Abstract
Microglia are the primary immune cells in the brain. Under pathological conditions, they become activated and participate in scavenging, inflammation and tissue repair in response to brain injury. While the function and underlying mechanism of activated microglia have been intensively studied in the past decades, physiological functions of resting microglia remain largely underestimated. In our recent work, by simultaneously monitoring both the motility of resting microglial processes and the activity of surrounding neurons in intact zebrafish optic tectum, we examined the interaction between resting microglia and neurons. Local increase in neuronal activity attracts resting microglial processes and drives them to contact neurons with high levels of activity. This process is mediated by neuronal release of “find-me” signals such as ATP via pannexin-1 hemichannels and requires small Rho GTPase Rac in microglia. Reciprocally, the microglia-neuron contact reduces both the spontaneous and visually evoked activities of contacted neurons. We here summarize and explain the key results in the context of our previous work.
Keywords: resting microglia, neuronal activity, microglia-neuron contact, pannexin-1 hemichannel, optic tectum, glutamate uncaging, in vivo two-photon imaging, calcium imaging, in vivo whole-cell recording, zebrafish
Microglia, under physiological conditions, spend most of the time in a “resting” state, with highly motile processes surveying surrounding neural tissues.1-3 However, it remains unknown why such energy-consuming behavior of resting microglia is required. Is the motility of resting microglial processes random or instructed by certain micro-environmental signals? What is the physiological function of this motility? Previous studies on resting microglia in the mouse cortex suggest a possible role of neural activity in regulating resting microglial motility.4,5 However, it is still unclear whether and how resting microglial processes find specific neuronal targets for making contacts and what the effect of such contact on neural functions is. Taking advantage of the larval zebrafish model with the transparency of its brain and the availability of genetic tools, we addressed these questions in the previous work.3
Demonstration of Contact between Resting Microglial Process and Neuronal Soma
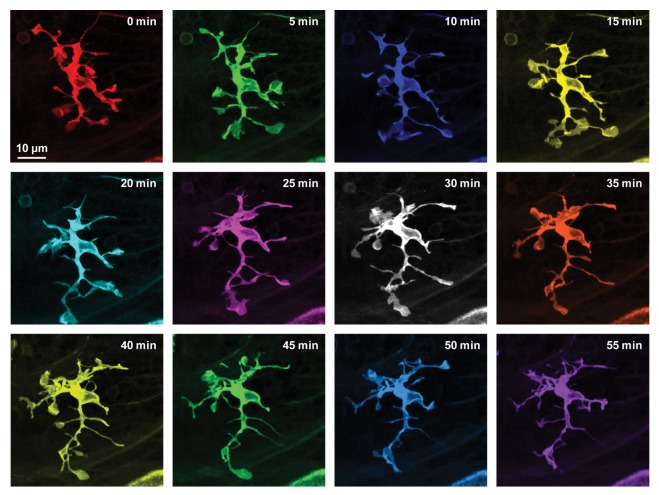
Using in vivo time-lapse confocal and two-photon imaging of the visual center optic tectum in living larval zebrafish, we first monitored changes in the morphology of microglia under resting state. Similar to the typical morphological properties of resting microglia in the mouse cortex,1,2 resting microglia in zebrafish are also highly branched with dynamic processes ended with either stick-like or bulbous tips (Fig. 1). These two differently shaped tips can be inter-converted as the process moves around. The bulbous ending forms rapidly through expansion from a stick-like ending, and stalls on the contacted neuronal soma for several minutes before gradually shrinking back again. The existence of resting microglia-neuron contact is further confirmed by using 3-dimensional reconstruction and transmission electron microscopy technique.
Figure 1. High dynamics of resting microglia in intact larval zebrafish. Series images of a resting microglial cell acquired every 5 with in vivo time-lapse confocal imaging of the optic tectum in a 6-dpf Tg(Apo-E:eGFP) zebrafish larva, in which eGFP is specifically expressed in microglia. Images at different time points are shown in different colors.
Neuronal Activity Steers the Motility of Resting Microglial Processes and Induces the Formation of Microglia-Neuron Contact
In previous studies, global increase or decrease of neural activity in vivo by using the GABAA receptor inhibitor bicuculline or the voltage-gated sodium channel blocker TTX, respectively, could oppositely regulate the dynamics of resting microglia.2,4,5 In zebrafish, we further proved that neural activity plays an instructive role in steering the motility of resting microglial processes and the formation of microglia-neuron contact. Using glutamate uncaging, we locally upregulated neuronal activity in a small brain region of the intact zebrafish larvae. Glutamate uncaging, a non-invasive approach, can efficiently induce a local increase of neural activity.6 We applied repetitive glutamate uncaging about 20 µm away from microglial soma, and found that the processes of resting microglia can gradually navigate toward the uncaging side at about 10 min after the uncaging onset. Furthermore, more bulbous tips are formed at the uncaging side, indicating an elevated formation of microglia-neuron contact in responding to the local increase of neuronal activity in vivo. Different from the microglia in culture and mammalian brain slices,7-9 we found no ionotropic glutamate receptors expressed on microglia in the optic tectum of zebrafish larvae using in vivo whole-cell recording, excluding the possibility that uncaged glutamate directly acts on resting microglia.
Importantly, the formation of resting microglial bulbous endings can also be regulated by natural sensory inputs, e.g., visual stimuli, which can globally increase neuronal activity in the optic tectum. However, after global downregulation of neural activity via pre-incubation of larvae with TTX, the number of bulbous endings is markedly decreased. All these results further confirm an instructive role of neuronal activity in regulating the dynamics of resting microglial processes and the formation of microglia-neuron contact.
Molecular and Cellular Mechanisms of Neuronal Activity-Induced Changes in Microglial Dynamics
Having shown that local elevation of neuronal activity can induce the formation of microglial bulbous endings wrapping neuronal somata, we next asked what might be mechanisms by which neurons with high activity “talk” to resting microglia. We found that this process requires the membrane depolarization-activated pannexin-1 hemichannels on tectal neurons and the ATP/P2 purinergic receptor signaling between neurons and microglia. Pannexin-1, a large pore-like hemichannel, is widely expressed in the central nervous system.10-13 The opening of pannexin-1 is gated by some cellular signals, such as membrane depolarization, intracellular calcium and so on.14 Small molecules, including ATP, NAD and PGE2, can be released through these hemichannels.10,13,15 Using whole-mount in situ hybridization and in vivo whole-cell recording, we found that the functional pannexin-1 hemichannels are expressed in tectal neurons but not microglia. After impairing the function of pannexin channels by drug treatment or morpholino-mediated genetic downregulation, glutamate uncaging-induced orientated movement of microglial processes and formation of bulbous contacts were prevented. Similar results were observed when we applied the ATP-hydrolyzing enzyme apyrase or the P2 purinergic receptor blocker suramin.
Neuronal activity steers resting microglial processes by facilitating the redistribution of the cytoskeleton protein small Rho GTPase Rac. Rac is known to be required for the membrane protrusion and migration of many types of cells,16 including zebrafish germ cells.17 Using confocal FRET imaging, we found that the Rac FRET intensity is significantly increased in microglial processes, especially at bulbous endings, at the uncaging side after repetitive glutamate uncaging. It is immediately followed by the oriented movement of microglial processes and the formation of bulbous endings. However, if we genetically inhibited endogenous Rac activity in microglia, those neuronal activity-induced microglial morphological changes could not be observed. Taken together, we identify the underlying mechanisms by which neurons with high activity signal to resting microglia.
Physiological Significance of Resting Microglia-Neuron Contact
Considering the facilitated formation of microglia-neuron contact induced by neuronal activity increase, we wondered what the physiological function of such contact is. By simultaneously monitoring changes in both the morphology of microglial processes and Ca2+ activity of tectal neurons, we found that microglial processes preferentially contact with neurons that exhibit high levels of spontaneous activity before contact. We then mosaically overexpressed the human inward rectifier K+ channel Kir2.1 (Kir), which is used to reduce the excitability of neurons, and a non-conducting mutant version of Kir2.1 (mKir) in tectal neurons.18,19 In comparison with mKir-expressing neurons, Kir-expressing neurons show a lower probability of microglial contact. These results further indicate that resting microglia preferentially contact neurons with higher activities, consistent with the data obtained with glutamate uncaging.
While accumulating evidence shows that resting microglia respond to neural activity, we surprisedly noticed that resting microglia can also in turn regulate neuronal activity. In the zebrafish optic tectum, we found that such resting microglia-neuron contact can downregulate both spontaneous and visually evoked activities in contacted neurons. Interestingly, the decreases in both the frequency and magnitude of spontaneous Ca2+ activity are positively correlated with the duration of microglia-neuron contact. What is the consequence on neuronal firing after loss of resting microglia? In response to two-photon laser-induced focal injury, some microglia quickly trans-locate to the injury site. Consistent with the speculation based on our previous findings, the spontaneous activity of neurons within microglia pre-existing territories is significantly elevated after the translocation of microglia to the injury site. Meanwhile, in other cases with the similar injury but no microglial translocation, the neuronal activity is not significantly changed. Thus, our study reveals a functional regulation of neuronal activity by resting microglia under physiological conditions, offering a new way for homeostatic regulation of neuronal activity.
Collectively, our work brings forth and demonstrates a novel reciprocal regulation between resting microglia and neurons in vivo. We not only demonstrate an instructive role of neuronal activity in resting microglial motility, but also reveal, for the first time, a previously unappreciated function of microglia in homeostatic regulation of neuronal activity in the healthy brain (Fig. 2). Considering the bi-directional modulation between neurons and microglia, this study also represents a new perspective in understanding the physiological function of resting microglia.
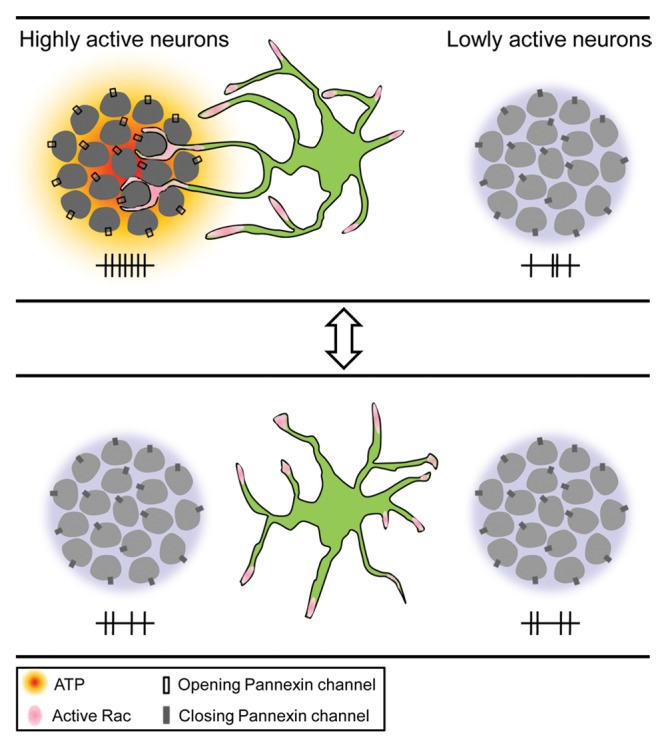
Figure 2. Working model. Highly active neurons attract resting microglial processes and induce the formation of microglia-neuron contact via ATP signaling (top). Such neuronal activity-driven microglia-neuron contact in turn reduces the activity of contacted neurons (bottom).
Acknowledgments
This work was supported by grants from the National Basic Research Program of China (2012CB945101, 2011CBA00400), Shanghai government (06dj14010, 07pj14107) and the Hundred Talents Program from Chinese Academy of Sciences.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/cib/article/24493
References
- 1.Davalos D, Grutzendler J, Yang G, Kim JV, Zuo Y, Jung S, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 2.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–8. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Du XF, Liu CS, Wen ZL, Du JL. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev Cell. 2012;23:1189–202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 4.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–80. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zito K, Scheuss V, Knott G, Hill T, Svoboda K. Rapid functional maturation of nascent dendritic spines. Neuron. 2009;61:247–58. doi: 10.1016/j.neuron.2008.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu LJ, Zhuo M. Resting microglial motility is independent of synaptic plasticity in mammalian brain. J Neurophysiol. 2008;99:2026–32. doi: 10.1152/jn.01210.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chen T, Koga K, Li XY, Zhuo M. Spinal microglial motility is independent of neuronal activity and plasticity in adult mice. Mol Pain. 2010;6:19. doi: 10.1186/1744-8069-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu GJ, Nagarajah R, Banati RB, Bennett MR. Glutamate induces directed chemotaxis of microglia. Eur J Neurosci. 2009;29:1108–18. doi: 10.1111/j.1460-9568.2009.06659.x. [DOI] [PubMed] [Google Scholar]
- 10.MacVicar BA, Thompson RJ. Non-junction functions of pannexin-1 channels. Trends Neurosci. 2010;33:93–102. doi: 10.1016/j.tins.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Ray A, Zoidl G, Weickert S, Wahle P, Dermietzel R. Site-specific and developmental expression of pannexin1 in the mouse nervous system. Eur J Neurosci. 2005;21:3277–90. doi: 10.1111/j.1460-9568.2005.04139.x. [DOI] [PubMed] [Google Scholar]
- 12.Vogt A, Hormuzdi SG, Monyer H. Pannexin1 and Pannexin2 expression in the developing and mature rat brain. Brain Res Mol Brain Res. 2005;141:113–20. doi: 10.1016/j.molbrainres.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Ransford GA, Fregien N, Qiu F, Dahl G, Conner GE, Salathe M. Pannexin 1 contributes to ATP release in airway epithelia. Am J Respir Cell Mol Biol. 2009;41:525–34. doi: 10.1165/rcmb.2008-0367OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruzzone R, Hormuzdi SG, Barbe MT, Herb A, Monyer H. Pannexins, a family of gap junction proteins expressed in brain. Proc Natl Acad Sci USA. 2003;100:13644–9. doi: 10.1073/pnas.2233464100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA. 2007;104:6436–41. doi: 10.1073/pnas.0611280104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridley AJ. Life at the leading edge. Cell. 2011;145:1012–22. doi: 10.1016/j.cell.2011.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Kardash E, Reichman-Fried M, Maître JL, Boldajipour B, Papusheva E, Messerschmidt EM, et al. A role for Rho GTPases and cell-cell adhesion in single-cell motility in vivo. Nat Cell Biol. 2010;12(sup pp 1-11):47–53. doi: 10.1038/ncb2003. [DOI] [PubMed] [Google Scholar]
- 18.Hua JY, Smear MC, Baier H, Smith SJ. Regulation of axon growth in vivo by activity-based competition. Nature. 2005;434:1022–6. doi: 10.1038/nature03409. [DOI] [PubMed] [Google Scholar]
- 19.Burrone J, O’Byrne M, Murthy VN. Multiple forms of synaptic plasticity triggered by selective suppression of activity in individual neurons. Nature. 2002;420:414–8. doi: 10.1038/nature01242. [DOI] [PubMed] [Google Scholar]