Abstract
Purpose
Prevention of chemotherapy-induced nausea and vomiting (CINV) remains an important goal for patients receiving chemotherapy. The objective of this study was to define, from the UK payer perspective, the cost-effectiveness of an antiemetic regimen using aprepitant, a selective neurokinin-1 receptor antagonist, for patients receiving chemotherapy for breast cancer.
Methods
A decision-analytic model was developed to compare an aprepitant regimen (aprepitant, ondansetron, and dexamethasone) with a standard UK antiemetic regimen (ondansetron, dexamethasone, and metoclopramide) for expected costs and health outcomes after single-day adjuvant chemotherapy for breast cancer. The model was populated with results from patients with breast cancer participating in a randomized trial of CINV preventative therapy for cycle 1 of single-day chemotherapy.
Results
During 5 days after chemotherapy, 64% of patients receiving the aprepitant regimen and 47% of those receiving the UK comparator regimen had a complete response to antiemetic therapy (no emesis and no rescue antiemetic therapy). A mean of £37.11 (78%) of the cost of aprepitant was offset by reduced health care resource utilization costs. The predicted gain in quality-adjusted lifeyears (QALYs) with the aprepitant regimen was 0.0048. The incremental cost effectiveness ratio (ICER) with aprepitant, relative to the UK comparator, was £10,847/QALY, which is well below the threshold commonly accepted in the UK of £20,000–£30,000/QALY.
Conclusion
The results of this study suggest that aprepitant is cost-effective for preventing CINV associated with chemotherapy for patients with breast cancer in the UK health care setting.
Keywords: antiemetic therapy, emesis, CINV
Introduction
The prevention of chemotherapy-induced nausea and vomiting (CINV) is an important element of supportive care in cancer. The occurrence of CINV is distressing for patients and can interfere with their daily activities, reducing quality of life and discouraging them from continuing with chemotherapy.1–5 Patients with CINV may require unplanned outpatient or inpatient medical care, adding to the cost of cancer therapy, and lost workdays can pose an economic burden for patients and their caregivers.6–8
Successful control of CINV in the first chemotherapy cycle is associated with reduced incidence of CINV in subsequent cycles.5,9,10 In addition, patients with no emesis in the acute phase (day 1 of chemotherapy) are less likely to have emesis in the delayed phase (day 2 onward);11 therefore, preventing CINV on the first day of the first chemotherapy is important.
Antiemetic therapy is prescribed according to the perceived emetogenic potential of chemotherapy. Patients with breast cancer usually receive adjuvant polychemotherapy with an anthracycline (doxorubicin or epirubicin) plus cyclophosphamide, with or without 5-fluorouracil, and in some cases in combination or sequence with a taxane.12,13 Anthracyclines and cyclophosphamide are each classified as moderately emetogenic chemotherapy (MEC), defined as causing emesis in 30%–90% of patients not receiving prophylactic therapy. However, when administered together their emetogenicity is increased.14 European consensus guidelines published in 2010 by the Multinational Association of Supportive Care in Cancer/European Society of Medical Oncology (MASCC/ESMO) recommend antiemetic regimens for preventing CINV associated with anthracycline and cyclophosphamide-based chemotherapy that are more efficacious than those recommended for other MEC regimens.15 Moreover, recent British and American guidelines classify combined anthracycline and cyclophosphamide-based regimens as highly emetogenic chemotherapy (HEC), defined as causing emesis in >90% of patients not receiving prophylactic therapy.16,17
For preventing acute CINV with anthracycline and cyclophosphamide-based chemotherapy, current MASCC/ESMO guidelines recommend a three-drug regimen of aprepitant (or fosaprepitant), a 5-HT3 receptor antagonist (5-HT3 RA), and dexamethasone on day 1.15 These guidelines recommend aprepitant on day 2 and day 3 for preventing delayed CINV.15 For other MEC regimens, the recommended MASCC/ESMO antiemetic regimen comprises palonosetron and dexamethasone on day 1 and dexamethasone on day 2 and day 3.15
Aprepitant is a selective neurokinin-1 receptor antagonist (NK-1 RA) that significantly improves the prevention of CINV for patients receiving HEC or MEC when administered in a three-drug regimen together with a 5-HT3 receptor antagonists (RA) and dexamethasone, as compared with a 5-HT3 RA and dexamethasone alone.11,18–23 This aprepitant regimen was effective, compared with the control regimen, in preventing CINV for patients with breast cancer for up to four cycles of anthracycline and cyclophosphamide-based chemotherapy.21,22 Rapoport et al23 reported that this aprepitant regimen was effective in preventing CINV for a diverse population of patients with a range of tumor types, receiving a variety of MEC regimens. Significantly more patients in their trial who received aprepitant, as compared with the control regimen, reported no vomiting and complete response to antiemetic therapy.
There is considerable variation in antiemetic regimens administered in clinical practice in the UK and, despite guideline recommendations for an aprepitant regimen to prevent CINV associated with anthracycline and cyclophosphamide-based chemotherapy,15,16 combinations of agents including 5-HT3 RA, dexamethasone, metoclopramide, and domperidone are often used. The objective of this cost-effectiveness analysis was to define, from the UK payer perspective, the cost-effectiveness of aprepitant, administered in combination with a 5-HT3 RA, and dexamethasone, for preventing CINV in patients receiving chemotherapy for breast cancer.
Methods
Overview and model design
A decision-analytic model was developed to estimate the expected costs and health outcomes over 5 days after single-day chemotherapy for breast cancer, when using an aprepitant-containing antiemetic regimen compared with an antiemetic regimen commonly used in UK clinical practice (Table 1). The aprepitant regimen meets current European and American antiemetic guidelines for anthracycline and cyclophosphamide-based chemotherapy,15,17 while the comparator regimen reflects current clinical practice in the UK, based on recommendations by the London Cancer New Drugs Group16 and feedback from UK clinicians.
Table 1.
Treatment regimens for prevention of CINV used in the model
| Antiemetic regimen | CINV prophylaxis | Day 1a
|
Days 2 and 3 | |
|---|---|---|---|---|
| Before chemotherapy | 8 hours after 1st dose | |||
| Aprepitant regimen | Aprepitant | 125 mg PO once | – | 80 mg PO OD |
| Ondansetron | 8 mg PO | 8 mg PO | – | |
| Dexamethasone | 12 mg PO once | – | – | |
| UK comparator regimen | Ondansetron | 8 mg PO once | – | – |
| Dexamethasone | 20 mg IV once | – | 8 mg PO OD | |
| Metoclopramide | – | – | 20 mg PO TID | |
| Clinical trial control regimen23 | Ondansetron | 8 mg PO | 8 mg PO | 8 mg PO BID |
| Dexamethasone | 20 mg PO once | – | – | |
| Alternative UK comparator regimen (sensitivity analysis) | Ondansetron | 8 mg PO once | – | 8 mg PO BID |
| Dexamethasone | 20 mg IV once | – | 8 mg PO OD | |
| Metoclopramide | – | – | 20 mg TID | |
Note:
Chemotherapy was administered on day 1.
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; PO, oral; OD, once daily; IV, intravenous; TID, three times a day; BID, two times daily; UK, United Kingdom.
The model was populated with clinical results and health care resource utilization from an analysis of patients with breast cancer in the clinical trial reported by Rapoport et al.23 The control regimen used in this clinical trial is not in line with current guidelines15,17 and is not the regimen commonly used in UK clinical practice. For this reason, we selected the UK comparator regimen as the comparator for our analysis (Table 1). Given the lack of efficacy data for the UK comparator regimen compared to the aprepitant regimen, the model assumes that the UK comparator regimen has efficacy equal to that shown by the trial comparator regimen (Table 1). We tested this assumption in sensitivity analyses varying the efficacy of the UK comparator regimen. The economic evaluation was conducted from the perspective of the UK National Health Service (NHS), in line with current clinical practice, and valued in British sterling (£). No discounting was applied to costs or health effects because of the short time horizon.
Data sources and assumptions
Reference clinical trial and patient population
The reference clinical trial23 used in the model was a doubleblind, randomized, multinational trial of CINV preventative therapy, completed in 2008 (National Clincial Trials registry number: NCT00337727). Enrolled patients were adults with a range of tumor types who were naïve to emetogenic chemotherapy and scheduled to receive cycle 1 of single-day MEC. In this study, anthracycline and cyclophosphamide-based regimens were considered to be MEC; therefore, patients receiving these combination regimens were included. Of the 949 patients screened, 848 were randomized, with 101 (10.6%) patients excluded during screening (please refer to original publication by Rapoport et al23 for further details). The average age of the 848 randomized patients was 57 years; 77% were female, and 69% were white. The most common malignancies were breast cancer (52%), colorectal cancer (20%), and lung cancer (13%). Patients were randomly assigned to receive either an aprepitant-containing regimen or the control regimen of ondansetron and dexamethasone (Table 1). The primary endpoint was the proportion of patients with no vomiting during 120 hours (5 days) after receiving chemotherapy, and the key secondary endpoint was the proportion of patients reporting complete response, defined as no emetic episodes and no use of rescue medication during the 5 days after initiation of chemotherapy. Self-completed patient diaries were used to record emetic episodes, use of rescue therapy, and nausea severity on a daily basis for 5 days. More than 98% of the patients completed their medication according to the protocol.
The trial included 438 patients with breast cancer, of whom 193/218 (88.5%) in the aprepitant group and 189/220 (85.9%) in the control group received anthracycline and cyclophosphamide-based chemotherapy.23 Remaining patients received a variety of MEC regimens. For the purpose of this analysis, we used results from 428 patients with breast cancer who received at least one dose of the study drug and had at least one post-treatment assessment (Merck Sharp and Dohme Ltd, data on file, 2011).23 Analysis of the subgroup of patients with breast cancer was not prespecified but was selected for this cost-effectiveness analysis to allow a homogeneous patient population. In patients with breast cancer, the efficacy of both control and aprepitant regimens was lower, while the relative efficacy with aprepitant was greater, than in patients with other tumor types, with respect to the primary and key secondary endpoints. These results could be expected, both due to the higher emetogenicity of anthracycline and cyclophosphamide-based regimens as compared with other MEC regimens and because almost all patients in the breast cancer group were women, who are known to be more susceptible to CINV.10
Resource utilization and costs
Health care resource utilization data, including health care contacts, medication, and rescue antiemetic therapy use, were based on information for patients with breast cancer enrolled in the reference clinical trial (Merck Sharp and Dohme Ltd, data on file, 2011).23 There were no differences between treatment groups in the incidence or categories of adverse events; therefore, specific adverse events were not incorporated into the model. Nonetheless, any health care resource utilization associated with adverse events would have been captured. Health care contacts included hospitalizations, emergency department visits, outpatient care by physicians and nurses, visits for laboratory tests, and home health care visits.
Costs assigned to health care resource use were derived from the Personal Social Services Research Unit for 201024 and the Department of Health NHS Reference Costs for 2009–2010.25 Drug costs were derived from the Monthly Index of Medical Specialties (May 2011),26 the British National Formulary (BNF 61, March 2011),27 and the NHS Drug Tariff (May 2011),28 and weighted based on market share (Merck Sharp and Dohme Ltd, data on file, 2012). All health care resource costs were specific to the UK.
Utilities and health outcomes
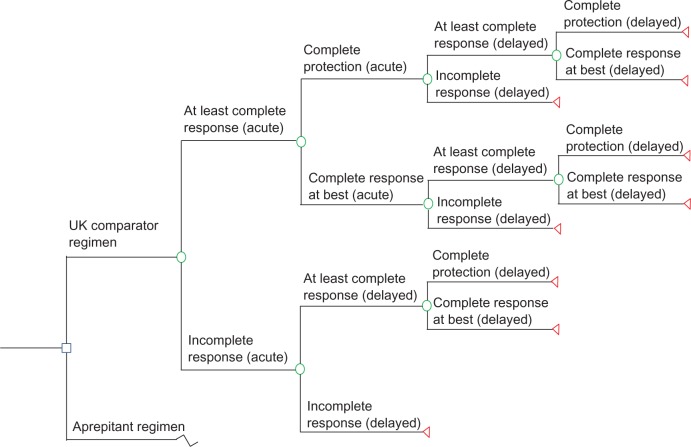
The model was populated with data from the first cycle of chemotherapy and included 5 days, counting the day of chemotherapy administration as day 1. The health outcome complete response (CR) was the key secondary endpoint in the reference clinical trial and, for the purpose of the model, was subdivided into 2 mutually exclusive health outcomes: complete protection (CP) and complete response at best (CRB). The health outcome incomplete response (IR) was assigned to patients who did not achieve CR. All outcomes are defined in Table 2. The outcomes C P, CRB, and IR were combined in the model for the acute phase (day 1) and the delayed phase (day 2–day 5) to produce nine possible health states, as depicted in Figure 1. Table 2 summarizes the probabilities for each of these health outcomes, derived from the reference trial results (Merck Sharp and Dohme Ltd, data on file, 2011).23
Table 2.
CINV-related health state probabilities based on modified intent-to-treata analyses of patients with breast cancer from a clinical trial of aprepitant23
| Health state outcome by phaseb
|
Clinical trial
|
||
|---|---|---|---|
| Acute phase (day 1) | Delayed phase (days 2–5) | Aprepitant regimen (n = 212c)
|
Control regimen (n = 216c)
|
| n (%) | n (%) | ||
| Complete protection | Complete protection | 123 (58.0) | 83 (38.4) |
| Complete response at best | 6 (2.8) | 10 (4.6) | |
| Incomplete response | 35 (16.5) | 49 (22.7) | |
| Complete response at best | Complete protection | 0 (0) | 2 (0.9) |
| Complete response at best | 6 (2.8) | 6 (2.8) | |
| Incomplete response | 10 (4.7) | 6 (2.8) | |
| Incomplete response | Complete protection | 3 (1.4) | 11 (5.1) |
| Complete response at best | 2 (0.9) | 3 (1.4) | |
| Incomplete response | 27 (12.7) | 46 (21.3) | |
Notes:
Patients included in modified intent-to-treat analyses were those who received moderately emetogenic chemotherapy, received at least one dose of study drug, and had at least one post-treatment assessment;
complete response, defined in the reference trial23 as no emesis and no rescue antiemetic therapy, was subdivided into two mutually exclusive health outcomes: (1) Complete protection was defined as no emesis; no rescue antiemetic therapy; and maximum nausea <25 mm on 100 mm visual analog scale scored from 0 (no nausea) to 100 (nausea as bad as it can be); and (2) complete response at best, which describes those who achieved complete response but not complete protection. Incomplete response was defined as some emesis or some use of rescue antiemetic therapy;
the reference clinical trial included a total of 438 patients with breast cancer.23 For the purpose of this analysis, we used results for 428 patients with breast cancer who received at least one dose of study drug and had at least one post-treatment assessment; 212 of these received the aprepitant regimen and 216 received the control regimen.
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; n, number.
Figure 1.
Model decision tree depicting the nine possible health states, each marked by a triangle, calculated directly from clinical trial data,23 that were included in the model.
Measures of utility are used in health economic evaluations to summarize quality of life in a particular health state. They are reported on a scale from 0 to 1, where 0 represents death and 1 represents perfect health. The utility value for a health state and the time spent in that health state are combined to produce the quality-adjusted lifeday (QALD) or quality-adjusted lifeyear (QALY). QALDs and QALYs are commonly used by health technology appraisal agencies globally as their preferred generic measure of health-related quality of life. For the model base case (ie, the modeled scenario which is expected to most closely reflect actual clinical practice), we assigned utilities to each health state in the model, as per Grunberg et al.29 They elicited QALY scores using a visual analog scale for chemotherapy states, in the absence and presence of nausea and vomiting. We assigned a value of 0.79 to the outcome CP, which corresponds to chemotherapy with no appreciable nausea or emesis, and a value of 0.27 to IR, a state in which emesis or nausea, or both, were present. We assigned a value of 0.594 to CRB, a state including nausea but not requiring rescue therapy, by normalizing the Börjeson et al30 mild nausea utility of 0.752 to the CP state (0.752 × 0.79). Utility weights, according to health states in the acute and delayed phases, are depicted in Table 3. Five-day QALY values were calculated by multiplying the full QALY value by 5/365.
Table 3.
Utility weights for CINV outcome (base case analysis)
| Health state in acute_delayed phase | 5-day QALYsa (base case) |
|---|---|
| IR_IR | 0.004 |
| IR_CRB | 0.007 |
| IR_CP | 0.009 |
| CRB_IR | 0.005 |
| CRB_CRB | 0.008 |
| CRB_CP | 0.010 |
| CP_IR | 0.005 |
| CP_CRB | 0.009 |
| CP_CP | 0.011 |
Notes:
5-day QALY = (QALY weight/365) ×5; QALY weights were as follows: IR, 0.27; CRB, 0.594; CP, 0.79.
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; CP, complete protection; CRB, complete response at best; IR, incomplete response.
The model was used to explore other CINV-related outcomes, associated costs, and gains in QALDs and QALYs. We calculated the incremental cost effectiveness ratio (ICER) as a measure of the cost-effectiveness of the aprepitant regimen relative to the UK comparator regimen, defined as the incremental cost divided by the incremental health gain in QALYs.
Sensitivity analyses
Sensitivity analyses are commonly used to assess uncertainty in economic models by evaluating the effect of differing model parameters, such as the utility values, costs, and efficacy, on the results of the economic analysis. We varied the utility assigned to CRB and IR by ±30%, and that assigned to CP between a lower bound equivalent to the CRB health state (0.594) and an upper bound of 0.90, as reported by Sun et al.31 We varied the preventative antiemetic drug costs by ±20% (rescue antiemetic drug costs were not varied). In addition, we evaluated results using an alternative UK comparator regimen (Table 1) in which ondansetron is continued for an additional 2 days, as is common practice in some UK centers. Finally, we performed threshold analyses to calculate the improvement in efficacy of the UK comparator regimen required to result in ICERs of £20,000/QALY and £30,000/QALY.
A probabilistic sensitivity analysis was conducted using bootstrap resampling techniques with 1000 iterations. Results of the probabilistic sensitivity analysis are presented as a cost-effectiveness acceptability curve, which illustrates the probability of the aprepitant regimen being cost-effective at different “willingness-to-pay” thresholds. Healthcare resource utilization by model health state was varied, according to the observed discrete distributions for each type of healthcare contact. Using the Grunberg et al29-based utility weights, utilities were varied using the resulting discrete distribution suggested by the trial population.
Analyses were performed using Microsoft Excel 2003 (Microsoft Corp, Redmond, WA, USA).
Results
Health outcomes
During 5 days after the first cycle of chemotherapy, 64% of patients receiving the aprepitant regimen and 47% of those receiving the comparator regimen had either CRB or CP against CINV (Merck Sharp and Dohme Ltd, data on file, 2011) (Table 4).23 More patients receiving the aprepitant regimen, versus the comparator regimen, remained emesis-free (70% versus 53%) and CINV-free (61% versus 43%) over the 5-day study period. The mean number of emetic events over the 5-day assessment period was 18.9% lower among patients treated with the aprepitant regimen than with the comparator regimen.
Table 4.
Summary of expected health outcomes and costs over 5 days after cycle 1 of chemotherapy23
| Aprepitant regimen (n = 212) | UK comparator regimena (n = 216) | Absolute differenceb (relative difference) | |
|---|---|---|---|
| Health outcome measure | |||
| Mean number emetic eventsc | 2.11 | 2.61 | −0.49 (−18.9%) |
| Complete responsed | 63.7% | 46.8% | 16.9% (36.2%) |
| Emesis-free over 5-day cycle | 69.8% | 52.8% | 17.0% (32.3%) |
| Mean number emesis-free daysc | 4.3 | 3.9 | 0.4 (9.5%) |
| CINV-free over 5-day cycle | 60.8% | 42.6% | 18.3% (42.9%) |
| Mean number CINV-free daysc | 3.9 | 3.4 | 0.5 (13.7%) |
| No impact on daily life due to nausea and vomiting | 69.8% | 56.5% | 13.3% (23.5%) |
| Quality-adjusted life days | 3.10 | 2.75 | 0.35 (12.6%) |
| Healthcare resource measure | |||
| Antiemetic regimen | |||
| Aprepitant | £47.43 | – | £47.43 |
| Ondansetron | £10.18 | £5.09 | £5.09 |
| Dexamethasone | £0.86 | £5.29 | −£4.44 |
| Metaclopramide | – | £0.42 | −£0.42 |
| Subtotal | £58.47 | £10.80 | £47.67 |
| Health-care resource | |||
| Outpatient cared | £6.71 | £7.22 | −£0.51 |
| Hospitalization | – | £36.32 | −£36.32 |
| Rescue medication | £0.57 | £1.09 | −£0.52 |
| Subtotal | £7.28 | £44.63 | −£37.35 |
| Total costs | £65.75 | £55.43 | £10.32 |
| Summary measures | |||
| Total aprepitant costs offset by HCR savings with use of aprepitant | £37.11 | ||
| Additional drug cost per emesis-free day gained with use of aprepitant | £28 | ||
| Additional drug cost per CINV-free day gained with use of aprepitant | £22 | ||
| Additional drug cost per emetic event avoided with use of aprepitant | £97 | ||
| Incremental cost-effectiveness ratio (ICER), £/QALY | £10,847 | ||
Notes:
The data shown here are from breast cancer patients who received the clinical trial control regimen in the reference clinical trial.23 For the purpose of this analysis, we have used this data as the best estimate of the efficacy of the UK comparator regimen, given the lack of efficacy data for the UK comparator regimen in comparison with an aprepitant regimen;
difference represents aprepitant regimen value minus comparator regimen value;
mean number over 5 days;
complete response includes patients with complete response at best or complete protection;
outpatient care includes visits to primary care physicians or medical specialists, visits for laboratory tests not mandated by the study protocol, home health care, and emergency room visits.
Abbreviations: CINV, chemotherapy-induced nausea and vomiting; £, pound sterling; HCR, health care resource; QALY, quality-adjusted life year; UK, United Kingdom.
The predicted gain in QALDs with the aprepitant regimen was 0.35 (Table 4), which equates to a gain of 0.0048 QALYs. There were no hospital days reported for the aprepitant arm, whereas 15 days were reported in the comparator arm amongst 3/216 (1.4%) of patients.
Base case costs and cost-effectiveness results
The projected drug and healthcare resource costs for the aprepitant and UK comparator regimens are presented in Table 4. As compared with the UK comparator regimen in the base case scenario, an average of £37.11 (78%) of the cost of aprepitant is offset by the reduction in health care resource utilization costs. Use of the aprepitant regimen was associated with an additional cost of £28 for each emesis-free day gained and £22 for each CINV-free day gained. The ICER with aprepitant, relative to the UK comparator, was £10,847/QALY.
Sensitivity analyses results
The results of sensitivity analyses demonstrate that ±30% changes in utility values for IR and CRB have minimal impact on the cost-effectiveness of aprepitant (Table 5). While the model is more sensitive to changing the utility values for CP, the cost-effectiveness of the aprepitant regimen remains below the threshold of £20,000/QALY over the range of utility values tested. A reduction of 20% in antiemetic regimen drug costs reduces the ICER with aprepitant, relative to the UK comparator regimen, from £10,847/QALY to £826/QALY, while a 20% increase in drug costs increases the ICER to £20,868/QALY.
Table 5.
Results of a sensitivity analysis varying QALY weights for the trial outcome measures
| Health state | QALY weight
|
ICER (£/QALY)
|
|||
|---|---|---|---|---|---|
| Base case | Lower bound | Upper bound | Lower bound | Upper bound | |
| Incomplete response | 0.27 | 0.189 | 0.35 | £9,440 | £12,746 |
| Complete response at best | 0.594 | 0.416 | 0.772 | £10,434 | £11,293 |
| Complete protection | 0.79 | 0.594 | 0.90 | £18,200 | £8,842 |
Abbreviations: QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio; £, pound sterling.
The efficacy of the UK comparator regimen was assumed in the base case analysis to be equivalent to that of the clinical trial control regimen. Threshold analysis results indicate that the efficacy of the UK comparator would need to improve by 9.9% or 16.2% for the ICER to reach a threshold of £20,000/QALY or £30,000/QALY, respectively.
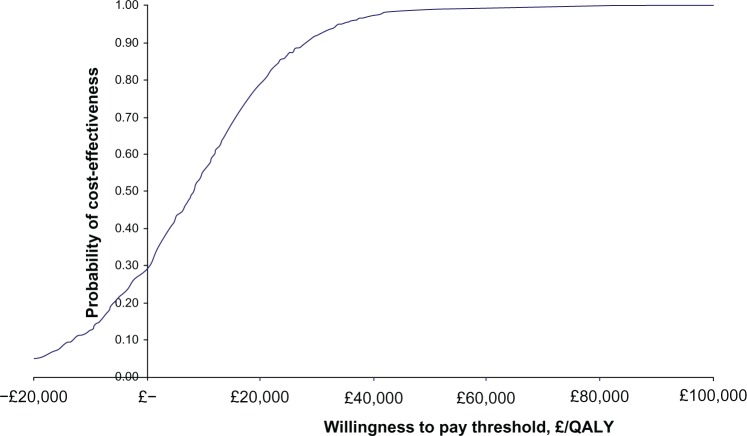
The cost-effectiveness acceptability curve indicates that the probability of the aprepitant regimen being cost-effective, compared with the UK comparator regimen, is 79% and 92% at “willingness-to-pay” thresholds of £20,000/QALY and £30,000/QALY, respectively (Figure 2).
Figure 2.
Cost-effectiveness acceptability curve depicting the expected marginal cost/QALY gained with aprepitant versus the UK clinical practice comparator regimen.
Note: The Y-axis shows the probability of the aprepitant regimen being cost-effective as a function of increasing levels of willingness to pay (shown on X-axis).
Abbreviations: £, pound sterling; QALY, quality-adjusted life year.
With use of the alternative UK comparator regimen (Table 1), an average of £57.47 (121%) of the cost of aprepitant is offset by reduced health care resource utilization costs. The aprepitant regimen is thus cost-saving compared with the alternative UK regimen.
Discussion
The aprepitant regimen had a positive impact on health outcomes and quality of life for patients with breast cancer receiving chemotherapy. In the base case analysis, an average of £37 or 78% of the cost of aprepitant was offset due to the better efficacy of the aprepitant regimen compared with the regimen representing the UK standard of care. The results of the cost-effectiveness analysis demonstrated that an aprepitant regimen, when compared with standard UK clinical practice, is cost-effective for preventing CINV in patients receiving chemotherapy for breast cancer with an ICER of £10,847/QALY. This ICER is well below the willingness-to-pay threshold of £20,000–£30,000/QALY that is commonly accepted in the UK.32 When the utility values, costs, and efficacy assigned to the regimens were varied in sensitivity analyses, the aprepitant regimen remained cost-effective, with ICERs below or equal to £20,000/QALY, with the exception of increasing the antiemetic regimen drug costs by 20%, which resulted in an ICER of £20,868/QALY.
Optimizing control of CINV in the first cycle of chemotherapy is already an important goal of cancer supportive care15,17 and is associated with a reduced incidence of CINV in subsequent chemotherapy cycles.5,9,10 Administration of the appropriate antiemetic regimen before cycle 1 of chemotherapy will become increasingly important as the provision of chemotherapy in the UK moves away from the traditional oncologist-led model toward nurse- and pharmacist-led chemotherapy clinics and home delivery.33 Use of aprepitant was associated with lower health care resource utilization compared with the comparator regimen, mainly due to fewer hospitalizations in the aprepitant group in the reference trial. Successful prevention of CINV has previously been shown to reduce outpatient health care resource use, including visits to a specialist and the emergency room.6,34
When compared with the alternative UK comparator regimen in which ondansetron is continued twice daily on day 2 and day 3 of the regimen, the aprepitant regimen was both more effective and cost-saving. This is likely because the efficacy of the alternative comparator regimen was not increased to account for the addition of ondansetron on day 2 and day 3, whereas the cost of the additional ondansetron was incorporated. This may be a reasonable assumption, however, because ondansetron has limited efficacy in preventing delayed CINV.35–38
One of the strengths of this study is the consistency of results across all outcomes in the model and in the sensitivity analyses. The aprepitant regimen applied in the study model is that used in clinical trials of anthracycline plus cyclophosphamide-based chemotherapy and other MEC regimens21,23 and represents the current standard of care for prevention of CINV associated with anthracycline plus cyclophosphamide-based chemotherapy as recommended by evidence-based, consensus guidelines for Europe and the US.15,17 Some patients included in this analysis received MEC regimens and, although the aprepitant regimen is not recommended for these patients in clinical guidelines, aprepitant is licensed for use with all MEC regimens.
There are some limitations in this economic analysis. The study published by Rapoport et al in 201023 was used as the reference trial, as it contains the most recent data regarding use of aprepitant in patients receiving chemotherapy for breast cancer and because the patient population was not limited to anthracycline and cyclophosphamide-based chemotherapy. However, there were no UK centers included in this study23 while the observed resource utilization was assumed to be applicable to a UK setting. Data from an earlier trial, which included patients with breast cancer restricted to anthracycline and cyclophosphamide-based chemotherapy,21 were not included in this analysis. We assumed for the purpose of the analysis that the efficacies of the trial and UK comparator regimens were the same. However, the UK regimen includes dexamethasone and metoclopramide in the delayed phase and so might be more effective than the trial comparator regimen in which only ondansetron is used in the delayed phase.15 The efficacy of the UK comparator regimen may have been underestimated; therefore, we varied the efficacy in sensitivity analyses and found that the efficacy would have to increase by 16% for the ICER to reach £30,000/QALY.
There are other potential limitations to this study. First, our analyses were based on UK list prices while actual prices may differ. Second, the results of the analysis were sensitive to hospitalizations, of which there were none in the aprepitant group and 0.069 hospital days per patient in the trial control group; therefore, if fewer hospitalizations are assumed with standard UK practice, the ICER increases. Third, the selectivity of patient eligibility criteria in the aprepitant clinical trials could also limit applicability to real-life clinical practice; for example, administration of an aprepitant regimen is untested for patients who experienced CINV in previous cycles of chemotherapy. Finally, we examined only one cycle of chemotherapy, and the cost-effectiveness of the aprepitant regimen over multiple chemotherapy cycles is uncertain. However, data from a prior study indicate that the antiemetic efficacy of the aprepitant regimen is maintained over multiple cycles of chemotherapy.22
Decision-analytic modeling has previously been used to study the cost-effectiveness of aprepitant for CINV prevention with MEC in Belgium.39 The aprepitant regimen was compared for patients receiving anthracycline and cyclophosphamide-based chemotherapy with the control regimen used in the Rapoport et al trial.23 The Belgian model, which drew on clinical data from a different trial22 and used health state preference measures different from those in the present study, found that the aprepitant regimen was associated with a gain of 0.014 QALYs and was the dominant approach from the perspective of the Belgian health care payer.
Further study is required to evaluate the use of aprepitant in the UK for preventing CINV associated with MEC over multiple cycles and in patients who experienced CINV in previous chemotherapy cycles. In addition, data are needed on the efficacy of the aprepitant regimen compared with current UK clinical practice. Finally, as our model only included direct medical costs of CINV, it would be of interest to also assess other medical costs and indirect costs, such as costs associated with missed work days for patients and their caregivers.
Control of CINV remains an important goal for patients receiving chemotherapy. In this time of growing demands on finite health care resources, assessment of the cost-effectiveness of therapies is becoming increasingly necessary. The aprepitant regimen has been shown to improve the control of CINV associated with a variety of MEC regimens, including those used among patients with breast cancer. The results of this study suggest that the use of aprepitant is cost-effective for preventing CINV associated with cycle 1 of single-day chemotherapy for patients with breast cancer in the UK health care setting.
Acknowledgments
This study was funded by Merck and Co, Inc, Whitehouse Station, NJ, USA. Medical writing and editorial assistance was provided by Elizabeth V Hillyer. This assistance was funded by Merck Sharp and Dohme Corp, a subsidiary of Merck and Co, Inc, Whitehouse Station, NJ, USA.
Footnotes
Disclosure
Samantha Humphreys and James Pellissier are employed by Merck Sharp and Dohme Ltd and Merck and Co, Inc, respectively. Alison Jones is a consultant to several pharmaceutical companies, including Merck Sharp and Dohme Ltd, Roche, Novartis, Celgene Corp, GlaxoSmithKline, Amgen, and Pfizer Inc. The authors have full control of all primary data and agree to allow the journal to review their data if requested.
References
- 1.Sun CC, Bodurka DC, Weaver CB, et al. Rankings and symptom assessments of side effects from chemotherapy: insights from experienced patients with ovarian cancer. Support Care Cancer. 2005;13(4):219–227. doi: 10.1007/s00520-004-0710-6. [DOI] [PubMed] [Google Scholar]
- 2.Kris MG. Why do we need another antiemetic? Just ask. J Clin Oncol. 2003;21(22):4077–4080. doi: 10.1200/JCO.2003.07.968. [DOI] [PubMed] [Google Scholar]
- 3.Ballatori E, Roila F, Ruggeri B, et al. The impact of chemotherapy-induced nausea and vomiting on health-related quality of life. Support Care Cancer. 2007;15(2):179–185. doi: 10.1007/s00520-006-0109-7. [DOI] [PubMed] [Google Scholar]
- 4.Glaus A, Knipping C, Morant R, et al. Chemotherapy-induced nausea and vomiting in routine practice: a European perspective. Support Care Cancer. 2004;12(10):708–715. doi: 10.1007/s00520-004-0662-x. [DOI] [PubMed] [Google Scholar]
- 5.Cohen L, de Moor CA, Eisenberg P, Ming EE, Hu H. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007;15(5):497–503. doi: 10.1007/s00520-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 6.Burke TA, Wisniewski T, Ernst FR. Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer. 2011;19(1):131–140. doi: 10.1007/s00520-009-0797-x. [DOI] [PubMed] [Google Scholar]
- 7.Ihbe-Heffinger A, Ehlken B, Bernard R, et al. The impact of delayed chemotherapy-induced nausea and vomiting on patients, health resource utilization, and costs in German cancer centers. Ann Oncol. 2004;15(3):526–536. doi: 10.1093/annonc/mdh110. [DOI] [PubMed] [Google Scholar]
- 8.Tina Shih YC, Xu Y, Elting LS. Costs of uncontrolled chemotherapy-induced nausea and vomiting among working-age cancer patients receiving highly or moderately emetogenic chemotherapy. Cancer. 2007;110(3):678–685. doi: 10.1002/cncr.22823. [DOI] [PubMed] [Google Scholar]
- 9.Morrow GR, Roscoe JA, Hickok JT, et al. Initial control of chemotherapy-induced nausea and vomiting in patient quality of life. Oncology (Williston Park) 1998;12(3 Suppl 4):32–37. [PubMed] [Google Scholar]
- 10.Hesketh PJ. Chemotherapy-induced nausea and vomiting. N Engl J Med. 2008;358(23):2482–2494. doi: 10.1056/NEJMra0706547. [DOI] [PubMed] [Google Scholar]
- 11.Warr DG, Grunberg SM, Gralla RJ, et al. The oral NK(1) antagonist aprepitant for the prevention of acute and delayed chemotherapy-induced nausea and vomiting: pooled data from 2 randomized, double-blind, placebo controlled trials. Eur J Cancer. 2005;41(9):1278–1285. doi: 10.1016/j.ejca.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 12.Peto R, Davies C, Godwin J, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomized trials. Lancet. 2012;379(9814):432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Institute for Health and Clinical Excellence (NICE) Early and locally advanced breast cancer: diagnosis and treatment. Full guideline (CG80) February2009[updated May 30, 2012]. Available from: http://www.nice.org.uk/CG0FullGuidelineAccessed Jun 2012
- 14.Grunberg SM, Osoba D, Hesketh PJ, et al. Evaluation of new antiemetic agents and definition of antineoplastic agent emetogenicity – an update. Support Care Cancer. 2005;13(2):80–84. doi: 10.1007/s00520-004-0718-y. [DOI] [PubMed] [Google Scholar]
- 15.Roila F, Herrstedt J, Aapro M, et al. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–v243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 16.University College Hospital NHS Foundation Trust Antiemetic Guidelines for Adult Patients Receiving Chemotherapy and Radiotherapy 2010Available from: http://www.medicinesresources.nhs.uk/upload/documents/Communities/London_CNDG/Antiemetic_guidelines_November_2010.pdfAccessed Jun 2012
- 17.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29(31):4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, doubleblind, placebo-controlled trial in patients receiving high-dose cisplatin – the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003;21(22):4112–4119. doi: 10.1200/JCO.2003.01.095. [DOI] [PubMed] [Google Scholar]
- 19.Poli-Bigelli S, Rodrigues-Pereira J, Carides AD, et al. Addition of the neurokinin 1 receptor antagonist aprepitant to standard antiemetic therapy improves control of chemotherapy-induced nausea and vomiting. Results from a randomized, double-blind, placebo-controlled trial in Latin America. Cancer. 2003;97(12):3090–3098. doi: 10.1002/cncr.11433. [DOI] [PubMed] [Google Scholar]
- 20.de Wit R, Herrstedt J, Rapoport B, et al. The oral NK(1) antagonist, aprepitant, given with standard antiemetics provides protection against nausea and vomiting over multiple cycles of cisplatin-based chemotherapy: a combined analysis of two randomized, placebo-controlled phase 3 clinical trials. Eur J Cancer. 2004;40(3):403–410. [PubMed] [Google Scholar]
- 21.Warr DG, Hesketh PJ, Gralla RJ, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol. 2005;23(12):2822–2830. doi: 10.1200/JCO.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 22.Herrstedt J, Muss HB, Warr DG, et al. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and emesis over multiple cycles of moderately emetogenic chemotherapy. Cancer. 2005;104(7):1548–1555. doi: 10.1002/cncr.21343. [DOI] [PubMed] [Google Scholar]
- 23.Rapoport BL, Jordan K, Boice JA, et al. Aprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with a broad range of moderately emetogenic chemotherapies and tumor types: a randomized, double-blind study. Support Care Cancer. 2010;18(4):23–431. doi: 10.1007/s00520-009-0680-9. [DOI] [PubMed] [Google Scholar]
- 24.Personal Social Services Research Unit Unit Costs of Health and Social Care 2010 Available from: http://www.pssru.ac.uk/project-pages/unit-costs/2010/index.phpAccessed Jun 2012
- 25.Department of Health NHS Reference Costs for 2009–2010 Appendix NSRC04: NHS trust and PCT combined reference cost schedules 2011Available from: https://www.gov.uk/government/publications/nhs-reference-costs-2009-2010Accessed Jun 2012
- 26.Monthly Index of Medical Specialities. [database on the Internet] London: Haymarket Medical Media; 2011Available from: http://www.mims.co.uk/go/mims_monthlyAccessed Jun 2012 [Google Scholar]
- 27.Joint Formulary Committee . British National Formulary. 61 edn. British Medical Association and the Royal Pharmaceutical Society of Great Britain; London, UK: Mar, 2011. [Google Scholar]
- 28.NHS Business Services Authority, NHS Prescription Services (compiled on behalf of the Department of Health) Drug Tariff. Norwich: TSO; May, 2011. 2011. [Google Scholar]
- 29.Grunberg SM, Boutin N, Ireland A, Miner S, Silveira J, Ashikaga T. Impact of nausea/vomiting on quality of life as a visual analogue scale-derived utility score. Support Care Cancer. 1996;4(6):435–439. doi: 10.1007/BF01880641. [DOI] [PubMed] [Google Scholar]
- 30.Börjeson S, Hursti TJ, Peterson C, et al. Similarities and differences in assessing nausea on a verbal category scale and a visual analogue scale. Cancer Nurs. 1997;20(4):260–266. doi: 10.1097/00002820-199708000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Sun CC, Bodurka DC, Donato ML, et al. Patient preferences regarding side effects of chemotherapy for ovarian cancer: do they change over time? Gynecol Oncol. 2002;87(1):118–128. doi: 10.1006/gyno.2002.6807. [DOI] [PubMed] [Google Scholar]
- 32.National Institute for Health and Clinical Excellence (NICE) Guide to the methods of technology appraisal June2008Available from: http://www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdfAccessed Jun 2012
- 33.National Chemotherapy Advisory Group Chemotherapy Services in England: Ensuring quality and safety UK Department of Health; 2009Available from: http://webarchive.nationalarchives.gov.uk/20130107105354. http://www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_104501.pdfAccessed Jun 2012 [Google Scholar]
- 34.Aapro M, Molassiotis A, Dicato M, et al. The effect of guideline-consistent antiemetic therapy on chemotherapy-induced nausea and vomiting (CINV): the Pan European Emesis Registry (PEER) Ann Oncol. 2012;23(8):1986–1992. doi: 10.1093/annonc/mds021. [DOI] [PubMed] [Google Scholar]
- 35.Kris MG, Hesketh PJ, Somerfield MR, et al. American Society of Clinical Oncology guideline for antiemetics in oncology: update 2006. J Clin Oncol. 2006;24(18):2932–2947. doi: 10.1200/JCO.2006.06.9591. [DOI] [PubMed] [Google Scholar]
- 36.Latreille J, Pater J, Johnston D, et al. Use of dexamethasone and granisetron in the control of delayed emesis for patients who receive highly emetogenic chemotherapy. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16(3):1174–1178. doi: 10.1200/JCO.1998.16.3.1174. [DOI] [PubMed] [Google Scholar]
- 37.Italian Group for Antiemetic Research Prevention of cisplatin-induced delayed emesis: still unsatisfactory. Support Care Cancer. 2000;8(3):229–232. [PubMed] [Google Scholar]
- 38.Gridelli C, Ianniello G, Ambrosini G, et al. A multicenter, double-blind, randomized trial comparing ondansetron versus ondansetron plus dexamethasone in the prophylaxis of cisplatin-induced delayed emesis. Int J Oncol. 1997;10(2):395–400. doi: 10.3892/ijo.10.2.395. [DOI] [PubMed] [Google Scholar]
- 39.Annemans L, Strens D, Lox E, Petit C, Malonne H. Cost-effectiveness analysis of aprepitant in the prevention of chemotherapy-induced nausea and vomiting in Belgium. Support Care Cancer. 2008;16(8):905–915. doi: 10.1007/s00520-007-0349-1. [DOI] [PubMed] [Google Scholar]