) and read against a standard curve made from a 1:1 solution of fructose and glucose.
2.5. Stability of blueberry anthocyanins and polyphenols
Multiple two-gram samples of freeze-dried blueberry polyphenol-enriched DSF were aliquoted into 50 ml screw-cap vials, divided into three sets and incubated at 4, 25 or 37°C. Samples from each temperature were removed at the indicated weeks and eluted 6x with 20 ml volumes of acidic methanol (methanol: water: acetic acid, 75:20:5). Total monomeric anthocyanins and total polyphenols in eluates were quantified as described above.
2.6. Preparation of blueberry polyphenol-enriched DSF for in vivo experiments
Anthocyanins from blueberry juice were sorbed to DSF (30 g/l) and the amount of anthocyanins bound to DSF was estimated as described above. As a control, DSF was also mixed with water acidified with citric acid to match the pH of the DSF-blueberry juice mixture (pH 3.5). The juice and water-treated DSF mixtures were centrifuged, and pellets were freeze- dried and powdered. Blueberry polyphenol-enriched DSF and the water-treated control DSF were formulated in 75% Labrasol (Gattefossé Corporation, NJ) for the in vivo experiments.
2.7. Acute hypoglycemic effect of blueberry polyphenol-enriched DSF in C57bl/6J DIO mice
The protocol was approved by Rutgers University Institutional Care and Use Committee and followed federal and state laws. Five-week-old male C57bl/6J mice (10–20 g) were purchased from Jackson Laboratory (Bar Harbor, Maine) and fed a regular diet ad libitum (Purina, #5015) during their one week acclimatization period. At 6 weeks, mice were placed on a very high-fat diet (VHFD; 60% kilocalories fat; Research Diets D12492) for 12 weeks, which led to insulin resistance and hyperglycemia. Body weights were measured weekly. Mice were randomly divided into experimental groups, fasted for 4 h, and then gavaged with the indicated doses of blueberry polyphenol-enriched DSF or DSF control (300 mg/kg) formulated in a 75% Labrasol-water solution (vehicle). Blood glucose readings were taken using a glucometer; animals were fasted during the testing period. Metformin® (300 mg/kg) was administered as positive control.
2.8. Bacterial culture preparation and antimicrobial assay
Staphylococcus aureus (ATCC 13301) grown on solid agar plates (Mueller Hinton Agar 2 (MHA, Sigma) were inoculated into 30 ml Mueller Hinton Broth 2 (MHB, Sigma) and cultured overnight on a shaker (130 rpm) at 37 °C. The overnight cultures were added to 10 ml of MHB and optical densities were compared with McFarland Turbitdity Standard No. 0.5 (BD) to obtain 1.5 × 108 cells/ml. Optical densities were measured at 590 nm on a Synergy HT Multi-Detection Microplate Reader. The bacterial suspensions were diluted a second time to obtain 105 cells/ml for the antimicrobial assays. Cranberry polyphenol-enriched DSF was prepared as described using 2x diluted cranberry concentrate and 10 g/l of DSF. Cranberry polyphenol-enriched DSF or DSF was added to MHA at concentrations of 0, 10, 20, 30 or 40 mg/ml. The unadjusted pH of the MHA and cranberry polyphenol-enriched matrix (10 mg/ml) in MHA were 7 and 4, respectively. The media were adjusted to pH 7 and 4 with 2 N citric acid or 5 M NaOH, respectively. The agar suspension was autoclaved at 121 °C for 15 min and 1 ml was aliquoted per well on a 24-well plate. Microbial suspensions (30 μl) were added to the surface of agar solidified media and incubated at 37 °C for 24 h.
2.9. Bacterial anti-adhesion assay and quantification of proanthocyanidins
The HRBC hemagglutination in vitro assay (Howell et al., 2005) was used to compare the bacterial anti-adhesion activity of cranberry polyphenol-enriched DFS and cranberry juice cocktail (CJC; Ocean Spray). Briefly, serial 2-fold dilutions of NaOH-neutralized cranberry polyphenol-enriched DFS and dried CJC (60 mg/ml starting concentrations) were prepared in phosphate buffered saline (PBS) and 30 μl of each dilution was incubated with 10 μl of uropathogenic P-fimbriated E. coli bacterial suspension (5 × 108 cfu/ml) for 10 min at room temperature. A 3% v/v suspension of human red blood cells (HRBC; A1Rh+) in PBS was prepared and 10 μl was added to the test suspensions for 20 min on a shaker. Inhibition of agglutination of HRBCs was evaluated microscopically. The minimum inhibitory concentration (MIC) is the dilution at which 50% agglutination is observed. Wells containing bacteria plus PBS, HRBC plus PBS, bacteria plus test material, and HRBC plus test material served as negative controls for agglutination; bacteria and HRBC served as a positive control for agglutination.
2.10. Quantification of proanthocyanidins from cranberry polyphenol-enriched DFS and CJC
Cranberry proanthocyanidins were eluted from DSF with methanol:acetone:water:acetic acid (40:40:19:1). The organic solvent was vacuum evaporated (Büchi, Switzerland) and the remaining aqueous extract was passed over a Sephadex LH-20 column pre-conditioned with 20% methanol and 1% acetic acid in water. The column was washed with the same acidified methanol to remove any anthocyanins and then the proanthocyanidins were eluted with 70% aqueous acetone. Proanthocyanidins were isolated from 240 ml of CJC by pouring the juice directly onto a Sephadex column following the same procedure as above. The solvent was evaporated and the weights of proanthocyanidins isolated from the cranberry polyphenol-enriched DFS and CJC, calculated and compared on a per weight (DFS) and per volume (CJC) basis. About 50% of cranberry proanthocyanidins could be eluted from the DSF compared to the calculated amount that was sorbed.
2.11. Statistics
Statistics were performed with STATISTICA v.10 (StatSoft). Normality of data was confirmed (Lilliefors test) prior to using the parametric methods. One-way ANOVA was used to determine significance among three or more groups followed by the indicated post-hoc test. Paired t-tests were performed within groups (before vs. after treatment).
3. Results
3.1. Capacity of different flours to sorb berry polyphenols
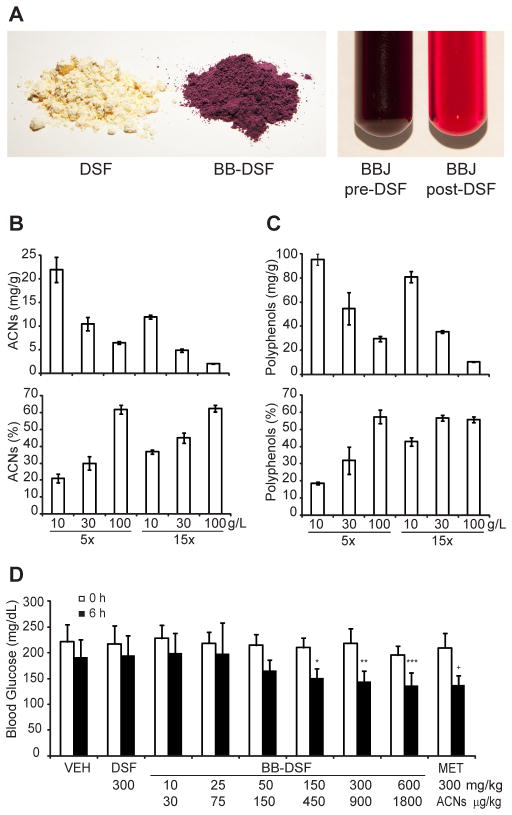
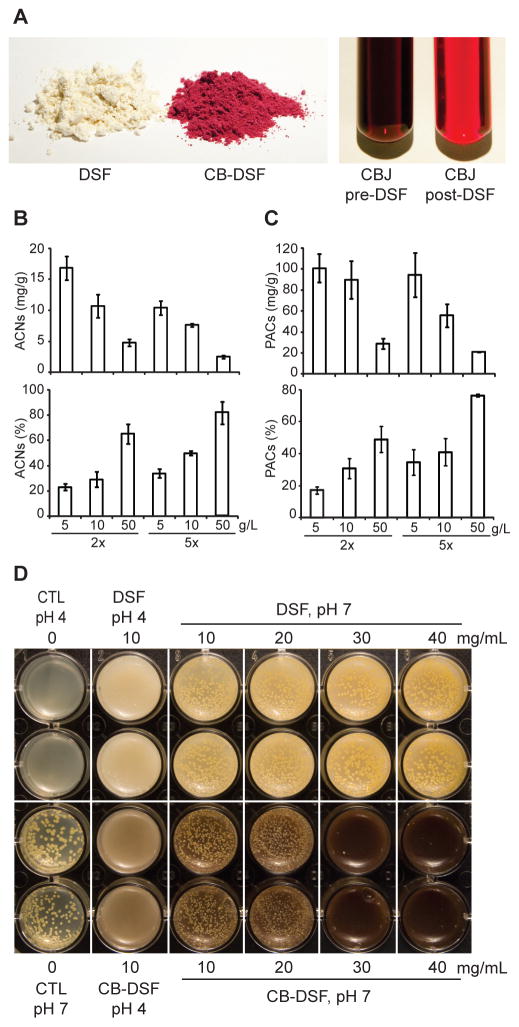
To determine whether proteins and other insoluble components of plant flours will bind plant polyphenols defatted soybean flour (DSF), white whole wheat flour, brown rice flour or corn flour was added to 50 ml of blueberry juice at a concentration of 5 g/l and mixed at room temperature for 5 min. The flour was recovered by centrifuging the mixture. Using the pH differential method (Lee et al., 2005), the total monomeric anthocyanins were quantified in juices before, as well as after the addition and removal of DSF; the difference represented the amount of anthocyanins sorbed by each flour. DSF, which had the highest protein content, had the highest capacity to sorb and concentrate blueberry anthocyanins, followed by wheat, rice and corn flours, respectively (Table 1). However, the protein content of the tested flours did not linearly correlate with the binding efficiency for blueberry anthocyanins. It is thus likely that carbohydrates, which are major components of seed flours, also participate in the sorption process. Different particle sizes of tested flours as well as variations in composition of storage proteins from different plants may also alter the efficiency of hypothesized electrostatic binding. Soy protein concentrate and soy protein isolate sorbed blueberry anthocyanins at least as well as DSF (Supplemental Fig. 1). All further experiments were performed with DSF, which efficiently sorbed the anthocyanins from both blueberry and cranberry fruit juices yielding uniformly colored berry polyphenol-enriched flour matrices (Figs. 1A and 2A, left) and pigment-depleted juices (Figs. 1A and 2A, right).
Table 1.
Sorption of anthocyanins from blueberry juice to flours with varying protein concentrations
| Matrix | % Protein1 | Anthocyanins (mg/g)2 |
|---|---|---|
| DSF | 47 | 4.2 ± 0.1 a |
| Wheat flour | 13 | 3.0 ± 0.2 b |
| Rice flour | 8.6 | 2.7 ± 0.1 b |
| Cornmeal | 5.3 | 1.8 ± 0.2 c |
Based on product nutrition label
Calculated as cyanidin-3-O-glucoside equivalents
Flour concentrations were 5 g/L in blueberry juice
Different letter subscripts indicate significant differences (ANOVA, Tukey post hoc)
Fig. 1.
Sorption of blueberry polyphenols to defatted soybean flour (DSF) and in vivo hypoglycemic activity of polyphenol-enriched DSF. (A) Left panel - untreated DSF and DSF enriched with blueberry anthocyanins and other polyphenols (BB-DSF). Right panel - blueberry juice before and after DSF treatment. (B) Concentration (top) and percentage (bottom) of blueberry anthocyanins (ACNs; cyanidin 3-O-glucoside equivalents) and (C) total polyphenols (gallic acid equivalents) sorbed to increasing amounts (g/l) of DSF. 5x and 15x refer to dilutions of blueberry juice concentrate (D) Blood glucose levels of C57bl/6 mice before and 6 h after treatment with 75% Labrasol (VEH), DSF, blueberry polyphenol-enriched DSF (BB-DSF) and Metformin® (MET). The second row of numbers represent the amount of anthocyanins (ACNs) delivered in the indicated dose of blueberry polyphenol-enriched DSF. Each bar represents mean ± SD (n = 5 to 15) of data combined from 3 independent experiments. * p= 0.005; ** p= 0.0009; *** p= 0.002, + p= 1.7×10−5 (Dunnett’s test relative to DSF group). Significance was confirmed within groups using two-tailed, paired t-tests.
Fig. 2.
Sorption of cranberry polyphenols to defatted soybean flour (DSF) and antibacterial activity of polyphenol-enriched DSF. (A) Left panel - untreated DSF and cranberry polyphenol-enriched DSF (CB-DSF). Right panel – cranberry juice before and after DSF treatment. (B) Concentration (top) and percentage (bottom) of cranberry anthocyanins (ACNs; cyanidin-3-O-glucoside equivalents) and (C) proanthocyanidins (PACs; catechin equivalents) sorbed to increasing amounts (g/l) of DSF. 2x and 5x refer to dilutions of cranberry juice concentrate (D) Ability of Staphylococcus aureus to form colonies on acidic (pH 4) or pH-adjusted (pH 7) solid medium (first column); on solid medium (pH 4) containing DSF or CB-DSF (second column) or on a pH-adjusted (pH 7) solid medium containing 10, 20, 30 or 40 mg/ml of DSF or CB-DSF (last four columns). Duplicate wells of each treatment are shown.
3.2. Sorption of berry polyphenols as a function of DSF concentration and berry concentrate dilution
To examine the relationship between flour sorption capacity, juice polyphenol content and flour concentration in the juice, increasing concentrations of DSF were mixed with 2x, 5x, or 15x dilutions of blueberry or cranberry juice concentrate. After mixing, the liquid phase was separated from the DSF as previously described and the concentration and percentage of anthocyanins, proanthocyanidins and total polyphenols sorbed to increasing amounts of DSF was determined. The concentration of berry phytochemicals sorbed to DSF was inversely proportional to the concentration of DSF in the juice and the level of juice dilution.
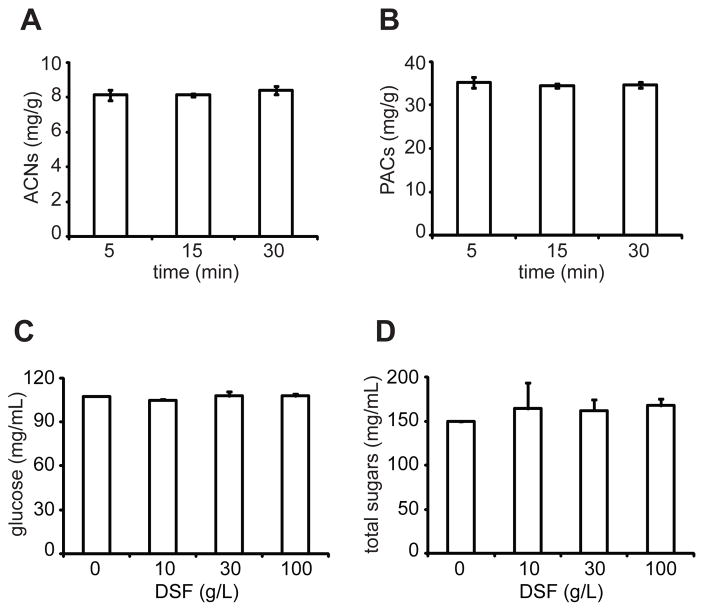
Depending on DSF concentration and juice dilution, the concentration of blueberry anthocyanins and other polyphenols sorbed to DSF ranged from 2 – 22 mg/g and 10 – 95 mg/g, respectively (Figs. 1B and 1C, top). DSF (100 g/l) was able to sorb up to 62% of anthocyanins and 57% of total polyphenols from 5x diluted blueberry juice concentrate (Figs. 1B and 1C, bottom). Blueberry fruits of the most commonly cultivated species, Vaccinium corymbosum (highbush blueberries), contain on average 0.95 mg of anthocyanins (cyandin 3-O-glucoside equivalents) and 1.8 mg of total polyphenols (gallic acid equivalents) per gram fresh weight (Ehlenfeldt & Prior, 2001). Therefore one gram of DSF can sorb and concentrate blueberry anthocyanins by ~20-fold and total polyphenols by ~50-fold. Based on the above reported averages, the recommended half cup (73g) serving of blueberries (http://www.fruitsandveggiesmatter.gov/month/berries.html) provides about 70 mg of anthocyanins and 131 mg of total polyphenols. Equivalent levels of anthocyanins and polyphenols can be delivered in as little as 3.2 g and 1.4 g of the most concentrated blueberry polyphenol-enriched DSF, respectively. The concentration of anthocyanins and proanthocyanidins in the cranberry polyphenol-enriched DSF ranged from 2.5 – 17 mg/g and 21 – 101 mg/g, respectively (Figs. 2B and 2C, top). Up to 82% of anthocyanins and 76% of proanthocyanidins were sorbed with the highest concentration of DSF and juice dilution (Figs. 2B and 2C, bottom). Fresh cranberry fruit contains ~0.35 mg/g anthocyanins (Wang & Stretch, 2001) and in this study we isolated 32 mg of proanthocyanidins from one 240 ml serving of commercially available cranberry juice cocktail (CJC). Therefore, one gram of the most saturated cranberry polyphenol-enriched DSF can deliver anthocyanins present in 49 g of fresh cranberry fruit and the amount of proanthocyanidins that would be available in three 240 ml servings of CJC. Increasing the incubation time from 5 min to 30 min did not significantly increase the concentration of blueberry anthocyanins or cranberry proanthocyanidins sorbed by DSF (Fig. 3A, B).
Fig. 3.
DSF sorbs berry polyphenols within 5 min, but does not sorb or concentrate sugars (A) The concentration of blueberry anthocyanins (ACNs) sorbed to DSF after mixing DSF (100 g/l) and 3x diluted blueberry concentrate for 5, 15 and 30 min. (B) The concentration of cranberry proanthocyanidins (PACs) sorbed to DSF after mixing DSF (30 g/l) and 5x diluted blueberry concentrate for 5, 15 and 30 min. Concentration of (C) glucose and (D) total sugars present in 5x diluted blueberry juice concentrate before (0 g/l DSF) and after addition and removal of 10, 30 and 100 g/l of DSF.
3.3. Compositional analysis of berry polyphenol-enriched DSF
LC-MS analysis of eluates confirmed that the DSF had sorbed compounds with masses and retention times corresponding to polyphenols from cranberry and blueberry fruit; native soybean compounds were also detected in the eluates (Table 2). In parallel, malvidin 3-O-glucoside and the proanthocyanidin A2 dimer were quantified by LC-MS in blueberry and cranberry juices, respectively, before and after addition and removal of DSF. DSF (100g/l) sorbed 51% of the malvidin 3-O-glucoside from 5x diluted blueberry juice concentrate and the concentration of malvidin 3-O-glucoside in the flour was 5.3 mg/g. Similarly, DSF (30 g/l) added to a 5x dilution of cranberry juice sorbed 55% of the proanthocyanidin A2 catechin dimer from the juice and the concentration of the A2 dimer in the DSF was 1.7 mg/g. Sorption of malvidin 3-O-glucoside and A2 dimer to DSF was confirmed by eluting polyphenols from the blueberry and cranberry polyphenol-enriched flours and subjecting the eluates to LC-MS.
Table 2.
Compounds eluted from blueberry (B) or cranberry (C)-enriched defatted soybean flour (DSF) or untreated DSF.
| Compounds | MS (m/z) ESI
|
fragments (m/z) | Rt (min) | |||
|---|---|---|---|---|---|---|
| M+ | M+1 | M−1 | ||||
|
| ||||||
| B, C | Cyanidin 3-O-galactoside | 449 | 287 | 9.65, 12.0 | ||
| C | Cyanidin 3-O-arabinoside | 419 | 287 | 14.9 | ||
| B | Delphinidin 3-O-arabinoside | 435 | 303 | 9.11 | ||
| B | Delphinidin 3-O-galactoside | 465 | 303 | 8.76 | ||
| B | Malvidin 3-O-arabinoside | 465 | 331 | 11.96 | ||
| B | Malvidin 3-O-galactoside | 493 | 331 | 11.03 | ||
| C | Peonidin-3-O-galactoside | 463 | 301 | 17.2 | ||
| C | Peonidin3-O-arabinoside | 433 | 301 | 19.2 | ||
| B | Petunidin 3-O-arabinoside | 449 | 317 | 10.55 | ||
| B | Petunidin 3-O-galactoside | 479 | 317 | 9.82 | ||
| C | Procyanidin A2 | 577 | 425, 287 | 18.5 | ||
| B | Procyanidin B1, B2 or B3 | 577 | nd | 15.7, 17.3, 18.0, 18.8 | ||
| B, C | Quercetin-3-O-arabinoside | 435 | 433 | 303 | 16.34, 28.0 | |
| B, C | Quercetin-3-O-galactoside | 465 | 463 | 303 | 15.11, 25.0 | |
| B, C | Quercetin-3-O-rhamnoside | 449 | 447 | 303 | 10.5, 30.3 | |
| B, C | Quercetin | 303 | 301 | 229 | 22.0, 33.5 | |
|
| ||||||
| DSF | Daidzin | 417 | 255 | 12.85 | ||
| Genistin | 433 | 271 | 15.67 | |||
| Acetyl-daidzin | 459 | 255 | 17.66 | |||
| Acetyl- genistin | 475 | 271 | 20.69 | |||
| Malonyl-daidzin | 503 | 255 | 15.89 | |||
| Malonyl-genistin | 519 | 271 | 18.38 | |||
| Malonyl-glycitin | 533 | 285 | 16.04 | |||
3.4. DSF effect on the concentration of sugars
To determine if sugars were sorbed we measured levels of glucose and total sugars in blueberry juice before and after the addition and removal of 10, 30 and 100 g/l of DSF. No significant differences in sugar content were found between untreated juice and juice supernatants after the addition and removal of DSF, indicating that DSF does not bind or concentrate sugars (Fig. 3C, D).
3.5. Stability of polyphenols sorbed to DSF
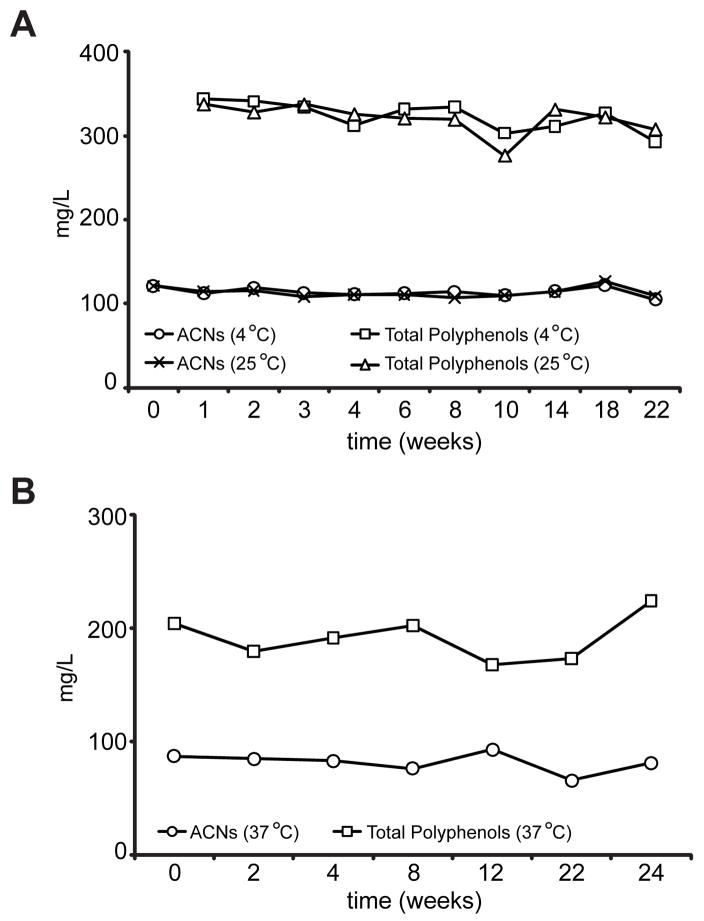
Anthocyanins are unstable and subject to degradation above neutral pH and at high temperatures (Castaneda-Ovando, Pacheco-Hernandez, Paez-Hernandez, Rodriguez & Galan-Vidal, 2009). Anthocyanins in pasteurized blueberry juice have a half-life of 184, 35 and 5 – 7.5 days at 4, 25 and 40 °C, respectively (Buckow, Kastell, Terefe & Versteeg, 2010; Kechinski, Guimaraes, Norena, Tessaro & Marczak, 2010). To determine the stability of anthocyanins and total polyphenols in blueberry polyphenol-enriched DSF, tubes containing 2 g aliquots of the enriched DSF were placed at 4, 25 or 37°C. On day 0 and after the indicated number of weeks blueberry phenolics were eluted from DSF with acidified methanol and the concentration of anthocyanins and total polyphenols in the eluates were quantified as described. The concentration of eluted blueberry anthocyanins and total polyphenols remained constant after at least 22 weeks of incubation at all temperatures with no visible change in colour indicating that they are stabilized when bound to DSF (Fig. 4 A, B).
Fig. 4.
Stability of blueberry anthocyanins and polyphenols bound to DSF (A) Concentration of anthocyanins (ACNs) and total polyphenols eluted from blueberry polyphenol-enriched DSF after the indicated number of weeks post-incubation at 4 °C or 25 °C. (B) Concentration of anthocyanins (ACNs) and total polyphenols eluted from blueberry polyphenol-enriched DSF after the indicated times post-incubation at 37 °C.
3.5. Hypoglycemic activity of blueberry polyphenol-enriched DSF
We investigated whether blueberry and cranberry polyphenols retained their biological activity after sorption to DSF. We have previously demonstrated that a column-purified anthocyanin-enriched fraction from blueberries reduced blood glucose levels in diet-induced obese hyperglycemic C57bl/6J mice with an efficacy comparable to Metformin® (Grace et al., 2009). We therefore tested whether blueberry polyphenol-enriched DSF would produce a similar effect in this animal model. Mice were fasted for 4 h, then blood glucose levels were measured before treatment and after 6 h the mice were gavaged with 10, 25, 50, 150, 300 and 600 mg/kg of blueberry polyphenol-enriched DSF, DSF, vehicle (75% Labrasol; VEH) or Metformin®. Blood glucose levels were significantly different between groups at 6 h post-treatment (ANOVA, p= 1.4×10−7), but not before treatment (ANOVA, p= 0.68). Compared to the VEH or DSF controls, the 150, 300 and 600 mg/kg doses of blueberry polyphenol-enriched DSF had a significant hypoglycemic effect at 6 h acting in a concentration-dependent manner (Fig. 1D). The anthocyanin concentration in the blueberry polyphenol-enriched DSF was 3 mg/g, therefore the 150, 300 and 600 mg/kg of the blueberry polyphenol-enriched DSF delivered 450, 900 and 1800 μg/kg doses of anthocyanins, respectively. The 150 to 600 mg/kg doses of blueberry polyphenol-enriched DSF used in this study delivered anthocyanin levels that were significantly lower than the dose delivered in a previous study where 500 mg/kg of column-purified anthocyanin-enriched fraction delivering 250 mg/kg of anthocyanins was used (Grace et al., 2009); however, similar hypoglycemic effects were observed. Several factors may contribute to the potent hypoglycemic properties of blueberry polyphenol-enriched DSF. Consumption of soy products is associated with lower incidence of obesity and type II diabetes, and purified soy isoflavones have been reported to improve glucose metabolism in diabetic mice (Cederroth & Nef, 2009). Therefore, blueberry and DSF components appear to act synergistically to produce the hypoglycemic effect. Bioavailability of anthocyanins in animal and human subjects is typically less than 1% of the intake (Del Rio, Borges & Crozier, 2010). Sorption of anthocyanins to DSF may improve the bioavailability of the anthocyanins, thus reproducing the hypoglycemic effect in the C57/Bl6 mouse model at lower doses. Anthocyanin metabolites produced by intestinal microflora (Del Rio et al., 2010; Kay, 2006) may contribute to the anti-diabetic effects of berries. It is possible that DSF partially protects bound anthocyanins and other polyphenols from degradation in the upper intestine thus enabling higher levels of these compounds to reach the lower intestine where they may be absorbed or metabolized to active compounds.
3.6. Antibacterial and anti-UTI activities of cranberry polyphenol-enriched DSF
To determine if cranberry components retained their antibacterial properties when bound to DSF, we evaluated the bactericidal effects of cranberry polyphenol-enriched DSF containing 11 mg/g of anthocyanins and 90 mg/g of proanthocyanidins against Staphylococcus aureus. Incorporation of the cranberry polyphenol-enriched DSF at a 10 mg/ml concentration in solid medium inhibited colony formation; however, at this dose the acidity of the medium greatly contributed to the bactericidal effects, not the presence of cranberry phytochemicals (Fig 2D, comparing bottom row 2nd and 3rd columns to DSF and controls at pH 4 and 7). At neutral pH, bacterial colony formation was prevented using 30 mg/g (3%) and 40 mg/g (4%) concentrations of cranberry polyphenol-enriched DSF, but not with the same concentrations of the DSF control (Fig 2D comparing 5th and 6th columns of top and bottom rows) indicating the antibacterial effect was due to cranberry constituents.
Agglutination of human red blood cells (HRBCs) by uropathogenic E. coli is used as a model for bacterial adhesion to uroepithelial cells (Howell et al., 2005) as P-fimbriated E. coli bind to glycolipid receptors common to both HRBCs and uroepithelial cells (Leffler & Svanborg-Eden, 1981). To determine if cranberry polyphenol-enriched DSF retained anti-adhesion/anti-UTI properties, we tested its ability to prevent E. coli-mediated agglutination of HRBCs in vitro. CJC shown to have positive results in this assay (Howell et al., 2005) and clinical trials (Avorn, Monane, Gurwitz, Glynn, Choodnovskiy & Lipsitz, 1994) served as a comparison. Two grams of cranberry polyphenol-enriched DSF (containing 32 mg/g of proanthocyanidins) was as effective in inhibiting HRBC agglutination as 240 ml (one serving) of CJC. Therefore DSF effectively captured and preserved the anti-adhesion constituents of cranberry, such as A-type proanthocyanidins.
4. Conclusions
We demonstrated that protein-rich flours, such as DSF, can effectively sorb, concentrate and stabilize bioactive polyphenols from juiced fruits and vegetables and separate them from sugars using a simple one-step mixing and separating procedure. We also observed that the blueberry and cranberry polyphenols retain their biological activity in the flour matrix. We hypothesize that the electrostatic interactions between flour and solute molecules and the large surface area of protein-rich DSF particles enable them to non-covalently bind moderately charged flavonoids, while highly polar sugars remain in solution. This process requires no chemical solvents or costly synthetic ion-exchange and affinity resins and creates value-added, nutritious, low-sugar protein-rich food ingredients.
Low glycemic index foods containing blueberry polyphenol-enriched DSF or other protein-rich ingredients made from soy, may be helpful for diabetes prevention and treatment, while the cranberry polyphenol-enriched DSF may provide a concentrated and low-sugar antibacterial alternative to sweetened cranberry juice. Future research should concentrate on further defining and optimizing the processes involved in sorption of polyphenols to soy and other flours, as well as studying the ability of these flours to bind other bioactive natural products such as terpenoids, steroids, alkaloids and peptides.
Most fresh fruits and vegetables are highly perishable and must be consumed within a few days unless refrigerated, frozen, dehydrated or preserved, therefore the DSF-sorption technology can be used to deliver the phytochemical benefits of fresh produce in a portable and shelf-stable format. Furthermore, this technology platform may be useful to capture bioactive phytochemicals from non-palatable plant parts, such as skins and peels, which are usually discarded.
This process of capturing, concentrating and preserving beneficial bioactive compounds from plants onto natural edible matrices, such as DSF, has the potential to create a new category of naturally fortified and scientifically validated food, dietary supplement (delivered as capsules or tablets) and cosmetic ingredients. Phytochemical-enriched natural matrices incorporated into familiar foods would provide a practical and convenient means of increasing dietary consumption of beneficial phytochemicals, such as polyphenols, and may be useful for the prevention and treatment of chronic and age-related disease.
Supplementary Material
Comparison of the percentage of blueberry anthocyanins sorbed by DSF, soy protein concentrate (SPC) and soy protein isolate (SPI). Each powder (5 g/l) was mixed with 50 ml of 5x diluted blueberry juice concentrate.
Highlights.
Defatted soybean flour (DSF) sorbs, concentrates and stabilizes berry polyphenols.
DSF does not sorb highly polar compounds, such as sugars.
Polyphenols sorbed to DSF retain their biological activity in vitro and in vivo.
Acknowledgments
The authors thank Andrew Oren, Kristen Moskal, Amanpreet Mashiana, Carly Rogers, Ruth Dorn, Flaubert Mbeunkui and Slavik Dushenkov for technical assistance.
DER designed blueberry experiments, performed statistical analysis and wrote the manuscript. MHG and NP performed cranberry experiments. PK performed animal experiments. DMC and AH performed cranberry antibacterial and anti-adhesion assay experiments, respectively. AP and FM did LC-MS analysis. BF helped with translational aspects of the research. IR and MAL are the principal investigators of the laboratories where the research was performed. All authors read and approved the final manuscript.
Abbreviations used
- ACN
anthocyanin
- PAC
proanthocyanidin
- DSF
defatted soybean flour
Footnotes
Disclosure Statement
DER, BF, MAL and IR have equity in Nutrasorb LLC, which has interest in developing polyphenol sorption technology.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amin ARMR, Kucuk O, Khuri FR, Shin DM. Perspectives for Cancer Prevention With Natural Compounds. Journal of Clinical Oncology. 2009;27(16):2712–2725. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avorn J, Monane M, Gurwitz JH, Glynn RJ, Choodnovskiy I, Lipsitz LA. Reduction of bacteriuria and pyuria after ingestion of cranberry juice. JAMA. 1994;271(10):751–754. doi: 10.1001/jama.1994.03510340041031. [DOI] [PubMed] [Google Scholar]
- Barbosa-Cesnik C, Brown MB, Buxton M, Zhang L, DeBusscher J, Foxman B. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52(1):23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzano LA. The high cost of not consuming fruits and vegetables. J Am Diet Assoc. 2006;106(9):1364–1368. doi: 10.1016/j.jada.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Buckow R, Kastell A, Terefe NS, Versteeg C. Pressure and temperature effects on degradation kinetics and storage stability of total anthocyanins in blueberry juice. J Agric Food Chem. 2010;58(18):10076–10084. doi: 10.1021/jf1015347. [DOI] [PubMed] [Google Scholar]
- Burns R. Methods for estimation of tannin in grain sorghum. Agronomy Journal. 1971;63:511–512. [Google Scholar]
- Castaneda-Ovando A, Pacheco-Hernandez MD, Paez-Hernandez ME, Rodriguez JA, Galan-Vidal CA. Chemical studies of anthocyanins: A review. Food Chemistry. 2009;113(4):859–871. [Google Scholar]
- Cederroth CR, Nef S. Soy, phytoestrogens and metabolism: A review. Molecular and Cellular Endocrinology. 2009;304(1–2):30–42. doi: 10.1016/j.mce.2009.02.027. [DOI] [PubMed] [Google Scholar]
- Del Rio D, Borges G, Crozier A. Berry flavonoids and phenolics: bioavailability and evidence of protective effects. British Journal of Nutrition. 2010;104:S67–S90. doi: 10.1017/S0007114510003958. [DOI] [PubMed] [Google Scholar]
- Ehlenfeldt MK, Prior RL. Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbus blueberry. Journal of Agricultural and Food Chemistry. 2001;49(5):2222–2227. doi: 10.1021/jf0013656. [DOI] [PubMed] [Google Scholar]
- Epp A, Larochelle A, Lovatsis D, Walter JE, Easton W, Farrell SA, Girouard L, Gupta C, Harvey MA, Robert M, Ross S, Schachter J, Schulz JA, Wilkie D, Ehman W, Domb S, Gagnon A, Hughes O, Konkin J, Lynch J, Marshall C. Recurrent urinary tract infection. J Obstet Gynaecol Can. 2010;32(11):1082–1090. doi: 10.1016/S1701-2163(16)34717-X. [DOI] [PubMed] [Google Scholar]
- Grace MH, Ribnicky DM, Kuhn P, Poulev A, Logendra S, Yousef GG, Raskin I, Lila MA. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, Vaccinium angustifolium Aiton. Phytomedicine. 2009;16(5):406–415. doi: 10.1016/j.phymed.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman AE, Butler LG. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981;256(9):4494–4497. [PubMed] [Google Scholar]
- Hagerman AE, Rice ME, Ritchard NT. Mechanisms of protein precipitation for two tannins, pentagalloyl glucose and epicatechin(16) (4 -> 8) catechin (procyanidin) Journal of Agricultural and Food Chemistry. 1998;46(7):2590–2595. [Google Scholar]
- Hanhineva K, Torronen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkanen H, Poutanen K. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11(4):1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell AB, Reed JD, Krueger CG, Winterbottom R, Cunningham DG, Leahy M. A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity. Phytochemistry. 2005;66(18):2281–2291. doi: 10.1016/j.phytochem.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Vol. 2011. CDC; 2011. http://apps.nccd.cdc.gov/5ADaySurveillance/ [Google Scholar]
- 2011 http://www.wildblueberries.com/health_benefits/glycemic.php.
- Jellin JM, Gregory P, Batz F, Hitchens K. Pharmacist’s Letter/Prescriber’s Letter Natural Medicines Comprehensive Database. Stockton, CA: Therapeutic Research Faculty; 2005. [Google Scholar]
- Jepson RG, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database Syst Rev. 2008;(1):CD001321. doi: 10.1002/14651858.CD001321.pub4. [DOI] [PubMed] [Google Scholar]
- Joseph J, Cole G, Head E, Ingram D. Nutrition, Brain Aging, and Neurodegeneration. Journal of Neuroscience. 2009;29(41):12795–12801. doi: 10.1523/JNEUROSCI.3520-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay CD. Aspects of anthocyanin absorption, metabolism and pharmacokinetics in humans. Nutrition Research Reviews. 2006;19(1):137–146. doi: 10.1079/NRR2005116. [DOI] [PubMed] [Google Scholar]
- Kechinski CP, Guimaraes PV, Norena CP, Tessaro IC, Marczak LD. Degradation kinetics of anthocyanin in blueberry juice during thermal treatment. J Food Sci. 2010;75(2):C173–176. doi: 10.1111/j.1750-3841.2009.01479.x. [DOI] [PubMed] [Google Scholar]
- Lacombe A, Wu VCH, Tyler S, Edwards K. Antimicrobial action of the American cranberry constituents; phenolics, anthocyanins, and organic acids, against Escherichia coli O157:H7. International Journal of Food Microbiology. 2010;139(1–2):102–107. doi: 10.1016/j.ijfoodmicro.2010.01.035. [DOI] [PubMed] [Google Scholar]
- Lee J, Durst RW, Wrolstad RE. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int. 2005;88(5):1269–1278. [PubMed] [Google Scholar]
- Leffler H, Svanborg-Eden C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau LC, Couture A, Spoor D, Benhaddou-Andaloussi A, Harris C, Meddah B, Leduc C, Burt A, Vuong T, Mai Le P, Prentki M, Bennett SA, Arnason JT, Haddad PS. Anti-diabetic properties of the Canadian lowbush blueberry Vaccinium angustifolium Ait. Phytomedicine. 2006;13(9–10):612–623. doi: 10.1016/j.phymed.2006.08.005. [DOI] [PubMed] [Google Scholar]
- Masuko T, Minami A, Iwasaki N, Majima T, Nishimura S, Lee YC. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal Biochem. 2005;339(1):69–72. doi: 10.1016/j.ab.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Neto CC. Cranberry and blueberry: evidence for protective effects against cancer and vascular diseases. Mol Nutr Food Res. 2007;51(6):652–664. doi: 10.1002/mnfr.200600279. [DOI] [PubMed] [Google Scholar]
- Puupponen-Pimia R, Nohynek L, Alakomi HL, Oksman-Caldentey KM. Bioactive berry compounds - novel tools against human pathogens. Applied Microbiology and Biotechnology. 2005;67(1):8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- Rawel HM, Czajka D, Rohn S, Kroll J. Interactions of different phenolic acids and flavonoids with soy proteins. Int J Biol Macromol. 2002;30(3–4):137–150. doi: 10.1016/s0141-8130(02)00016-8. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- Sobota AE. Inhibition of Bacterial Adherence by Cranberry Juice - Potential Use for the Treatment of Urinary-Tract Infections. Journal of Urology. 1984;131(5):1013–1016. doi: 10.1016/s0022-5347(17)50751-x. [DOI] [PubMed] [Google Scholar]
- Stull AJ, Cash KC, Johnson WD, Champagne CM, Cefalu WT. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J Nutr. 2010;140(10):1764–1768. doi: 10.3945/jn.110.125336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of AMP-activated protein kinase in diabetic mice. J Nutr. 2010;140(3):527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- Wang SY, Stretch AW. Antioxidant capacity in cranberry is influenced by cultivar and storage temperature. J Agric Food Chem. 2001;49(2):969–974. doi: 10.1021/jf001206m. [DOI] [PubMed] [Google Scholar]
- Williams RJ, Spencer JP, Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36(7):838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Wu XL, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. Journal of Agricultural and Food Chemistry. 2005;53(7):2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of the percentage of blueberry anthocyanins sorbed by DSF, soy protein concentrate (SPC) and soy protein isolate (SPI). Each powder (5 g/l) was mixed with 50 ml of 5x diluted blueberry juice concentrate.